


The new FDA guidance on biosimilars is designed to streamline the approval process for these products, ensuring their safe and effective use as substitutes for established biologics. By emphasizing rigorous analytical, animal, and clinical studies, this guidance not only fosters competition in the biologics market but also has the potential to reduce development costs and timelines. This, in turn, enhances patient access to affordable treatment options. However, challenges remain due to existing patent protections for branded biologics, which must be navigated to fully realize these benefits.
The recent updates to the FDA guidance on biosimilars mark a pivotal shift in the landscape of biologic therapies, with the primary aim of enhancing patient access to affordable medication. By streamlining the approval process and reducing development costs, these new regulations pave a promising pathway for manufacturers to introduce biosimilars that closely mirror existing biologics, all while ensuring safety and efficacy remain uncompromised. However, as the industry navigates the complexities of market entry alongside the enduring dominance of branded products, one pressing question emerges: can these regulatory changes genuinely foster a competitive environment that benefits both manufacturers and patients alike?
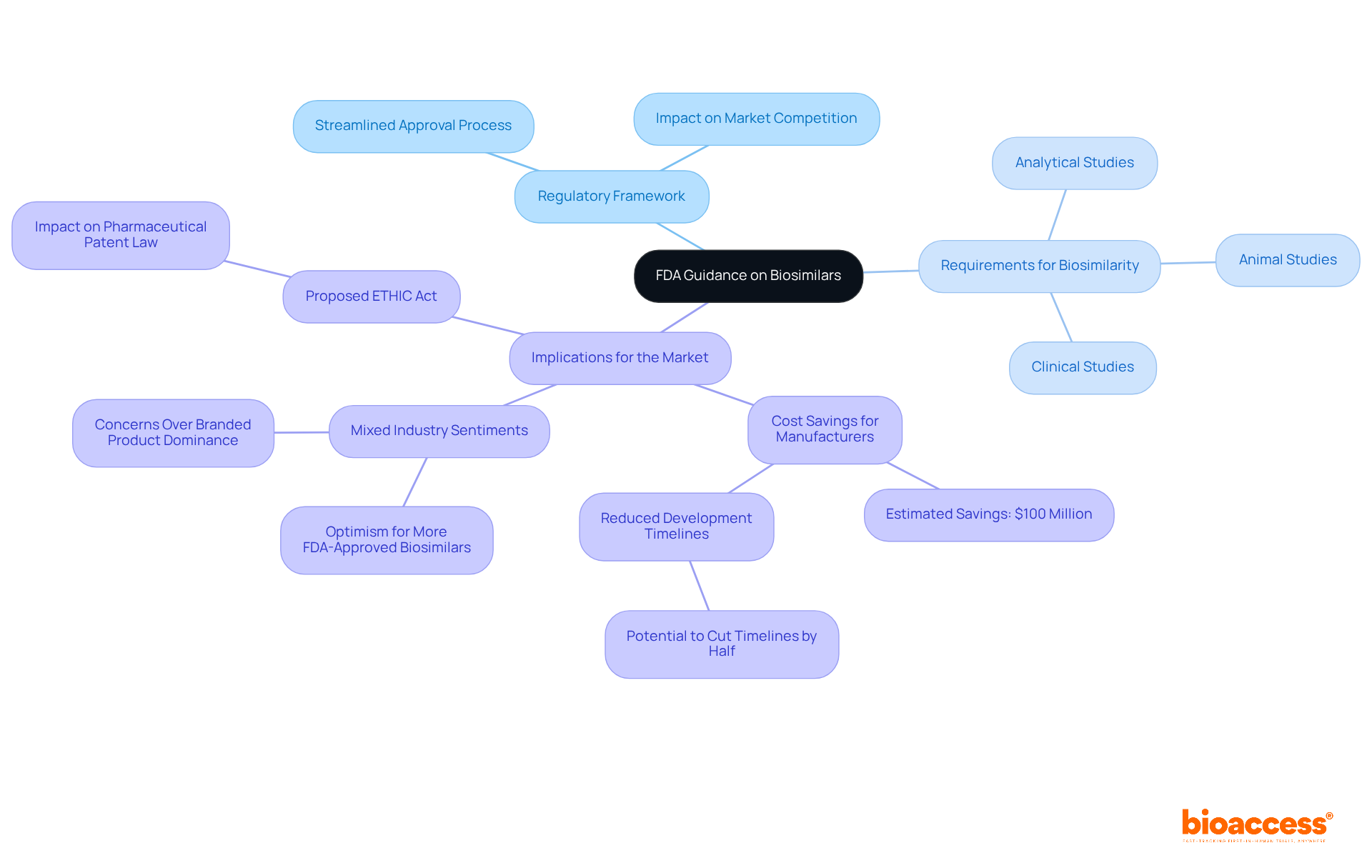
The new FDA guidance on biosimilars marks a significant regulatory framework established by the U.S. Food and Drug Administration (FDA) to streamline the approval and market entry of biosimilar products. These biologic medical products closely resemble an already approved reference product, showing no clinically meaningful differences in safety, purity, and potency. The revised recommendations clearly outline the requirements for demonstrating biosimilarity, which encompass:
This structured approach guarantees that biosimilars can be safely and effectively used as substitutes for their reference counterparts.
This direction is crucial for fostering competition within the biologics market, which is anticipated to enhance patient access to affordable medications. Notably, the new FDA guidance could potentially save manufacturers around $100 million in development costs and cut biosimilar development timelines by half. As FDA Commissioner Marty Makary pointed