


The article centers on the critical understanding of sterile fill finish, emphasizing its definition, evolution, and compliance within the pharmaceutical industry. Sterile fill finish is essential for guaranteeing the safety and effectiveness of injectable drugs, as it involves placing a clean product into its final container under controlled conditions. This process is vital for safeguarding the integrity of clinical research and ensuring regulatory approvals.
The sterile fill finish process represents a critical final step in ensuring the safety and efficacy of pharmaceutical products, particularly injectables. With the rising demand for biologics and complex therapies, it is essential for stakeholders in clinical research and manufacturing to grasp the intricacies of this process. However, as regulations evolve and technological advancements emerge, industry players face the challenge of navigating the complexities of sterile fill finish to maintain compliance and ensure product integrity. This exploration delves into the definition, historical evolution, and key components of sterile fill finish, revealing its pivotal role in drug development and regulatory adherence.
Aseptic completion signifies the ultimate and vital phase in the sterile fill finish of aseptic pharmaceutical items. This process involves placing a clean product into its final container under meticulously controlled conditions as part of the sterile fill finish to ensure cleanliness. It is essential for ensuring that the sterile fill finish of pharmaceuticals, especially injectables, is safe for patient administration. The importance of sterile fill finish extends into clinical research, where it plays a crucial role in assessing the quality and effectiveness of the drug being studied.
A strong aseptic completion process, including a sterile fill finish, not only protects the integrity of clinical trials but also guarantees that the results are trustworthy, which is essential for regulatory approvals and subsequent market entry for innovative therapies. With bioaccess®'s expert services, including regulatory approval assistance and patient recruitment, clinical research can be accelerated, allowing Medtech, Biopharma, and Radiopharma startups to move to the next phase of their studies 40% faster. As industry leaders emphasize, maintaining stringent aseptic protocols during the sterile fill finish is crucial to prevent microbial contamination, thereby extending the drug's shelf life and ensuring patient safety.
With over 200 biologics currently available and more than 10,000 candidates undergoing clinical evaluation, the demand for expertise in sterile fill finish is more pronounced than ever, particularly in the context of rising biologic therapies and complex chronic conditions requiring injectable treatments. The effect of sterile fill finish on drug effectiveness in clinical studies cannot be exaggerated, as it directly affects the results that regulatory agencies depend on for product authorization.
