


Understanding the complex landscape of clinical research in Bulgaria highlights the crucial role that Contract Research Organizations (CROs) play in regulatory submissions. As the demand for streamlined research processes continues to rise, CROs provide essential expertise that not only boosts the efficiency of study management but also guarantees compliance with rigorous regulatory frameworks.
However, the intricacies of regulatory submissions pose significant challenges. This raises an important question: how can CROs effectively navigate these obstacles to ensure successful clinical trials in Bulgaria?
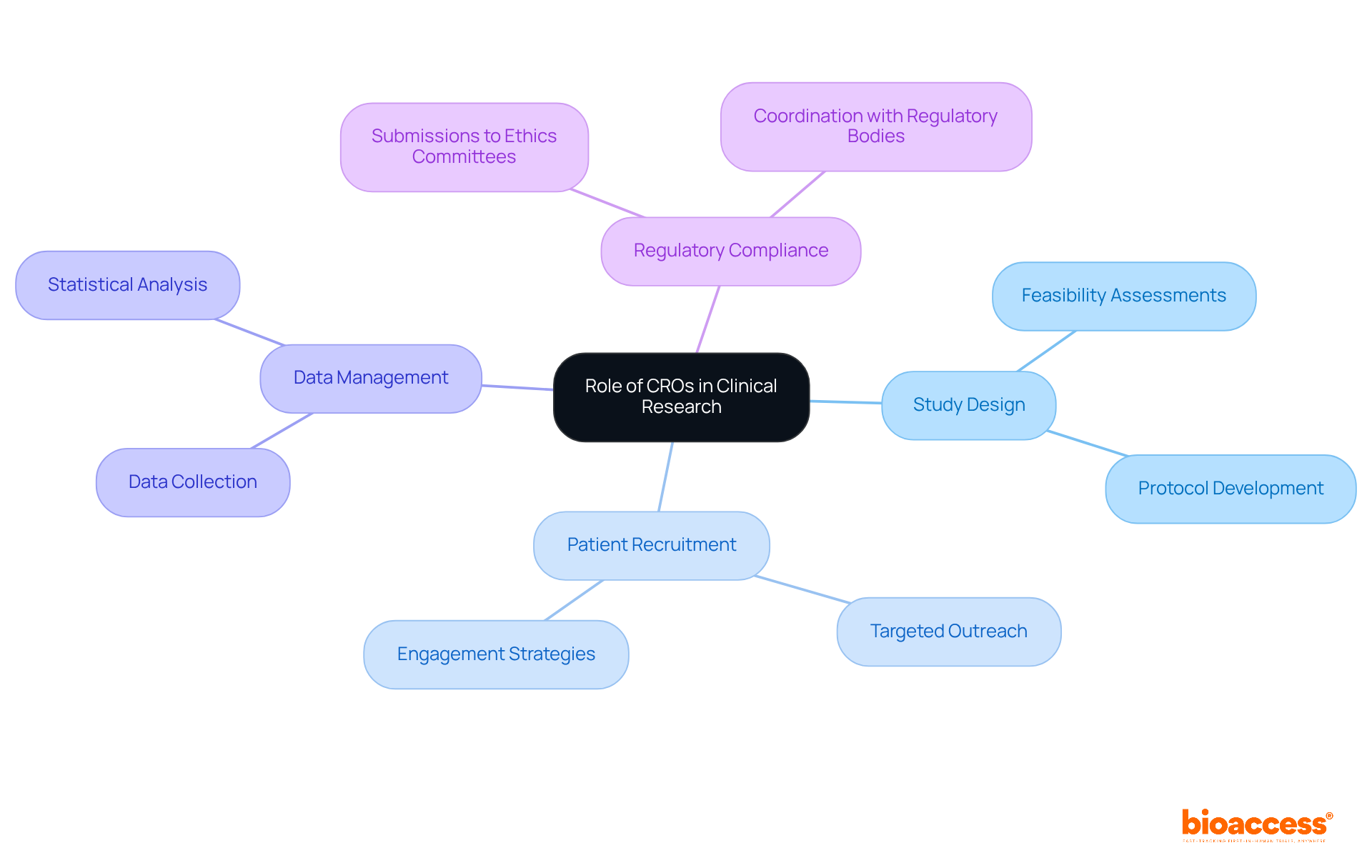
Contract Research Organizations (CROs) play a crucial role in the administration of research studies, overseeing a range of responsibilities that include:
They act as essential links between sponsors and oversight bodies, ensuring that studies adhere to established protocols and guidelines. CROs play a crucial role in regulatory submissions in Bulgaria, leveraging their expertise in therapeutic areas to navigate the complexities of research involving human subjects. Notably, nearly 75% of research studies are conducted by CROs, underscoring their significance in the industry.
