


The clinical research landscape presents a complex web of regulations, approvals, and stakeholder interactions, where the success of a trial hinges on meticulous planning and execution. Central to this intricate process is the study start-up specialist, a role that has become increasingly vital as the demand for efficient study initiation grows. With approximately 31% of research sites citing slow initiation as a significant hurdle, how can these specialists effectively navigate challenges to ensure timely and compliant trial launches?
Exploring the multifaceted responsibilities and essential skills of study start-up specialists reveals not only their importance but also the potential for innovation in clinical research. Their expertise is crucial in overcoming obstacles and streamlining processes, ultimately leading to more successful trial outcomes. As the landscape evolves, the need for these specialists to adapt and innovate becomes even more pressing, ensuring that clinical research can meet the demands of a rapidly changing environment.
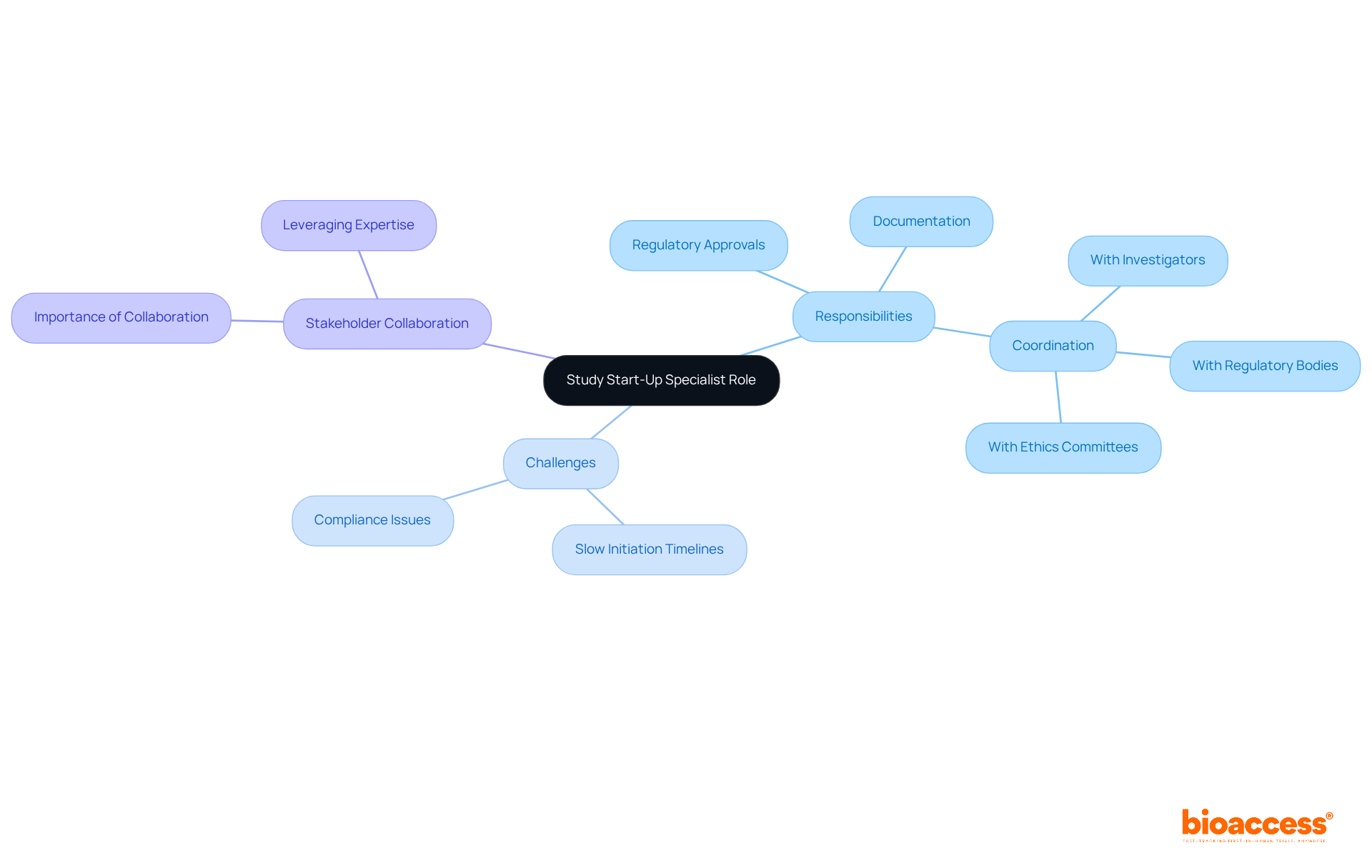
A study start-up specialist is essential in the clinical research landscape, primarily overseeing the initiation phase of clinical trials. This role involves managing the critical path to activation, ensuring that all necessary regulatory approvals, preparations, and documentation are completed efficiently and in compliance with applicable regulations. As subject matter experts, SSU Specialists coordinate with various stakeholders, including investigators, regulatory bodies, and ethics committees, facilitating a seamless transition from planning to execution.
Given that approximately 31% of research locations identify slow study initiation timelines as a significant obstacle, the involvement of a study start-up specialist is vital in minimizing delays and enhancing overall study efficiency. Their involvement not only streamlines processes but also significantly impacts the timely delivery of medical studies, ultimately contributing to advancements in healthcare.
In this context, collaboration among stakeholders is crucial. By leveraging the knowledge and skills of study start-up specialists, clinical research can overcome initiation challenges, ensuring that studies progress smoothly and efficiently.
