


The primary focus of this article is to elucidate the objectives and significance of Phase 3 clinical studies within the drug development process. Phase 3 trials are paramount for validating the efficacy and safety of new treatments, as they encompass large participant groups, rigorous methodologies, and stringent regulatory oversight. Ultimately, these trials play a crucial role in influencing the approval and accessibility of new therapies to the public. Understanding the intricacies of Phase 3 studies is essential for stakeholders in clinical research, as it underscores the collaborative efforts required to navigate the complexities of bringing innovative treatments to market.
Understanding the intricate landscape of clinical trials is essential for grasping how new medical treatments are evaluated and approved. Among the various stages, clinical study phase 3 stands out as a pivotal juncture, where the efficacy and safety of a drug are rigorously tested in diverse populations. This phase not only serves as a benchmark for drug approval but also acts as a battleground for innovative approaches in medical research.
Readers will delve into the objectives, methodologies, and regulatory roles that define this critical phase, uncovering the challenges that can impact its success.
What makes phase 3 trials so crucial in the landscape of clinical research?
Clinical studies are systematically categorized into distinct phases, each serving a crucial role in evaluating new medical interventions.
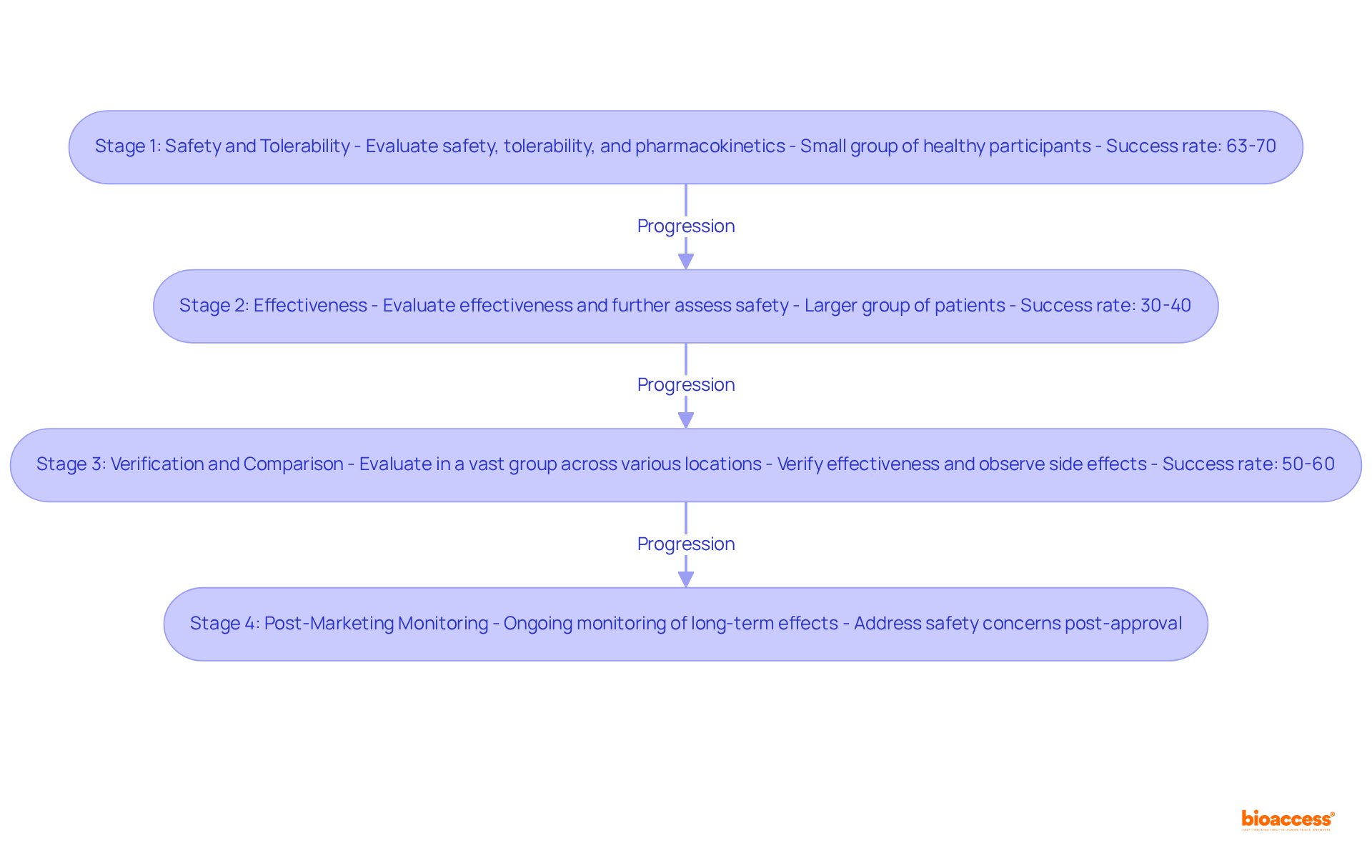
Stage 1: This preliminary stage evaluates the safety, tolerability, and pharmacokinetics of a medication in a small group of healthy participants or patients. The primary goal is to determine the appropriate dosage and identify any side effects. Roughly 63%-70% of medications successfully clear this stage, which mainly emphasizes safety.
