


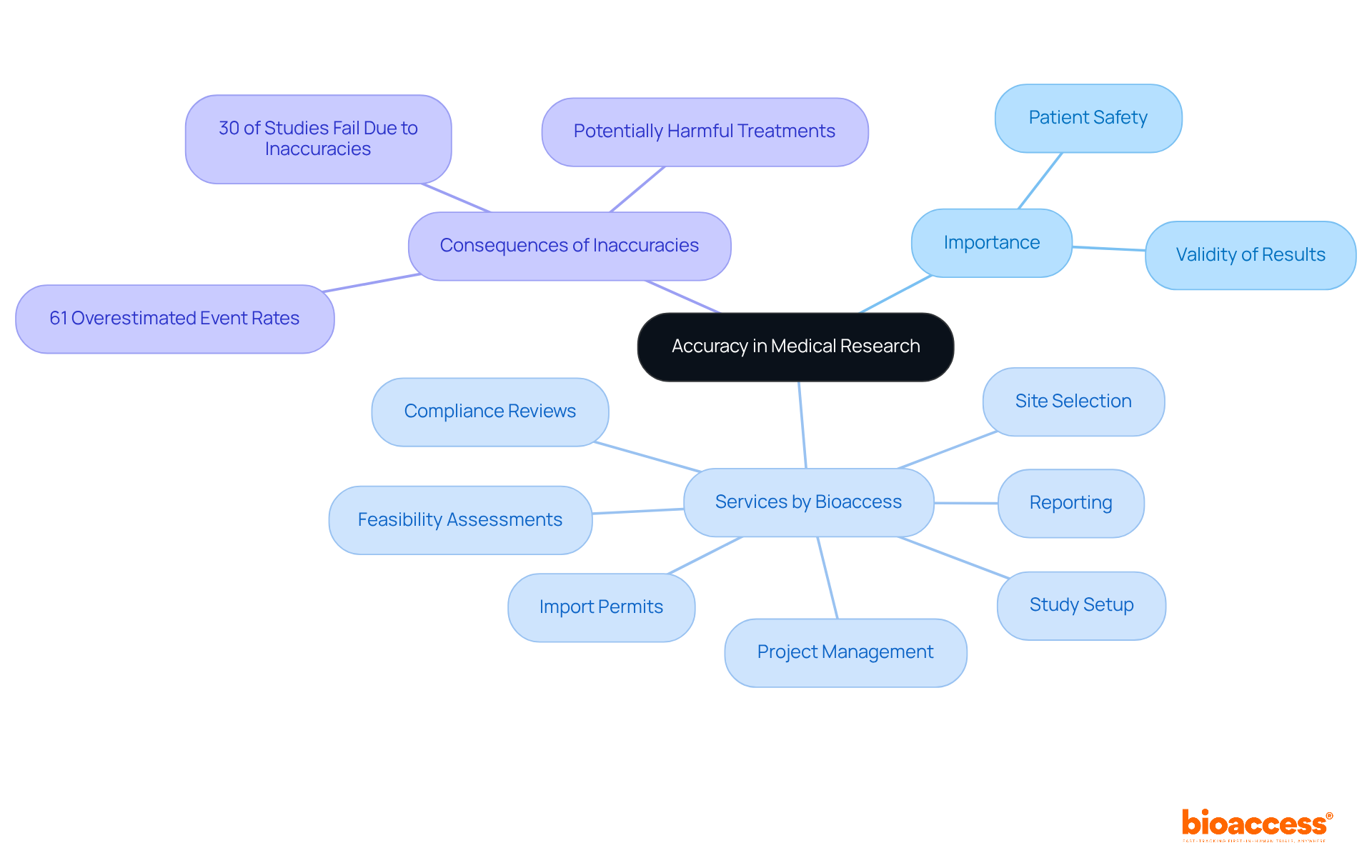
The article centers on the critical measure of accuracy in clinical research, highlighting its essential role in ensuring the reliability of study results and safeguarding patient safety. Achieving high accuracy is paramount for valid research findings; inaccuracies can lead to misleading conclusions and potentially harmful outcomes. Evidence indicates that a significant percentage of medical studies fail due to such inaccuracies, underscoring the importance of precision in this field. This understanding not only enhances the integrity of research but also fosters trust among stakeholders, reinforcing the necessity for meticulous attention to detail in clinical trials.