CROs like bioaccess not only handle operational tasks but also engage in strategic planning, enabling sponsors to focus on their core competencies. This collaborative model has proven effective; studies indicate that companies relying on CROs experience improved study completion rates and reduced costs. As the research landscape evolves, the demand for CRO services is expected to grow, with the market projected to reach USD 164.3 billion by 2035, driven by increasing complexity in studies and the need for specialized expertise.
Bioaccess offers comprehensive services for first-in-human medical device clinical studies, encompassing:
Successful partnerships between sponsors and CROs can lead to enhanced study efficiency. For example, a European biotech firm reduced its time-to-offer from over 50 days to 36 days by collaborating with a CRO that implemented a dedicated Full-Time Equivalent (FTE) model for regulatory and data management services. This highlights the importance of selecting a CRO that understands the role of CROs in regulatory submissions in Bulgaria, as they can adapt to specific needs and scale operations as required, ensuring that studies are conducted effectively and ethically.
Bulgaria operates under the EU Clinical Studies Regulation (EU No 536/2014), which standardizes the approval process for studies across EU member nations. This regulation requires that all research study applications be submitted through the Clinical Trials Information System (CTIS), a unified electronic portal designed to streamline entries and enhance efficiency. The Bulgarian Drug Agency (BDA) oversees this approval process, ensuring compliance with ethical standards and participant safety.
As of 2025, the BDA has significantly shortened study review durations, aiming for approvals within 35 days. This notable enhancement is intended to accelerate the commencement of research, making it crucial for Contract Research Organizations (CROs) like bioaccess to understand the role of CROs in regulatory submissions in Bulgaria. It directly influences timelines, documentation requirements, and ethical considerations necessary for successful project applications. Bioaccess® connects innovative Medtech, Biopharma, and Radiopharma startups with leading research locations, offering expert services that include:
Typically, the BDA examines applications within 60 days. If no objections arise, testing can commence, underscoring the importance of prompt and precise submissions. With approximately 12,000 patients participating in research studies each year in Bulgaria, and the nation ranking 4th in the EU for the number of testing centers, Bulgaria presents a robust environment for medical research. This is further supported by a highly skilled workforce and a favorable legal framework, making collaboration essential for success in this dynamic field.
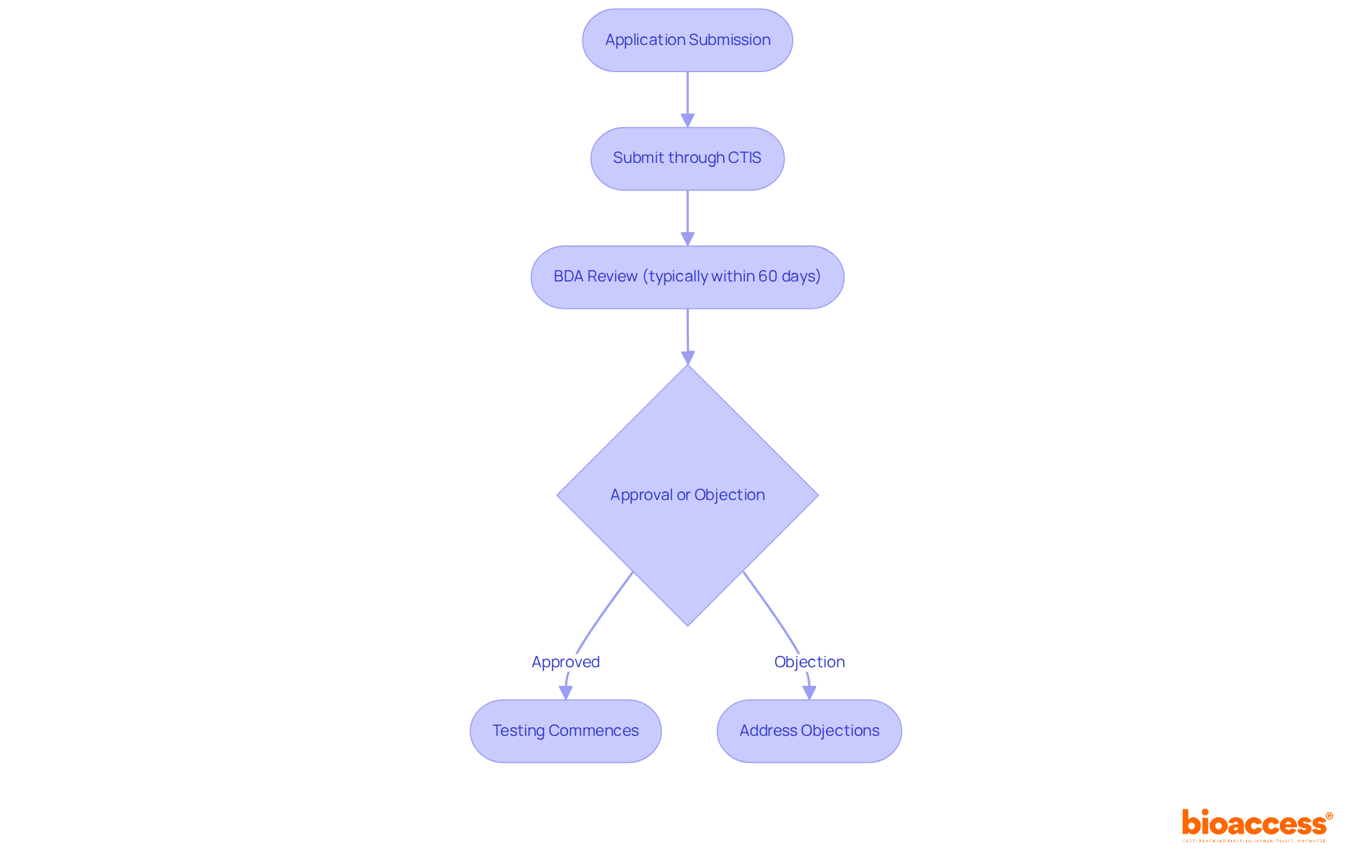
Preparation of Documentation: The first step in the clinical research process is the meticulous gathering of essential documents, including the clinical research protocol, Investigator's Brochure, and informed consent forms. The role of CROs in regulatory submissions in Bulgaria is pivotal for ensuring that these documents meet the stringent standards set by the Bulgarian Drug Agency (BDA), which typically evaluates applications within 60 days. A well-prepared application not only streamlines the process but also significantly mitigates the risk of delays in obtaining approval.
Submission to the BDA: Once the documentation is complete, CROs proceed to submit the application through the Clinical Trials Information System (CTIS). This submission encompasses comprehensive details about the study's objectives, methodologies, and safety protocols for participants. The CTIS enhances transparency and efficiency in regulatory compliance, making the application process more straightforward.
Ethics Committee Review: Following submission, the application undergoes scrutiny by an independent ethics committee, which evaluates the ethical considerations of the study. The role of CROs in regulatory submissions in Bulgaria includes facilitating effective communication between the ethics committee and the sponsor, promptly addressing any concerns that may arise during the review. Timely responses to feedback are crucial, as the ethics committee's evaluations can significantly impact the overall timeline for initiating the study.
Approval and Commencement: After receiving approval from both the BDA and the ethics committee, the study can commence. Throughout the process, the role of CROs in regulatory submissions in Bulgaria is to ensure that all compliance standards are consistently met, including ongoing reporting and adherence monitoring. This proactive approach not only upholds the integrity of the assessment but also nurtures a collaborative relationship with regulatory bodies, ultimately increasing the likelihood of successful outcomes.