out, this could lead to substantial cost reductions for advanced treatments, especially in areas like rare diseases, oncology, and autoimmune diseases. However, specialists caution that while these recommendations may speed up the approval process for similar biological products, the dominance of branded biologics due to existing patent protections may still pose challenges to market entry. A recent case study highlighted mixed industry sentiments regarding the new FDA guidance, with some experts expressing optimism about the emergence of alternatives approved by the FDA, while others warned that branded products would likely continue to dominate the market.
Overall, the FDA's updated biosimilar regulations are poised to play a vital role in shaping the future of biologic therapies, ultimately benefiting patients through improved access to cost-effective treatment options. Furthermore, the proposed ETHIC Act could significantly influence pharmaceutical patent legislation, further impacting the landscape of similar biologics and market access.
The FDA's recommendations on follow-on biologics emerge in response to the growing demand for biologic treatments, which account for a substantial share of healthcare spending. As we look ahead to 2025, spending on biologics is expected to rise further, highlighting the urgent need for cost-effective alternatives. The new FDA guidance aims to streamline the biosimilar approval process to enhance patient access to these essential therapies, marking a significant shift towards a more efficient regulatory framework. This framework is designed to uphold rigorous safety and efficacy standards while accelerating the introduction of affordable options into the market.
For instance, the recent recommendations allow for the approval of certain biological products without the typically required comparative efficacy studies. This change could save developers millions in costs and years in development time. Such adjustments are vital for manufacturers, healthcare providers, and patients alike, as the new FDA guidance fosters increased competition and lowers prices in the biologics market. Expert analysis underscores that these regulatory enhancements are crucial for expanding market access and ensuring that patients benefit from the availability of lower-cost alternatives.