Stage 2: In this stage, the medication is given to a larger group of patients to evaluate its effectiveness and further assess safety. This stage is crucial for determining whether the medication has the intended therapeutic effect, with only about 30%-40% of substances moving forward successfully to Stage 3.
Stage 3: Also known as clinical study phase 3, this stage acts as a crucial step by evaluating the medication in a vast group across various locations. The main goals are to verify effectiveness, observe side effects, and compare the medication to standard treatments. Successful finalization of the clinical study phase 3 is frequently a requirement for regulatory endorsement, with approximately 50%-60% of substances that reach this clinical study phase 3 eventually obtaining FDA authorization.
Stage 4: Also referred to as post-marketing monitoring, this stage takes place after a medication has been authorized for public use. It involves ongoing monitoring of the drug's long-term effects and effectiveness in the general population, addressing any safety concerns that may arise post-approval.
Grasping these stages is crucial, as they together guarantee that new therapies are safe and effective prior to entering the market. Each stage builds on the discoveries of the earlier one, making the clinical study phase 3 a crucial step in the journey from research to real-world application. As of 2023, there are 42,947 clinical study phase 3 registrations, highlighting the significance of the clinical study phase 3 in the medicine development landscape.
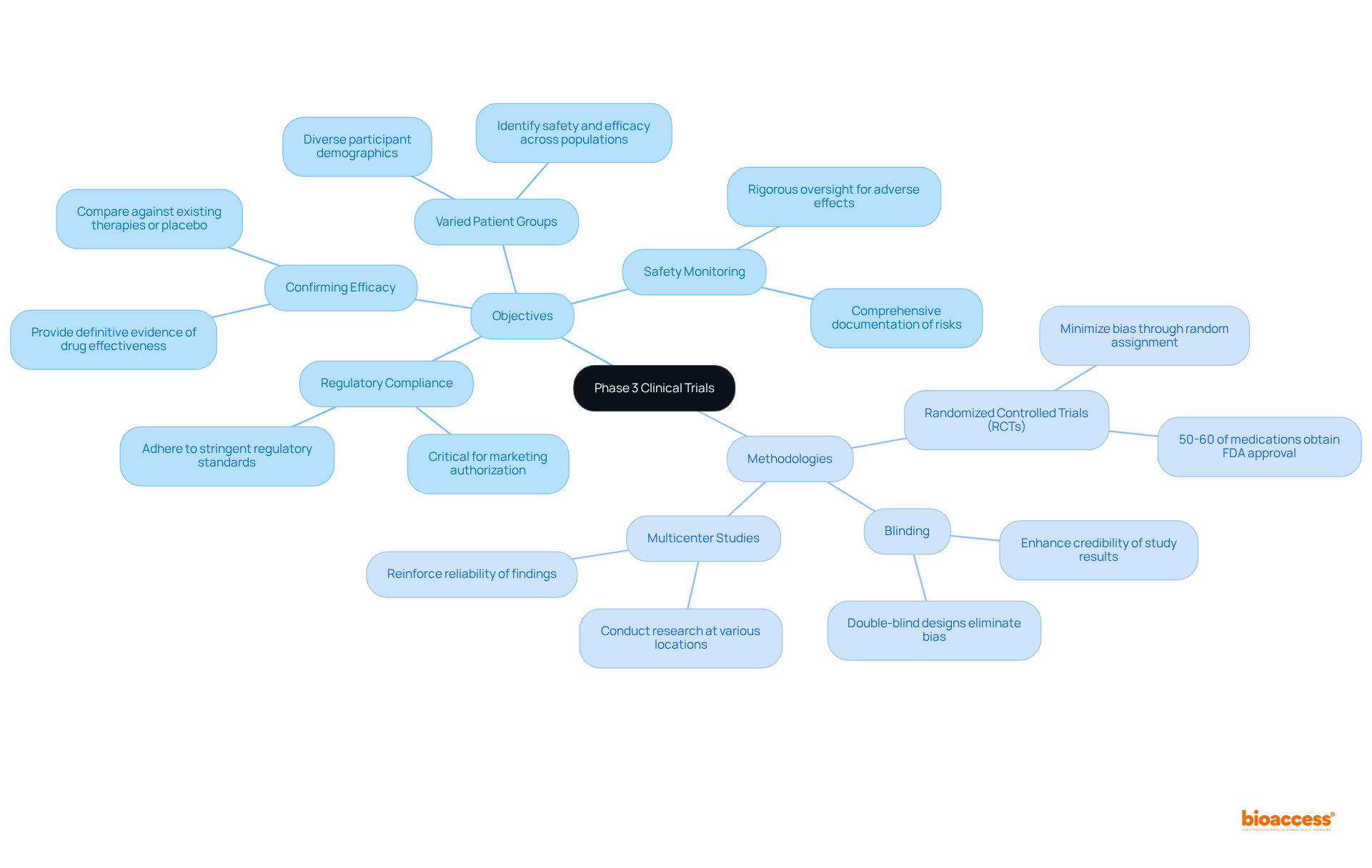
Clinical study phase 3 are meticulously structured to achieve specific objectives and employ robust methodologies that ensure a thorough evaluation of new treatments. The primary objectives include:
Confirming Efficacy: The foremost aim is to provide definitive evidence of the drug's effectiveness in treating the targeted condition, comparing it against existing therapies or a placebo.