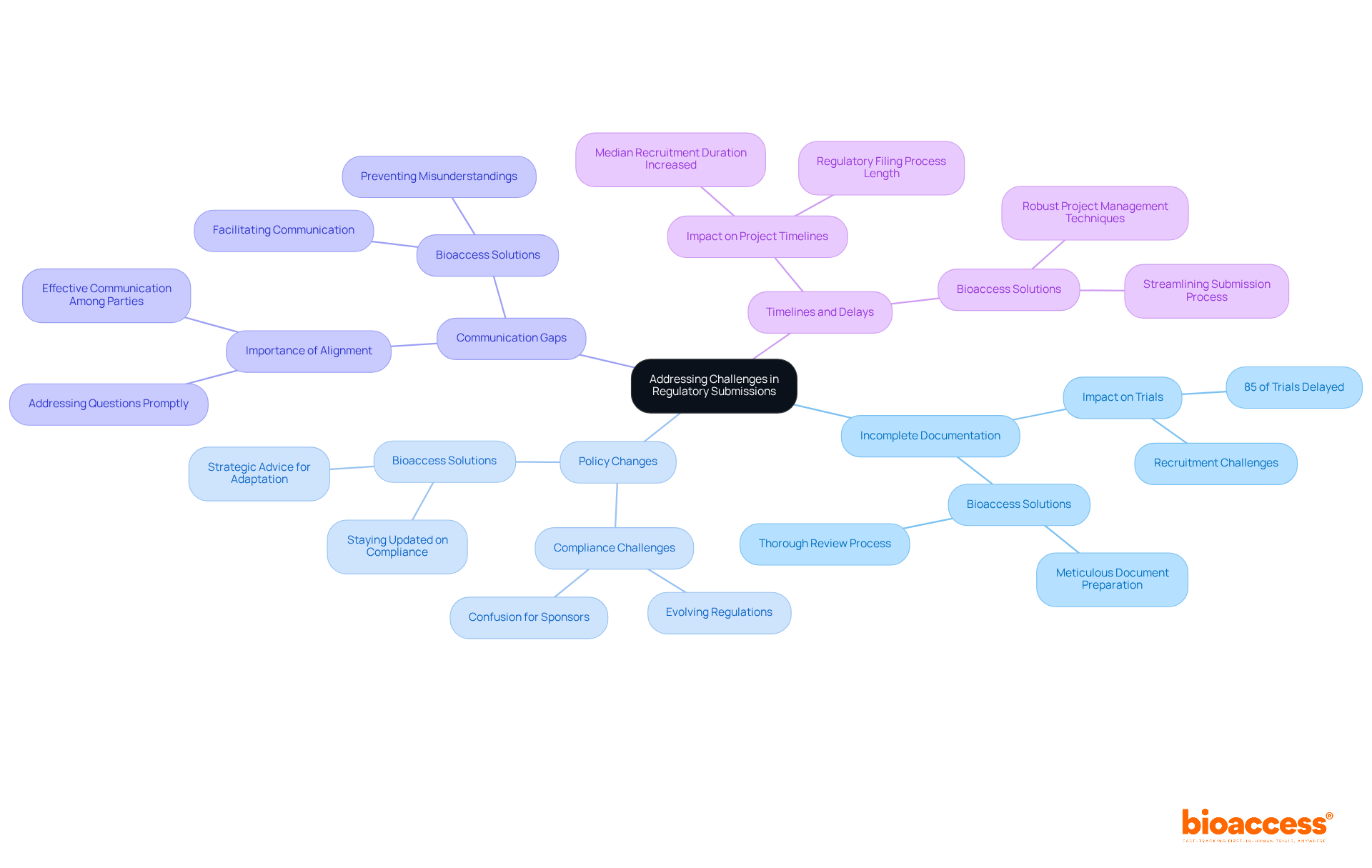
Incomplete Documentation: In clinical studies, incomplete or flawed documentation poses a significant obstacle that can severely impede progress. Studies indicate that 85% of clinical trials experience delays, often due to recruitment challenges and documentation errors. Bioaccess plays an essential role of CROs in regulatory submissions in Bulgaria by ensuring that all necessary documents are meticulously prepared and reviewed prior to submission. This diligence minimizes the risk of delays and enhances the overall efficiency of the trial process.