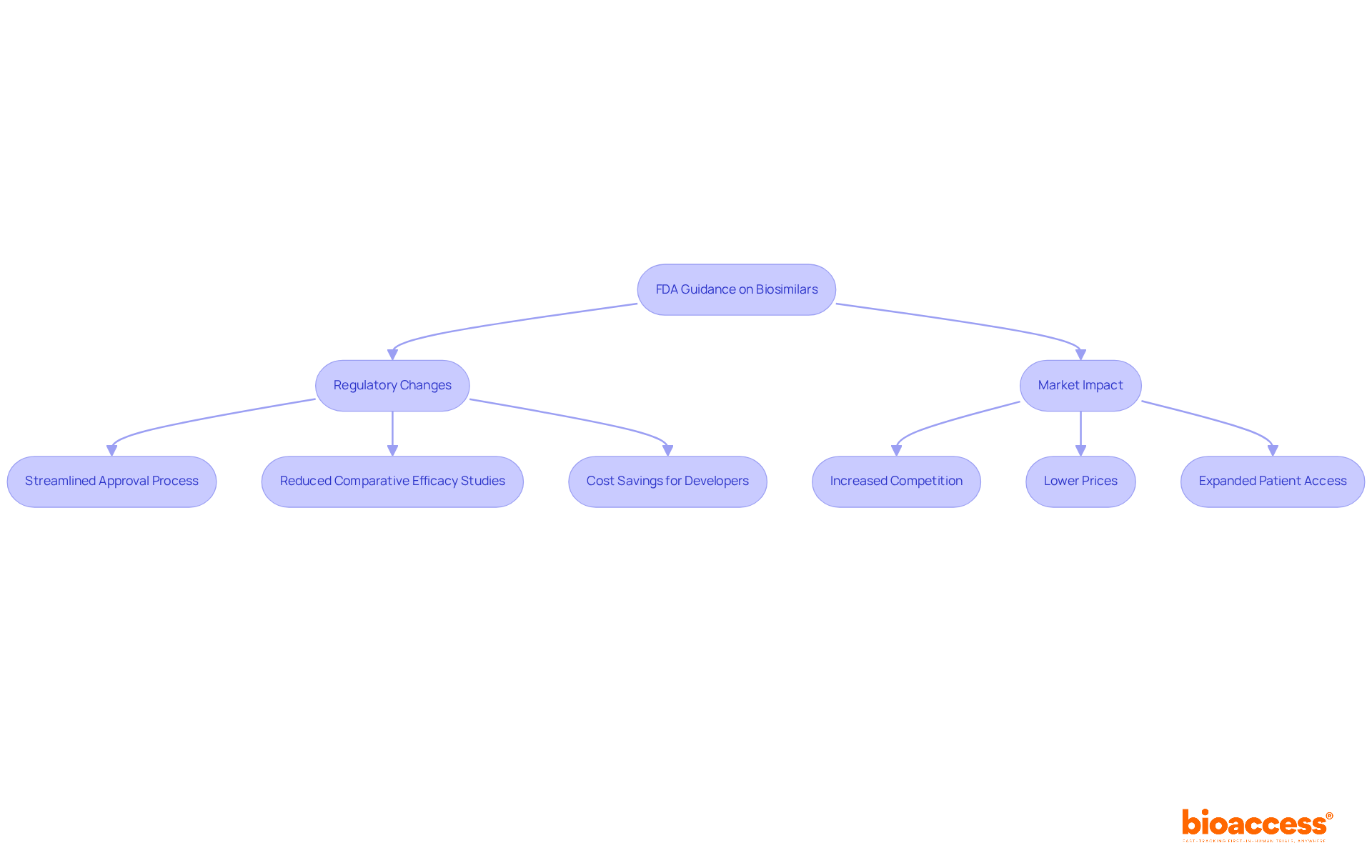
The origins of FDA recommendations on similar biologics date back to the Biologics Control Act of 1902, which laid the foundation for regulating biologics in the United States. This pivotal act established safety and efficacy standards for biological products, setting the stage for future regulatory advancements. However, it was the Biologics Price Competition and Innovation Act (BPCIA) of 2009 that formally created a pathway for biosimilar approval, allowing the FDA to implement a structured process for these products.
In 2012, the FDA began issuing draft advisory documents that were aligned with the new FDA guidance, outlining the scientific and regulatory considerations for biological product development. Over the years, stakeholder input and evolving scientific knowledge have refined these protocols, incorporating the new FDA guidance, which has led to the current detailed recommendations that address the complexities of similar product approval. This iterative process has been essential in adapting to advancements in the field, ensuring that guidelines remain relevant and effective.
