

Randomized Controlled Trials (RCTs) are the cornerstone of clinical research, essential for determining the efficacy and safety of various interventions. By employing rigorous methodologies that minimize bias and enhance reliability, RCTs yield invaluable insights that shape medical guidelines and treatment protocols. However, the complexities and challenges associated with conducting these trials raise critical questions:
Exploring the multifaceted benefits and implications of RCTs reveals their indispensable value in advancing healthcare. They not only provide a framework for assessing interventions but also highlight the ongoing need for innovation in research practices. As we delve deeper into the Medtech landscape, it becomes clear that addressing these challenges is vital for the future of clinical research.
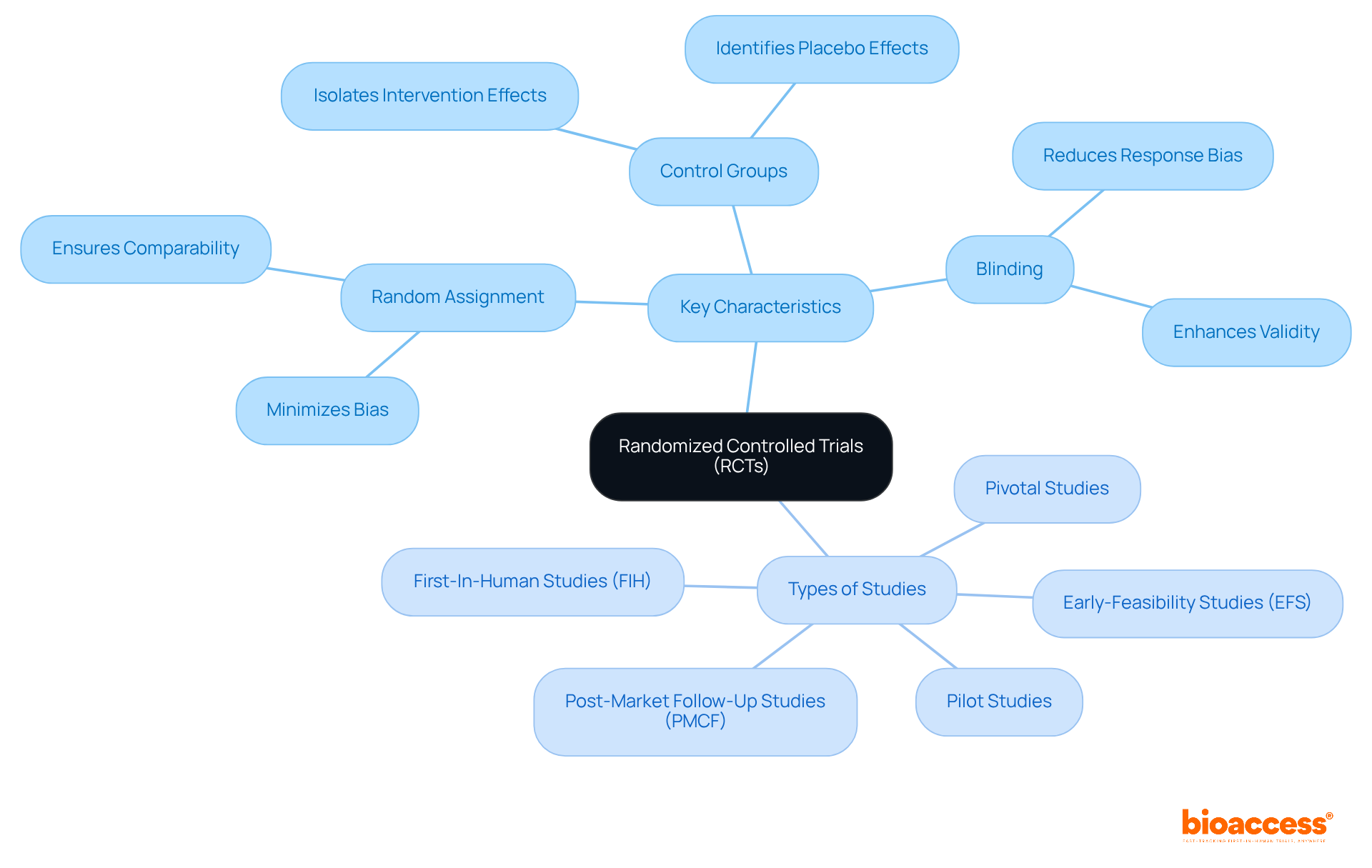
A Randomized Controlled Trial (RCT) stands as a cornerstone in clinical research, meticulously designed to assess the efficacy or safety of an intervention. By randomly assigning participants to either a treatment group or a control group, this process minimizes bias, ensuring comparability between groups. As a result, the outcomes can be directly attributed to the intervention, rather than external factors. In 2025, the significance of RCTs remains paramount, with thousands conducted globally each year, underscoring their vital role in advancing medical knowledge.
Key characteristics of RCTs - such as random assignment, control groups, and blinding - are essential for isolating the effects of an intervention. These elements provide reliable evidence for decision-making in healthcare. Experts in healthcare studies emphasize the benefit of randomized controlled trials (RCTs), which not only enhance the reliability of results but also play a crucial role in developing evidence-based methods across various fields, including medicine, psychology, and education.