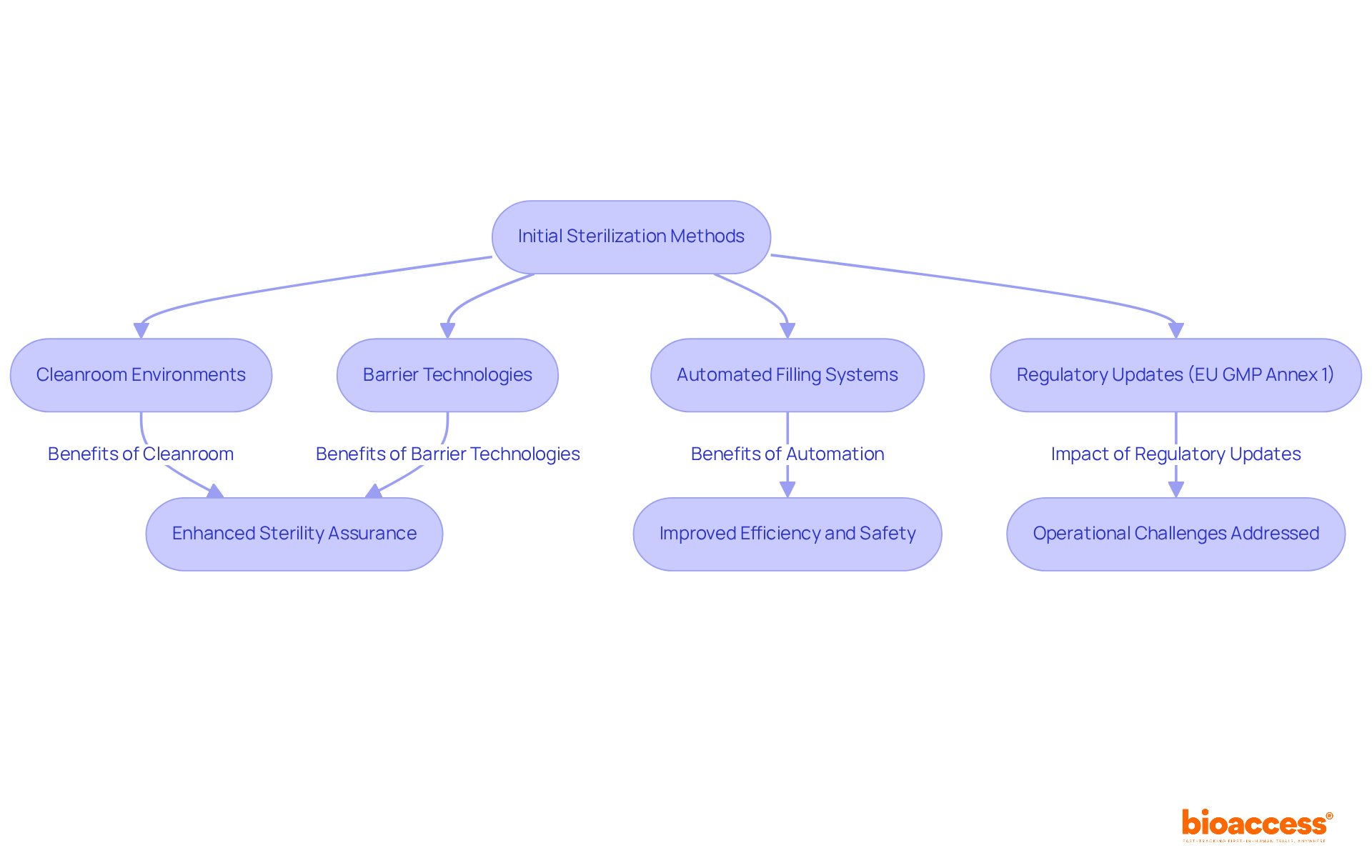
The advancement of sterile fill finish packaging procedures has significantly progressed since the initial stages of pharmaceutical production, which relied on basic sterilization methods. Over the decades, technological advancements and a deeper understanding of microbiology have driven the development of more sophisticated methods. Key innovations include:
These advancements have greatly enhanced the sterility assurance of the sterile fill finish process.
Recent regulatory updates, particularly the EU GMP Annex 1 revision, have introduced stricter controls and innovative approaches to ensure product safety and compliance. These modifications reflect an increasing acknowledgment of the critical nature of the fill-finish phase, the final step in drug manufacturing where purified liquids are placed into containers. Errors during this phase can result in significant project delays, increased costs, and even jeopardize the success of pharmaceutical companies.
As of 2025, the industry continues to witness advancements in sterile fill finish technology, focusing on enhancing efficiency and safety. For instance, the case study titled 'Technological Advancements in Sterile Fill Finish' illustrates how innovations over the past five years have addressed operational challenges, ensuring that the sterile fill finish process remains robust and reliable. Additionally, the case study on "Operational Obstacles in Pharmaceutical Manufacturing" emphasizes the importance of overcoming these challenges, highlighting the need for specialized facilities and equipment to prevent production bottlenecks. This ongoing evolution underscores the necessity for pharmaceutical manufacturers to stay informed of best practices and regulatory requirements in the sterile fill finish domain. As Josh Russell, VP of Technical Sales, remarked, 'The sterile fill finish step is the last and one of the most essential stages of the production process, during which the drug formulation - often a clean liquid - is moved into its final container.
The sterile fill finish process fundamentally relies on several key components, including filling equipment, sterilization methods, and stringent environmental controls. Isolators and Restricted Access Barrier Systems (RABS) play a pivotal role in establishing aseptic environments that significantly mitigate contamination risks. These advanced technologies foster a controlled environment where human intervention is minimized, thereby enhancing sterility. For instance, modern clean rooms achieve recovery counts of approximately 0.5% of total samples taken, underscoring their effectiveness in maintaining a contamination-free environment.