Safety Monitoring: These studies involve rigorous oversight for adverse effects, ensuring that any potential risks are identified and documented comprehensively.
Varied Patient Groups: Stage 3 studies typically incorporate a diverse participant demographic to evaluate how distinct groups react to the treatment. This diversity is crucial for comprehending its applicability across populations, as it helps identify safety issues and efficacy across different demographics.
Regulatory Compliance: The data collected must adhere to stringent regulatory standards, as this information is critical for obtaining marketing authorization from health authorities.
With bioaccess's innovative approach, treatment-naive cardiology or neurology cohorts can be enrolled 50% faster than traditional Western sites, resulting in significant savings of $25K per patient with FDA-ready data—eliminating rework and delays. This capability enhances the efficiency of clinical study phase 3, ensuring that these studies effectively achieve their objectives.
In addition to these goals, bioaccess provides extensive service capabilities encompassing feasibility studies, site selection, compliance reviews, experiment setup, project management, and reporting. These services are essential for guaranteeing that assessments are carried out efficiently and in accordance with regulatory standards.
The methodologies employed in Phase 3 trials generally include:
By adhering to these goals and methods, along with utilizing bioaccess's comprehensive service options, the clinical study phase 3 is crucial in confirming new therapies, ultimately resulting in enhanced patient outcomes and progress in medical understanding. The significant failure rate of oncology medications in Stage 3 evaluations underscores the necessity for creative study designs and patient selection approaches.