Precision in clinical research transcends mere technicality; it stands as a fundamental pillar of patient safety and treatment efficacy. As the medical community increasingly acknowledges the vital role of accurate data in crafting effective healthcare solutions, the stakes have reached unprecedented heights. Alarmingly, around 30% of medical studies falter due to inaccuracies. This raises a pressing question: how can researchers guarantee that their findings are both reliable and advantageous? This article embarks on an exploration of the intricate measure of accuracy in clinical research, examining its evolution, significance, and the enduring challenges in the pursuit of true precision in studies.
Precision in medical research is defined as the extent to which a measured value aligns with the actual value. This metric is fundamental as a measure of accuracy, significantly impacting the reliability of study results and patient safety. In research studies, achieving a high measure of accuracy is crucial, as it ensures that the data collected accurately reflects the real effects of a treatment or intervention. Such precision is essential for making informed decisions regarding patient care. Inaccurate measurements can undermine the validity of research findings, resulting in a poor measure of accuracy that leads to potentially harmful consequences for patients and misleading conclusions about treatment efficacy.
At bioaccess, our comprehensive research study management services encompass:
These services are designed to enhance the precision of clinical studies by ensuring meticulous management of all process elements. Expert opinions highlight the importance of accuracy, noting that approximately 30% of medical studies fail due to inaccuracies, which underscores the critical need for a measure of accuracy in meticulous information management. Case studies further illustrate this issue: one study revealed that over 61% of cardiovascular experiments overestimated expected event rates, potentially resulting in underpowered studies and hindering advancements in patient care. Moreover, the median observed event rate in these studies was considerably lower than the estimated rate of 11.0%, indicating a median deviation of -12.3%. Such discrepancies can lead to inconclusive results, a high failure rate in pharmaceutical development, and ultimately impact the measure of accuracy.
Furthermore, the adoption of electronic information capture (EDC) systems has been shown to reduce entry mistakes by as much as 50%, thereby enhancing information quality and precision. This technological advancement is vital for maintaining the integrity of medical research, as the measure of accuracy in precise data gathering is essential not only for regulatory compliance but also for ensuring that the conclusions drawn from studies are trustworthy and beneficial for patient safety. Ultimately, emphasizing precision in medical studies is critical for fostering confidence in health research and ensuring that innovative therapies can be safely and effectively delivered to patients.
The development of precision metrics in clinical studies traces back to the early days of clinical experiments, where fundamental observational techniques were the norm. As the field advanced, the necessity for sophisticated statistical techniques and measurement tools became evident. A pivotal moment occurred with the introduction of Good Clinical Practice (GCP) guidelines in the late 20th century, which underscored the importance of thorough information gathering and precision in reporting. Today, accuracy measures, specifically the measure of accuracy, are integral to trial design, with metrics such as sensitivity, specificity, and predictive values routinely utilized to assess the reliability of diagnostic tests and treatment outcomes. This evolution reflects a heightened awareness of the critical role that information integrity plays in ensuring patient safety and advancing medical knowledge.
Organizations like bioaccess®, a leading CRO in Latin America, are instrumental in this landscape, adeptly navigating complex regulatory environments and providing tailored solutions for Medtech startups. Their recent achievement of ACRP certification further underscores their commitment to elevating research standards in the region. Historical case studies, such as those evaluating the applicability of real-world information, highlight the ongoing challenges and advancements in the measure of accuracy. Furthermore, statistics indicate a significant increase in the adoption of GCP guidelines, demonstrating a collective commitment to enhancing the quality and reliability of research.