Despite these developments, challenges continue to arise, particularly the necessity for substantial evidence to demonstrate differences from reference products and the complexities of market access. These ongoing issues highlight the importance of continuous dialogue among stakeholders and the need for collaboration in navigating the evolving landscape of similar biological product approvals.
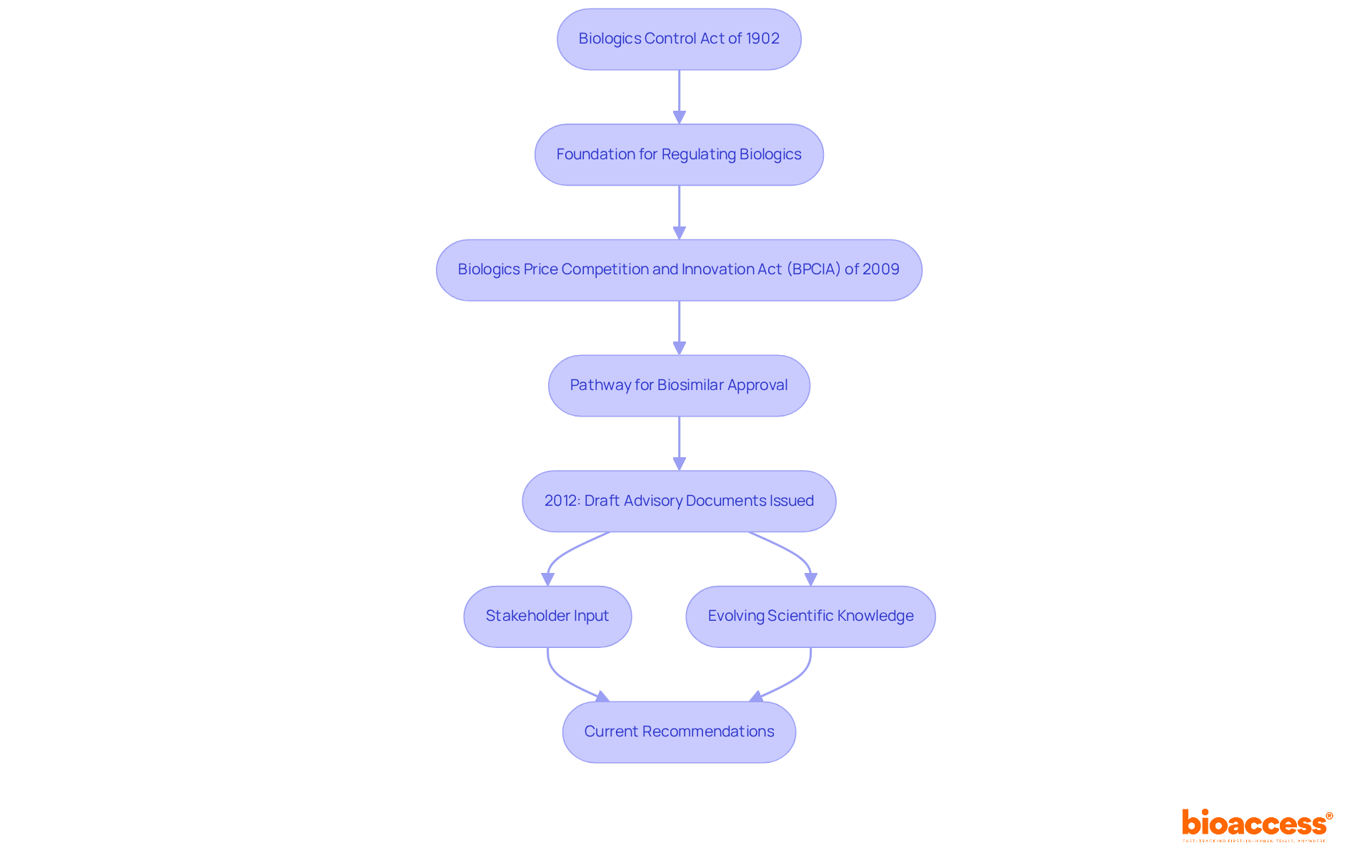
The recent FDA guidance on biosimilars emphasizes several critical characteristics necessary for regulatory compliance, as outlined in the new FDA guidance. A primary focus is on demonstrating analytical similarity, which requires manufacturers to conduct comprehensive evaluations comparing the biosimilar product to its reference counterpart. This analytical assessment is crucial for proving that the biologic product performs comparably in terms of quality, safety, and efficacy.