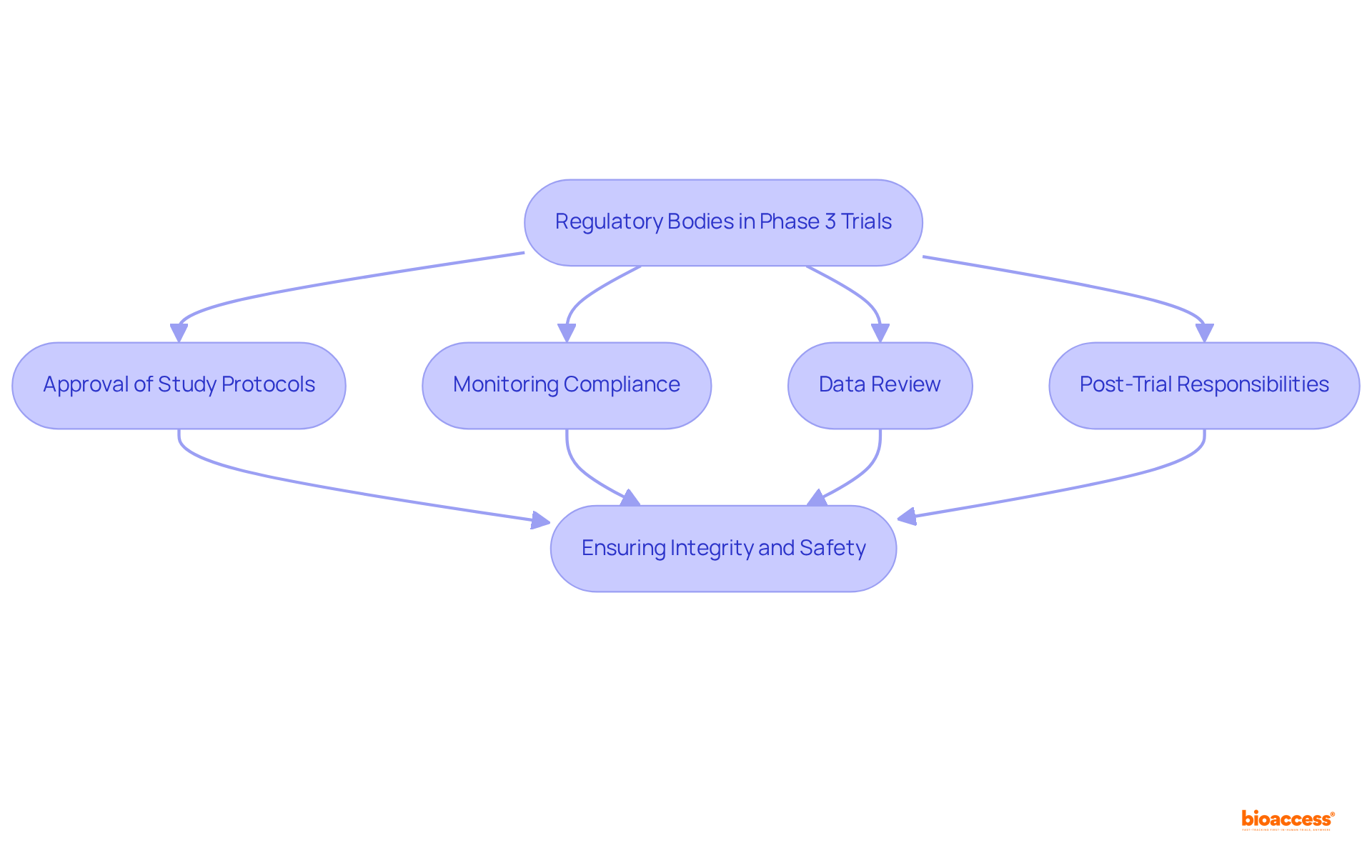
Regulatory agencies play an essential role in supervising Stage 3 studies, ensuring they are conducted ethically and scientifically. Their key responsibilities include:
Approval of Study Protocols: Prior to the commencement of a clinical study phase 3, the study protocol must be submitted to regulatory agencies for thorough review and approval. This process guarantees that the study design is robust and prioritizes participant safety.
Monitoring Compliance: Regulatory authorities oversee ongoing studies to ensure adherence to established guidelines and regulations. This includes regular inspections and audits of study locations to confirm compliance with Good Clinical Practice (GCP). Companies such as bioaccess® offer comprehensive clinical study management services, including feasibility assessments, site selection, and compliance evaluations, to support this process.
Data Review: Regulatory agencies evaluate the data collected during the clinical study phase 3 to assess the treatment's safety and effectiveness. This information is critical for making informed decisions regarding marketing authorization. Notably, approximately 58%-65% of Stage 3 studies progress successfully; however, only about 10%-12% of medications entering clinical evaluations receive FDA approval, underscoring the stringent criteria enforced.
Post-Trial Responsibilities: After the conclusion of Stage 3 studies, regulatory authorities may require ongoing observation and documentation of adverse events as part of the post-marketing surveillance process. bioaccess® ensures that all reporting requirements are met, facilitating a seamless transition into post-trial phases.