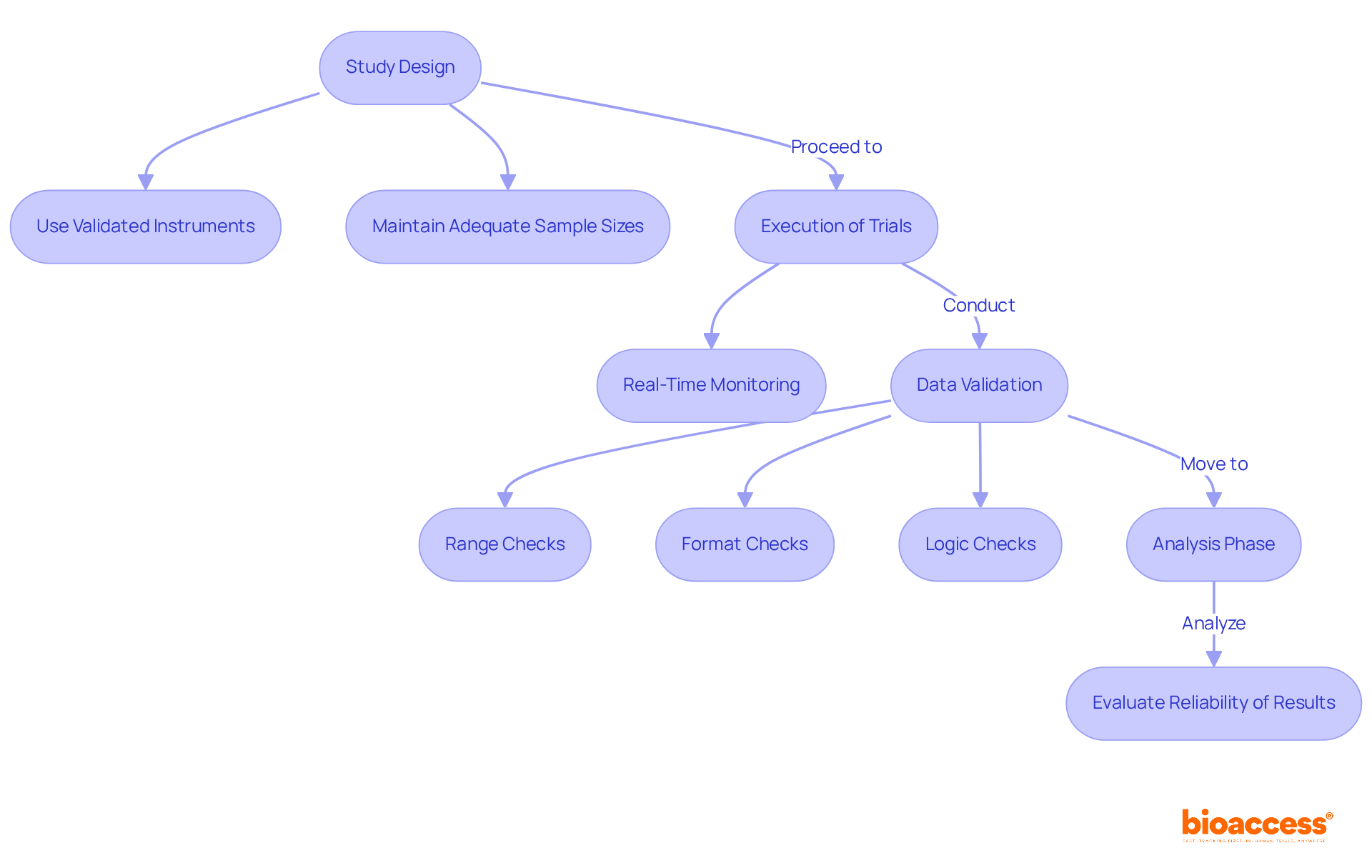
Precision metrics are essential to the clinical research process, influencing every phase from study design to information analysis. During the design phase, researchers must utilize validated instruments for information collection, ensuring that the tools employed yield reliable results. Maintaining adequate sample sizes is crucial for achieving statistical power, enhancing the validity of the study's conclusions. In the execution of trials, real-time monitoring becomes vital as a measure of accuracy, allowing for the prompt identification and correction of discrepancies. This proactive approach improves information integrity and aids in regulatory compliance.