At bioaccess, we leverage over 20 years of expertise in managing studies, including:
Our commitment ensures that clients receive comprehensive support throughout the research process. As we navigate the complexities of clinical trials, collaboration becomes essential. How can we work together to overcome the challenges in clinical research? Let's explore the next steps in advancing your research initiatives.
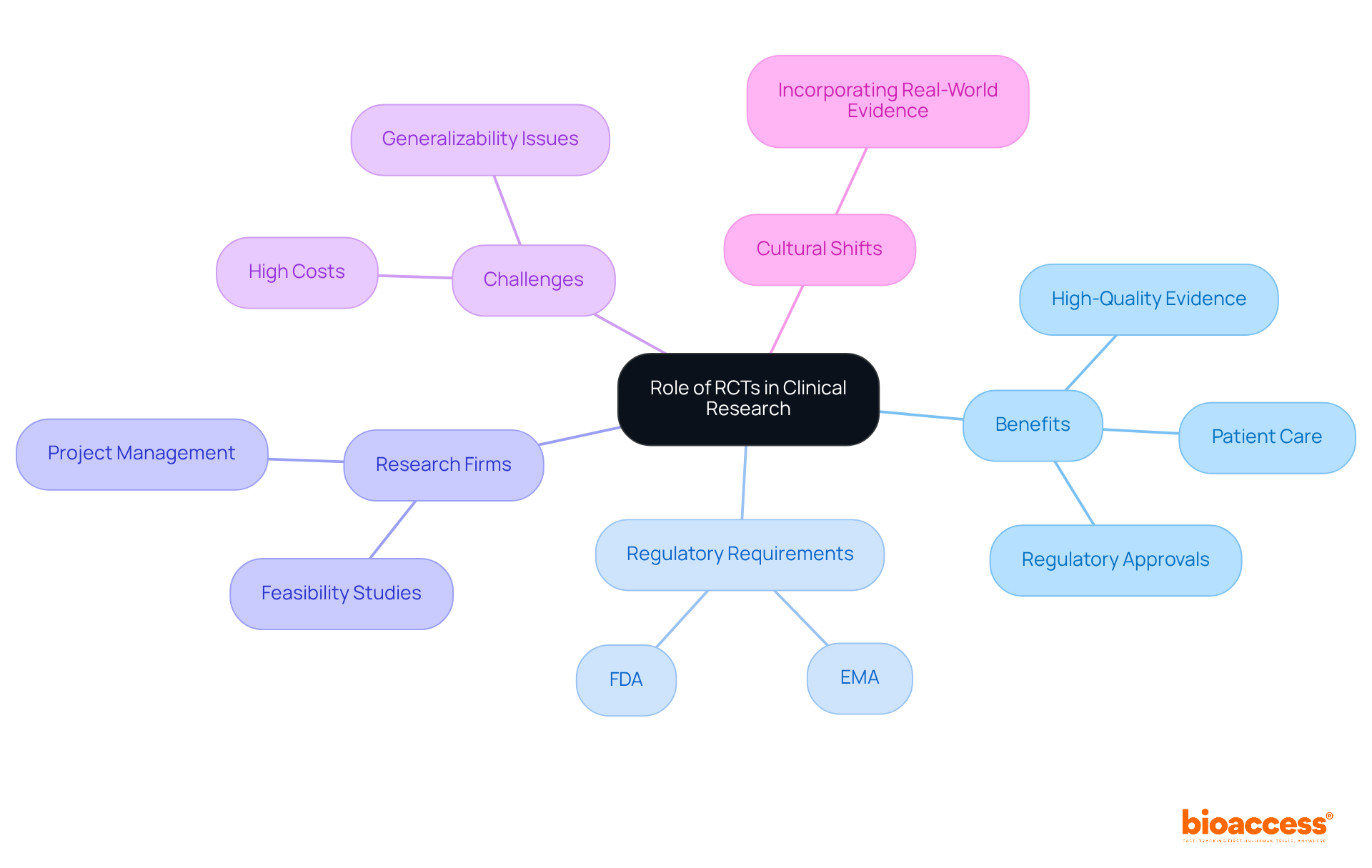
The role of randomized controlled studies in clinical research has undergone a remarkable transformation over the past decade. Initially, the field leaned heavily on observational studies, which, despite their inherent value, often introduced biases that could skew findings. The rise of randomized controlled trials (RCTs) marked a pivotal shift, highlighting the benefit of randomized controlled trials by establishing a rigorous framework for hypothesis testing. The benefit of randomized controlled trials is that they are indispensable for regulatory approvals, providing the high-quality evidence necessary to validate the safety and effectiveness of new treatments.
Regulatory authorities, including the FDA and EMA, require RCT data as a prerequisite for the approval of new drugs and medical devices. This requirement underscores the critical importance of the benefit of randomized controlled trials in shaping medical guidelines and best practices, ensuring that healthcare providers are equipped with the most reliable evidence for informed treatment decisions. Over the past ten years, the integration of RCTs into the regulatory framework has not only enhanced the reliability of medical studies but also facilitated the swift introduction of innovative treatments to the market, demonstrating the benefit of randomized controlled trials for patient care.