By understanding the role of regulatory organizations, stakeholders can appreciate the rigorous oversight that ensures the integrity of Stage 3 studies and the safety of new therapies. Recent discussions in the field highlight the significance of ethical oversight, with experts asserting that maintaining high standards in regulatory compliance is vital for fostering public trust in the drug development process.
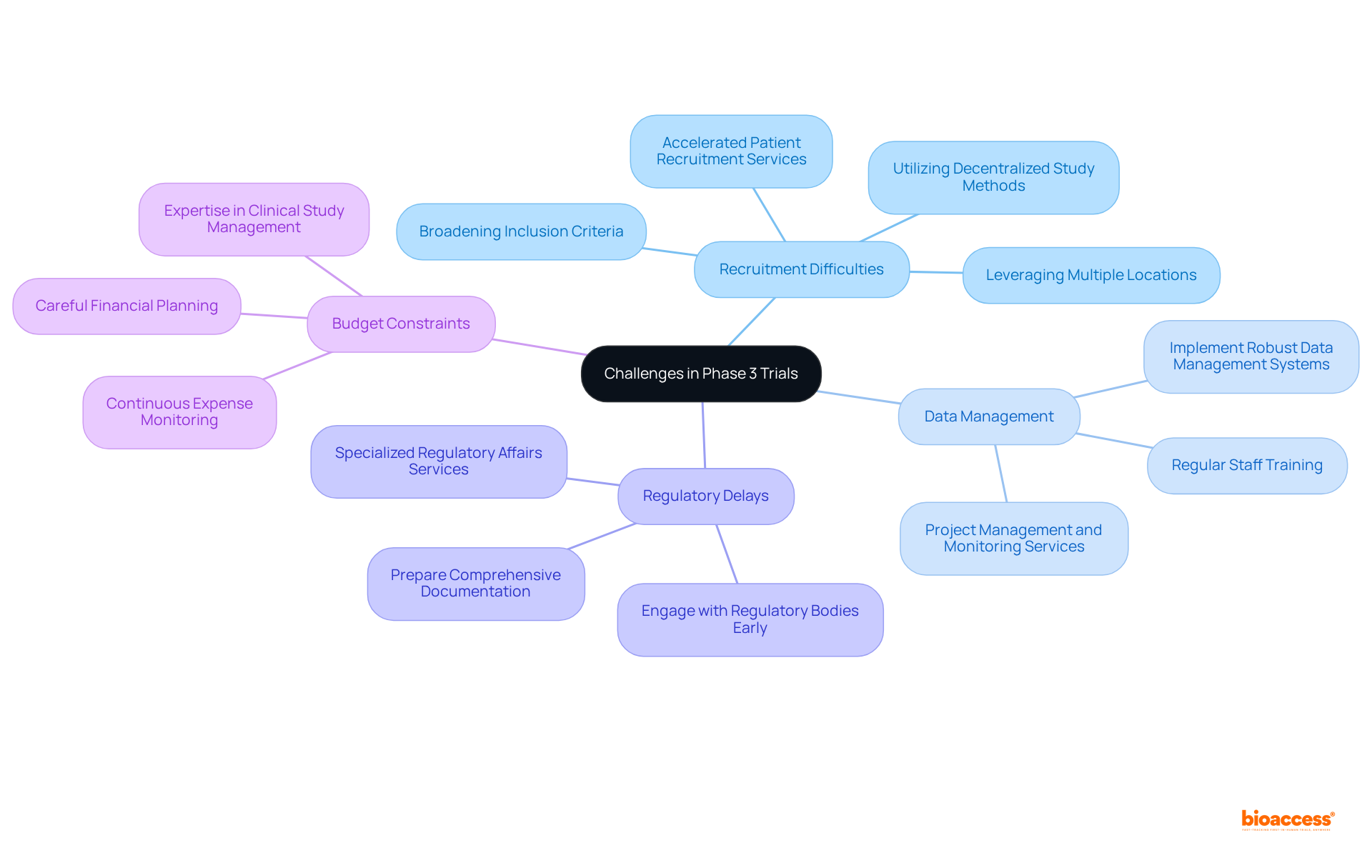
Phase 3 trials are critical for validating the efficacy and safety of new treatments; however, they present several challenges that can hinder success:
Recruitment Difficulties: Enrolling a sufficient number of participants remains a significant hurdle, especially for rare diseases or conditions with stringent eligibility criteria. Approximately 80% of clinical studies encounter delays or closures due to recruitment challenges. To counter this, strategies such as broadening inclusion criteria, leveraging multiple locations, and utilizing decentralized study methods can enhance participant access and diversity. bioaccess™ offers accelerated patient recruitment services, significantly improving enrollment efficiency.