Moreover, advanced monitoring systems are integral to this process, continuously sampling air for viable organisms and non-viable particulates to ensure compliance with regulatory standards. The integration of these technologies not only enhances the sterile fill finish of the final product but also adheres to Good Manufacturing Practices (GMP), which are essential for regulatory compliance. As the industry evolves, there is a notable shift towards entirely automated, robotics-driven filling systems, reflecting a broader trend of enhanced efficiency and adaptability in sterile fill finish operations. This evolution is further emphasized by the growing demand for aseptic manufacturing capabilities, particularly for biologics that cannot undergo terminal sterilization, making the role of isolators and RABS more critical than ever.

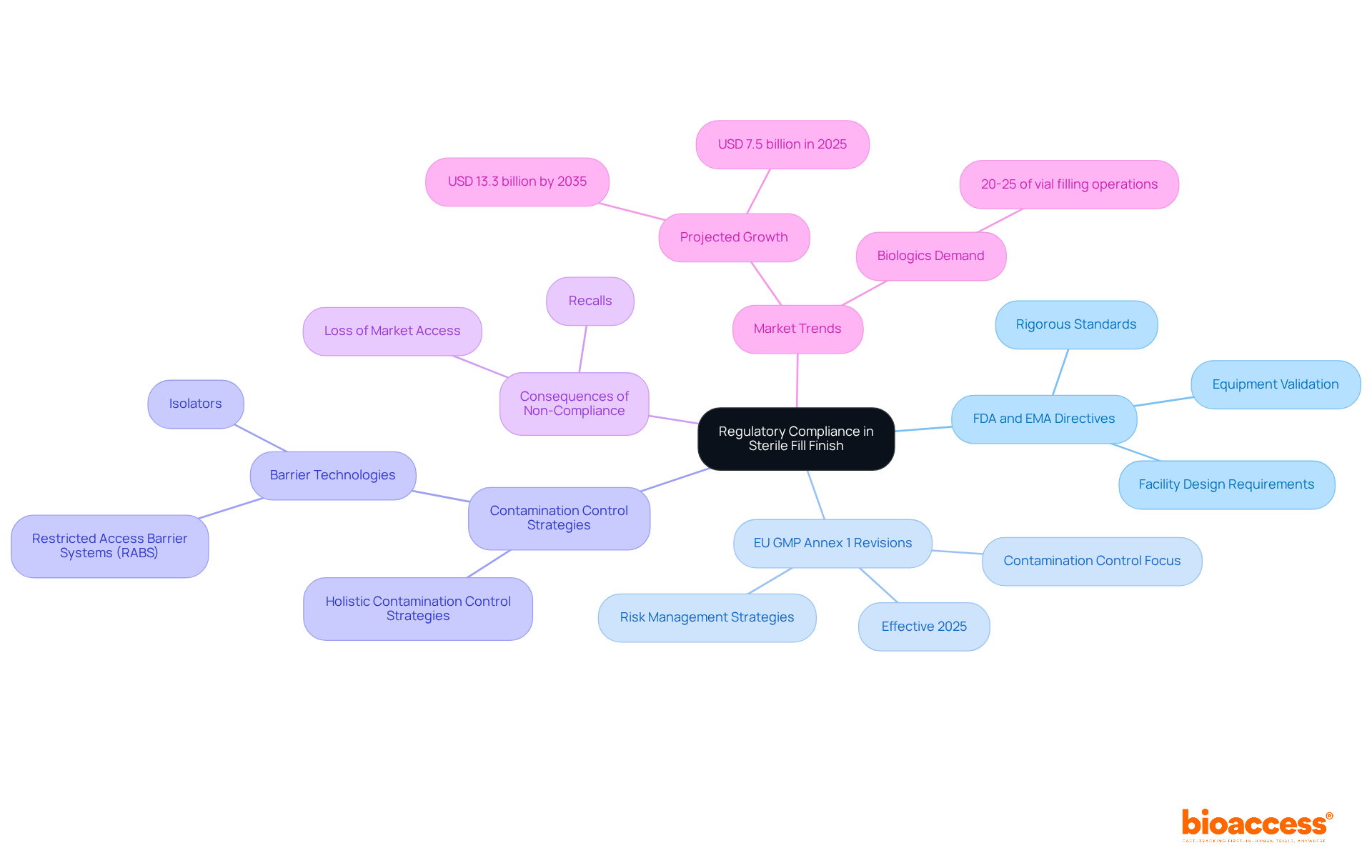
Regulatory adherence in aseptic completion is fundamentally influenced by directives from the FDA and EMA, which specify rigorous standards for facility design, equipment validation, and process controls to guarantee the sterility of pharmaceutical items. The recent revisions to the EU GMP Annex 1, effective in 2025, introduce critical new mandates focusing on contamination control and risk management. These changes compel manufacturers to adopt more rigorous practices, including the implementation of holistic Contamination Control Strategies and modern barrier technologies like Restricted Access Barrier Systems (RABS) and isolators.