Moreover, the new FDA guidance emphasizes the necessity for comparative clinical studies to address any lingering uncertainties regarding the product's performance. For example, the FDA mandates a three-way pairwise comparison when both US-licensed and EU-approved reference products are available, underscoring the importance of robust evidence in the biosimilar approval process.
Additionally, ongoing post-marketing monitoring is highlighted as a vital component to ensure the sustained safety and efficacy of these products in real-world settings. This proactive monitoring is essential for upholding the high standards expected by regulators and patients alike, ensuring that biosimilars not only meet initial approval criteria but also continue to provide therapeutic benefits over time.
The recent FDA guidance on biosimilars marks a significant advancement in the regulatory landscape, aimed at streamlining the approval process and enhancing market access for these vital biologic products. By setting forth clear requirements for demonstrating biosimilarity, the FDA seeks to foster competition and ultimately broaden patient access to more affordable treatment options.
Key insights from the article underscore the structured approach adopted by the FDA, which encompasses analytical, animal, and clinical studies to guarantee the safety and efficacy of biosimilars. The potential for substantial cost savings and shortened development timelines is encouraging for manufacturers, while the persistent challenges posed by branded biologics highlight the market's complexity. Moreover, the historical context of this guidance illustrates a long-standing evolution in the regulation of biologics, underscoring the necessity for continuous adaptation to scientific advancements.
As the biosimilar market evolves, the significance of these regulatory changes cannot be overstated. They not only pave the way for more cost-effective therapies but also reinforce the commitment to patient safety and efficacy. Stakeholders across the healthcare spectrum must engage in ongoing dialogue to effectively navigate this evolving landscape, ensuring that the benefits of these new regulations translate into tangible access and affordability for patients in need.
What is the new FDA guidance on biosimilars?
The new FDA guidance on biosimilars is a regulatory framework established by the U.S. Food and Drug Administration (FDA) to streamline the approval and market entry of biosimilar products that closely resemble an already approved reference product without clinically meaningful differences in safety, purity, and potency.
What are the requirements for demonstrating biosimilarity according to the new guidance?
The requirements for demonstrating biosimilarity include analytical studies, animal studies, and clinical studies.
How does the new guidance impact the biologics market?
The new guidance is expected to foster competition within the biologics market, enhancing patient access to affordable medications.
What potential cost savings could manufacturers experience due to the new FDA guidance?
Manufacturers could potentially save around $100 million in development costs and reduce biosimilar development timelines by half.
What areas of treatment could benefit from cost reductions due to this guidance?
Cost reductions could significantly impact advanced treatments in areas like rare diseases, oncology, and autoimmune diseases.
Are there any challenges to market entry for biosimilars despite the new guidance?
Yes, specialists caution that the dominance of branded biologics due to existing patent protections may still pose challenges to market entry for biosimilars.
What are the mixed industry sentiments regarding the new FDA guidance?
Some experts express optimism about the emergence of FDA-approved alternatives, while others warn that branded products are likely to continue dominating the market.
How might the proposed ETHIC Act influence the biosimilars landscape?
The proposed ETHIC Act could significantly influence pharmaceutical patent legislation, impacting the landscape of similar biologics and market access.