Specific validation checks—such as:
are vital components of the data validation process, ensuring that data entries meet predefined criteria. Moreover, precision measures play a critical role during the analysis phase, where statistical methods evaluate the reliability of results. For instance, in diagnostic trials, metrics such as sensitivity and specificity are essential for assessing the effectiveness of new diagnostic tools. By maintaining a high measure of accuracy, researchers can confidently draw conclusions that inform medical practice and ultimately enhance patient outcomes.
Statistics indicate that robust information validation practices can significantly enhance the credibility of study findings, with research showing that effective monitoring can reduce entry errors by up to 30%. Real-world instances, such as the application of automated validation tools in large-scale clinical trials, illustrate the tangible advantages of these practices, ensuring that the information gathered is both accurate and dependable. Additionally, maintaining detailed records of discrepancies and their resolutions is essential for transparency and data integrity.
Achieving a high measure of accuracy in clinical research is not merely a technical requirement; it is a fundamental pillar that supports the entire structure of medical inquiry and patient care. The integrity of research findings hinges on the precision of data collection and analysis, which directly influences the safety and efficacy of treatments. Without accurate measurements, the validity of research is compromised, potentially leading to harmful consequences for patients and misguided conclusions about therapeutic interventions.
Throughout this article, key arguments have underscored the critical role of accuracy in clinical studies. From the historical evolution of measurement techniques to the implementation of Good Clinical Practice (GCP) guidelines, the journey toward enhanced precision has been marked by significant milestones. The importance of employing validated instruments, maintaining adequate sample sizes, and conducting rigorous data validation checks has been emphasized as essential practices that ensure the reliability of study outcomes. Moreover, the integration of technology, such as electronic data capture systems, has proven to significantly reduce errors and bolster the quality of information.
Reflecting on the significance of accuracy in clinical research reveals its far-reaching implications for the medical field. It is imperative for researchers, institutions, and stakeholders to prioritize precision in every phase of study design and execution. By committing to rigorous accuracy measures, the research community can foster greater trust in clinical findings, enhance patient safety, and ultimately drive advancements in healthcare. The call to action is clear: embrace accuracy as a non-negotiable standard in clinical research to ensure that innovative therapies are both safe and effective for all patients.
What is the definition of precision in medical research?
Precision in medical research is defined as the extent to which a measured value aligns with the actual value, serving as a fundamental metric of accuracy.
Why is accuracy important in medical research?
Accuracy is crucial in medical research because it impacts the reliability of study results and patient safety, ensuring that data collected accurately reflects the real effects of a treatment or intervention.
What can happen if measurements in medical research are inaccurate?
Inaccurate measurements can undermine the validity of research findings, leading to poor measures of accuracy, potentially harmful consequences for patients, and misleading conclusions about treatment efficacy.
What services does bioaccess provide to enhance precision in clinical studies?
Bioaccess offers comprehensive research study management services including feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting.
What is the failure rate of medical studies due to inaccuracies?
Approximately 30% of medical studies fail due to inaccuracies, highlighting the critical need for meticulous information management.
Can you provide an example of inaccuracies in cardiovascular studies?
One study found that over 61% of cardiovascular experiments overestimated expected event rates, with the median observed event rate being considerably lower than the estimated rate of 11.0%, indicating a median deviation of -12.3%.
How do discrepancies in study estimates affect pharmaceutical development?
Discrepancies can lead to inconclusive results, a high failure rate in pharmaceutical development, and ultimately impact the measure of accuracy in research.
What role do electronic data capture (EDC) systems play in improving accuracy?
EDC systems can reduce entry mistakes by as much as 50%, enhancing information quality and precision, which is vital for maintaining the integrity of medical research.
Why is emphasizing precision in medical studies critical?
Emphasizing precision is critical for fostering confidence in health research and ensuring that innovative therapies can be safely and effectively delivered to patients.