Non-compliance with these regulations can lead to severe consequences, such as recalls and loss of market access, emphasizing the importance of adhering to these standards in the sterile fill finish. Statistics indicate that approximately 20-25% of vial filling operations are now dedicated to biologics, reflecting the growing demand for compliance in this sector. Furthermore, case studies reveal that companies investing in advanced aseptic technologies have significantly improved their operational efficiency and product traceability, thereby enhancing their compliance with regulatory standards.
As the market for aseptic fill-finish manufacturing is projected to grow from USD 7.5 billion in 2025 to USD 13.3 billion by 2035, the emphasis on regulatory adherence will only intensify, making it essential for manufacturers to stay ahead of evolving guidelines.
The sterile fill finish process is a cornerstone of the pharmaceutical industry, ensuring that injectable products are prepared under conditions that guarantee their safety and efficacy. This critical phase not only protects patient health but also upholds the integrity of clinical research, ultimately influencing regulatory approvals and market readiness for new therapies.
Throughout this article, we have explored key aspects of sterile fill finish, including its definition, historical evolution, technological advancements, and the stringent regulatory compliance required to maintain safety standards. Innovations such as:
have transformed the landscape, making the process more efficient and reliable. The emphasis on regulatory adherence, particularly with recent updates from the FDA and EMA, underscores the necessity for pharmaceutical manufacturers to adopt rigorous practices to avoid costly non-compliance issues.
In light of the growing demand for biologics and the complexities of modern drug development, the importance of mastering sterile fill finish cannot be overstated. As the industry continues to evolve, staying informed about best practices and regulatory requirements will be vital for manufacturers aiming to succeed in this competitive landscape. Embracing these advancements not only enhances product safety but also fosters innovation, ultimately leading to better health outcomes for patients worldwide.
What is sterile fill finish?
Sterile fill finish refers to the final phase in the aseptic processing of pharmaceutical products, where a clean product is placed into its final container under controlled conditions to ensure cleanliness and safety for patient administration.
Why is sterile fill finish important in clinical research?
Sterile fill finish is crucial in clinical research as it helps assess the quality and effectiveness of the drug being studied, protects the integrity of clinical trials, and ensures that results are trustworthy for regulatory approvals and market entry.
How does a strong aseptic completion process impact clinical trials?
A strong aseptic completion process, including sterile fill finish, guarantees the integrity of clinical trials, leading to reliable results that are essential for regulatory approvals and the introduction of new therapies to the market.
What role does bioaccess® play in clinical research related to sterile fill finish?
Bioaccess® offers expert services, including regulatory approval assistance and patient recruitment, which can accelerate clinical research processes, allowing startups in Medtech, Biopharma, and Radiopharma to progress their studies 40% faster.
What are the consequences of not maintaining stringent aseptic protocols during sterile fill finish?
Failing to maintain stringent aseptic protocols can lead to microbial contamination, which may compromise patient safety, reduce the drug's shelf life, and affect the overall effectiveness of the drug in clinical studies.
Why is there an increasing demand for expertise in sterile fill finish?
The demand for expertise in sterile fill finish is rising due to the growing number of biologics available and the large number of candidates undergoing clinical evaluation, particularly for complex chronic conditions that require injectable treatments.
How does sterile fill finish affect drug effectiveness in clinical studies?
The sterile fill finish directly impacts the results of clinical studies, which regulatory agencies rely on for product authorization, highlighting its significance in ensuring the effectiveness of the drug being evaluated.