Firms like bioaccess play a vital role in this process by offering comprehensive research management services, including feasibility studies, site selection, compliance reviews, study setup, project management, and reporting. These services ensure that RCTs are conducted efficiently and effectively, contributing to the overall success of clinical research initiatives. However, it is crucial to acknowledge that less than one-third of new drug indications had postapproval studies demonstrating superior efficacy, highlighting the ongoing need for rigorous assessment.
Moreover, while the FDA's approval process permits drugs to be approved with limited evidence, the balance between safety and efficacy remains a paramount consideration. As Eduardo Hariton emphasizes, appropriate conduct in RCTs, including intention-to-treat analysis and allocation concealment, is essential for preserving the integrity of trial results. Additionally, the cultural shift towards incorporating real-world evidence (RWE) into medical practice is becoming increasingly significant in the evolving landscape of healthcare studies.
Despite their importance, RCTs face challenges such as high costs and concerns regarding generalizability, which must be acknowledged to present a balanced view of their role in medical research.
Randomized controlled studies stand out as a cornerstone of clinical research, providing the benefit of randomized controlled trials that enhance their relevance. By minimizing bias through randomization, these studies ensure that any observed differences in outcomes are directly linked to the intervention, rather than confounding variables. This methodological rigor not only bolsters the reliability of results but also allows for precise assessments of treatment efficacy. Moreover, the inclusion of blinding - where both participants and researchers remain unaware of group assignments - further reduces bias, ensuring that expectations do not skew outcomes.
The structured framework of randomized controlled trials facilitates robust statistical analysis, empowering researchers to draw valid conclusions about treatment effectiveness. Additionally, RCTs allow for the exploration of secondary outcomes, such as quality of life and adverse effects, providing a comprehensive view of the intervention's impact. The reliability of results from RCTs is particularly noteworthy; their stringent design often influences medical practice and policy decisions. For instance, recent trials have demonstrated the effectiveness of innovative cancer therapies, leading to updates in treatment protocols and ultimately enhancing patient outcomes.
In the realm of bioaccess's comprehensive research study management services - including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting - the advantages of RCTs are amplified. With over 20 years of experience managing diverse studies such as Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, bioaccess is exceptionally equipped to navigate the complexities of clinical investigations in Latin America. Their project management services ensure that studies are conducted efficiently and effectively, thereby enhancing the validity and applicability of RCT findings. This expertise not only drives successful trial outcomes but also fosters local economies through job creation, economic growth, healthcare improvements, and international collaboration.
In summary, the combination of randomization, blinding, and a clear analytical framework showcases the benefit of randomized controlled trials, positioning them as the gold standard in clinical research and significantly enhancing the validity and applicability of their findings. Collaboration with experienced partners like bioaccess is essential for navigating the challenges of clinical research and achieving impactful results.

Randomized Controlled Trials (RCTs) stand as a cornerstone in clinical research, offering a structured and unbiased method for assessing the effectiveness of interventions. By utilizing randomization, control groups, and blinding, RCTs effectively minimize bias and bolster the reliability of findings. This makes them indispensable for informed medical decision-making and securing regulatory approvals.
Key insights throughout this article underscore the transformative impact of RCTs on advancing medical knowledge. Their methodological rigor empowers researchers to draw valid conclusions regarding treatment efficacy. Moreover, the integration of RCTs into regulatory frameworks guarantees that healthcare providers have access to trustworthy evidence. The comprehensive support from organizations like bioaccess highlights the critical role of collaboration in navigating the complexities of clinical trials, ultimately driving successful research outcomes.
Given the substantial benefits that RCTs contribute to clinical research, it is imperative for stakeholders in the healthcare sector to prioritize their implementation. Embracing the rigorous standards of RCTs not only elevates the quality of research but also stimulates innovation in patient care. As the clinical research landscape evolves, a steadfast commitment to utilizing RCTs will be pivotal in shaping future medical practices and enhancing health outcomes for patients globally.
What is a Randomized Controlled Trial (RCT)?
A Randomized Controlled Trial (RCT) is a research method used to assess the efficacy or safety of an intervention by randomly assigning participants to either a treatment group or a control group, minimizing bias and ensuring comparability.
Why are RCTs important in clinical research?
RCTs are vital in clinical research as they provide reliable evidence for decision-making in healthcare, allowing outcomes to be directly attributed to the intervention rather than external factors.
What are the key characteristics of RCTs?
The key characteristics of RCTs include random assignment of participants, the presence of control groups, and blinding, all of which help isolate the effects of the intervention.
How many RCTs are conducted globally each year?
Thousands of RCTs are conducted globally each year, highlighting their significance in advancing medical knowledge.
In what fields are RCTs utilized?
RCTs are utilized across various fields, including medicine, psychology, and education, to develop evidence-based methods.
What types of studies does bioaccess manage?
Bioaccess manages several types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
What is the role of collaboration in clinical research?
Collaboration is essential in clinical research to navigate the complexities of clinical trials and to overcome challenges in advancing research initiatives.