Data Management: The management of extensive data from various sites can lead to inconsistencies and errors. Implementing robust data management systems, alongside regular training for staff, is essential to maintain data integrity and ensure compliance with regulatory standards. bioaccess™ provides extensive project management and monitoring services to ensure data accuracy throughout the study.
Regulatory Delays: The regulatory environment can cause setbacks in the start of investigations and data submission. Engaging with regulatory bodies early and preparing comprehensive documentation can streamline processes and mitigate potential setbacks. bioaccess™ specializes in regulatory affairs, ensuring compliance with local requirements and expediting the approval process.
Budget Constraints: The financial requirements of Stage 3 studies are considerable, with typical expenses approximately $21 million. Budget overruns can jeopardize the study's success, making careful financial planning and continuous expense monitoring crucial. With bioaccess™'s expertise in clinical study management, stakeholders can better manage costs and allocate resources effectively.
By acknowledging these challenges and leveraging the comprehensive services offered by bioaccess™, including feasibility studies and compliance reviews, stakeholders can better prepare for the complexities of Phase 3 trials and implement strategies to enhance the likelihood of successful outcomes.
Understanding the intricacies of clinical study phase 3 is essential for grasping how new therapies transition from research to real-world application. This phase is not merely another step in drug development; it represents a rigorous verification of a medication's effectiveness and safety across diverse populations, ultimately paving the way for regulatory approval and public use.
Throughout the article, we have highlighted the pivotal objectives of phase 3 trials, including:
The discussion has underscored the methodologies employed, such as randomized controlled trials and multicenter studies, which enhance the reliability of findings. Furthermore, the role of regulatory bodies in overseeing these trials ensures that ethical and scientific standards are met, protecting participant safety and maintaining public trust.
The challenges faced during phase 3 trials, including recruitment difficulties and budget constraints, are significant yet surmountable with strategic planning and innovative solutions. As the landscape of clinical trials continues to evolve, stakeholders are encouraged to leverage comprehensive management services and adaptive methodologies to enhance the likelihood of successful outcomes. The importance of phase 3 clinical trials cannot be overstated; they are a cornerstone of effective medical advancement, ensuring that only the safest and most effective treatments reach patients in need.
What are the phases of clinical studies?
Clinical studies are categorized into four distinct phases: Stage 1 (safety and tolerability), Stage 2 (effectiveness and further safety assessment), Stage 3 (verification of effectiveness and comparison to standard treatments), and Stage 4 (post-marketing monitoring).
What is the purpose of Stage 1 in clinical studies?
Stage 1 evaluates the safety, tolerability, and pharmacokinetics of a medication in a small group of healthy participants or patients. The main goal is to determine the appropriate dosage and identify any side effects.
How successful are medications in Stage 1?
Approximately 63%-70% of medications successfully clear Stage 1, which primarily emphasizes safety.
What occurs during Stage 2 of clinical studies?
In Stage 2, the medication is given to a larger group of patients to evaluate its effectiveness and further assess safety, determining whether it has the intended therapeutic effect.
What is the success rate for medications moving from Stage 2 to Stage 3?
Only about 30%-40% of substances successfully move forward to Stage 3 from Stage 2.
What is the focus of Stage 3 in clinical studies?
Stage 3 evaluates the medication in a vast group across various locations, aiming to verify effectiveness, observe side effects, and compare the medication to standard treatments.
What is the significance of Stage 3 for regulatory approval?
Successful completion of Stage 3 is often a requirement for regulatory endorsement, with approximately 50%-60% of substances that reach this stage eventually obtaining FDA authorization.
What is Stage 4 in clinical studies?
Stage 4, or post-marketing monitoring, occurs after a medication has been authorized for public use. It involves ongoing monitoring of the drug's long-term effects and effectiveness in the general population.
Why is it important to understand the stages of clinical studies?
Understanding these stages is crucial as they ensure that new therapies are safe and effective before entering the market, with each stage building on the discoveries of the previous one.
How many clinical study phase 3 registrations were there as of 2023?
As of 2023, there were 42,947 clinical study phase 3 registrations, highlighting its significance in the medicine development landscape.