Policy Changes: The oversight landscape is in a constant state of flux, leading to confusion and compliance challenges for sponsors. Bioaccess is essential in navigating these changes, reflecting the role of CROs in regulatory submissions in Bulgaria by staying abreast of the latest compliance developments and providing strategic advice to sponsors on how to adapt their proposals. This proactive approach mitigates the risks associated with non-compliance, which can result in further delays and complications.
Communication Gaps: Effective communication among sponsors, Bioaccess, and oversight bodies is vital for the success of clinical studies. Bioaccess facilitates this communication, ensuring that all parties remain aligned and that any questions or concerns are addressed promptly. Given the complexities introduced by evolving regulations, clear communication is crucial to prevent misunderstandings that could lead to delays.
Timelines and Delays: The regulatory filing process can be extensive, and any delays can jeopardize project timelines. Recent statistics reveal that the median recruitment duration for U.S. Phase III studies has increased from approximately 13 months to 18 months. Bioaccess employs robust project management techniques to streamline the submission process, ensuring that deadlines are met and studies commence as planned. By addressing documentation issues and enhancing communication, Bioaccess significantly contributes to maintaining the integrity and efficiency of clinical trials. Looking ahead to 2025, the impact of incomplete documentation is expected to remain a critical concern, necessitating ongoing vigilance and adaptation from all stakeholders.
The pivotal role of Contract Research Organizations (CROs) in regulatory submissions in Bulgaria is crucial. By bridging the gap between sponsors and regulatory bodies, CROs ensure that clinical research studies are conducted efficiently, ethically, and in compliance with stringent regulations. Their expertise not only enhances the quality of submissions but also accelerates the approval process, ultimately contributing to the advancement of medical research.
Throughout this discussion, several key insights illustrate the multifaceted responsibilities of CROs:
CROs like Bioaccess demonstrate their value in optimizing the regulatory submission process. Their strategic involvement significantly reduces the risk of delays, enhances study efficiency, and fosters successful partnerships between sponsors and regulatory authorities.
As the landscape of clinical research continues to evolve, the demand for skilled CROs is expected to rise. Embracing their expertise is essential for sponsors aiming to navigate the intricate regulatory framework effectively. By prioritizing collaboration and leveraging the specialized knowledge of CROs, stakeholders can ensure that clinical trials not only meet compliance standards but also contribute to groundbreaking advancements in healthcare. Investing in these partnerships today is a crucial step toward shaping a more efficient and innovative future in medical research.
What are the main responsibilities of Contract Research Organizations (CROs) in clinical research?
CROs are responsible for study design, patient recruitment, data management, and regulatory compliance, acting as links between sponsors and oversight bodies.
How significant are CROs in the clinical research industry?
Nearly 75% of research studies are conducted by CROs, highlighting their crucial role in the industry.
What expertise do CROs leverage in Bulgaria?
CROs in Bulgaria leverage their expertise in therapeutic areas to navigate the complexities of research involving human subjects and assist in regulatory submissions.
How do CROs benefit sponsors in clinical research?
CROs handle operational tasks and engage in strategic planning, allowing sponsors to focus on their core competencies, which can lead to improved study completion rates and reduced costs.
What is the projected market growth for CRO services?
The market for CRO services is projected to reach USD 164.3 billion by 2035, driven by increasing complexity in studies and the need for specialized expertise.
What specific services does Bioaccess offer for first-in-human medical device clinical studies?
Bioaccess offers study design, feasibility assessments, submissions to ethics committees and health authorities, and project management.
Can you provide an example of how a CRO partnership improved study efficiency?
A European biotech firm reduced its time-to-offer from over 50 days to 36 days by collaborating with a CRO that implemented a dedicated Full-Time Equivalent (FTE) model for regulatory and data management services.
Why is it important to choose the right CRO for regulatory submissions in Bulgaria?
Selecting a CRO that understands the regulatory landscape in Bulgaria is essential as they can adapt to specific needs and scale operations effectively, ensuring studies are conducted ethically and efficiently.