The study start-up specialist plays a pivotal role in the early phases of clinical studies, especially as the clinical research landscape becomes increasingly complex and regulated. With a staggering 77% of registered research categorized as interventional, the demand for roles such as the study start-up specialist has surged. This position is essential for a study start-up specialist to ensure that studies commence on schedule, which is a critical factor in achieving research objectives and meeting regulatory deadlines.
The intricate web of regulatory requirements, site selection, and stakeholder engagement is adeptly navigated by study start-up specialists, making them indispensable to the success of clinical trials. Their contributions directly impact the speed and efficiency of study initiation, which can significantly affect overall research timelines and enhance patient access to groundbreaking therapies. Notably, 80% of studies face delays due to recruitment challenges, underscoring the vital role of study start-up specialists in mitigating such setbacks.
As research studies evolve, marked by a 61% increase in eligibility criteria from 2001 to 2015, the role of the study start-up specialist becomes even more crucial in managing these complexities and ensuring successful outcomes. Furthermore, the partnership between bioaccess™ and Caribbean Health Group, announced on March 29, 2019, aims to position Barranquilla as a key hub for medical research in Latin America, with support from Colombia's Minister of Health. This initiative not only enhances the local research environment but also aligns with the global trend, where the market for contract research outsourcing is projected to reach $90.4 billion by 2030.
Moreover, collaborations like that of GlobalCare Clinical Studies with bioaccess™ demonstrate the potential to reduce recruitment time by over 50% and achieve retention rates of 95%. This highlights the significant impact of study start-up specialists in adapting to decentralized research methodologies, which can shorten study timelines by 30%. As the clinical research field continues to evolve, the importance of study start-up specialists in driving efficiency and innovation is increasingly recognized.
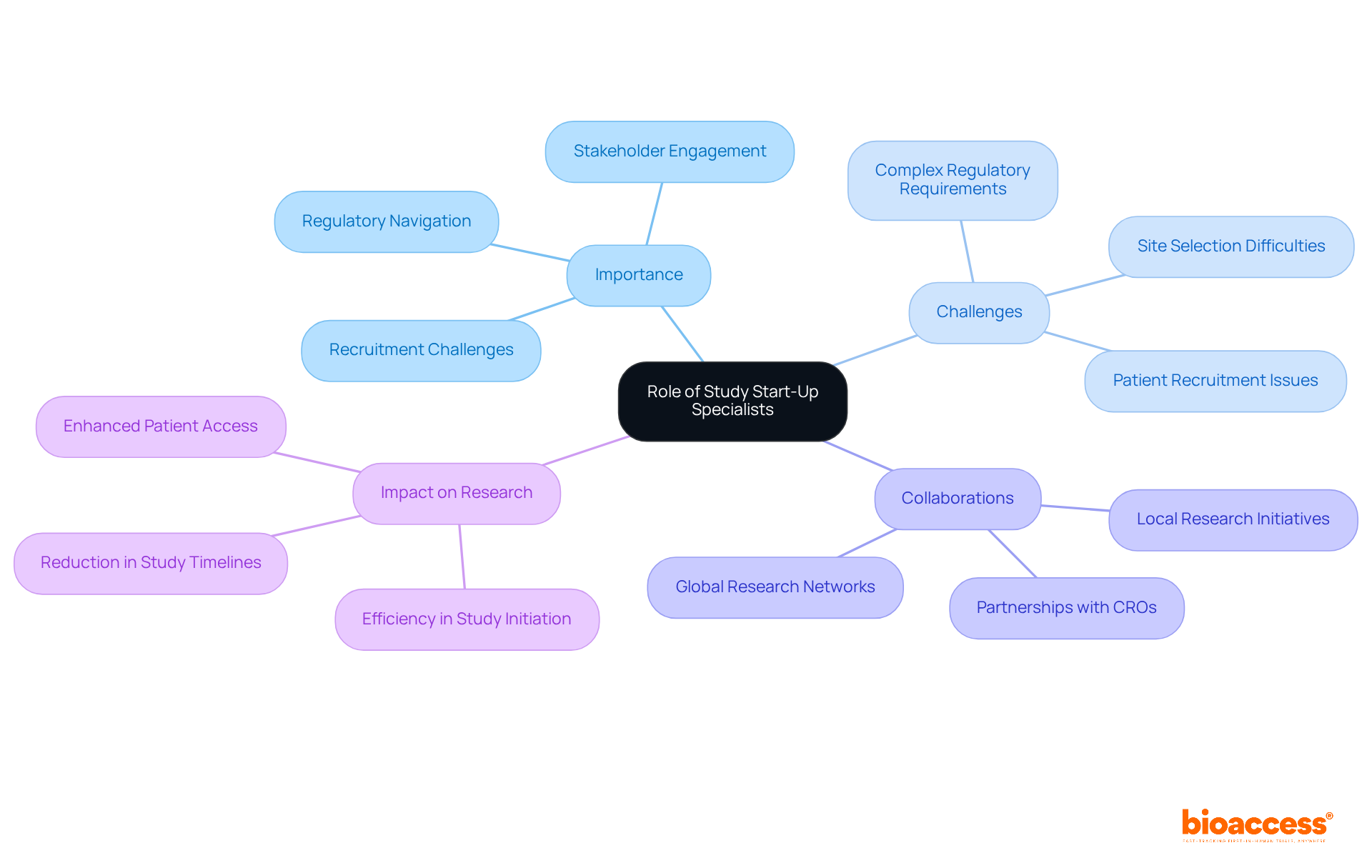
Key responsibilities of a Study Start-Up Specialist are crucial for the successful initiation of clinical trials:
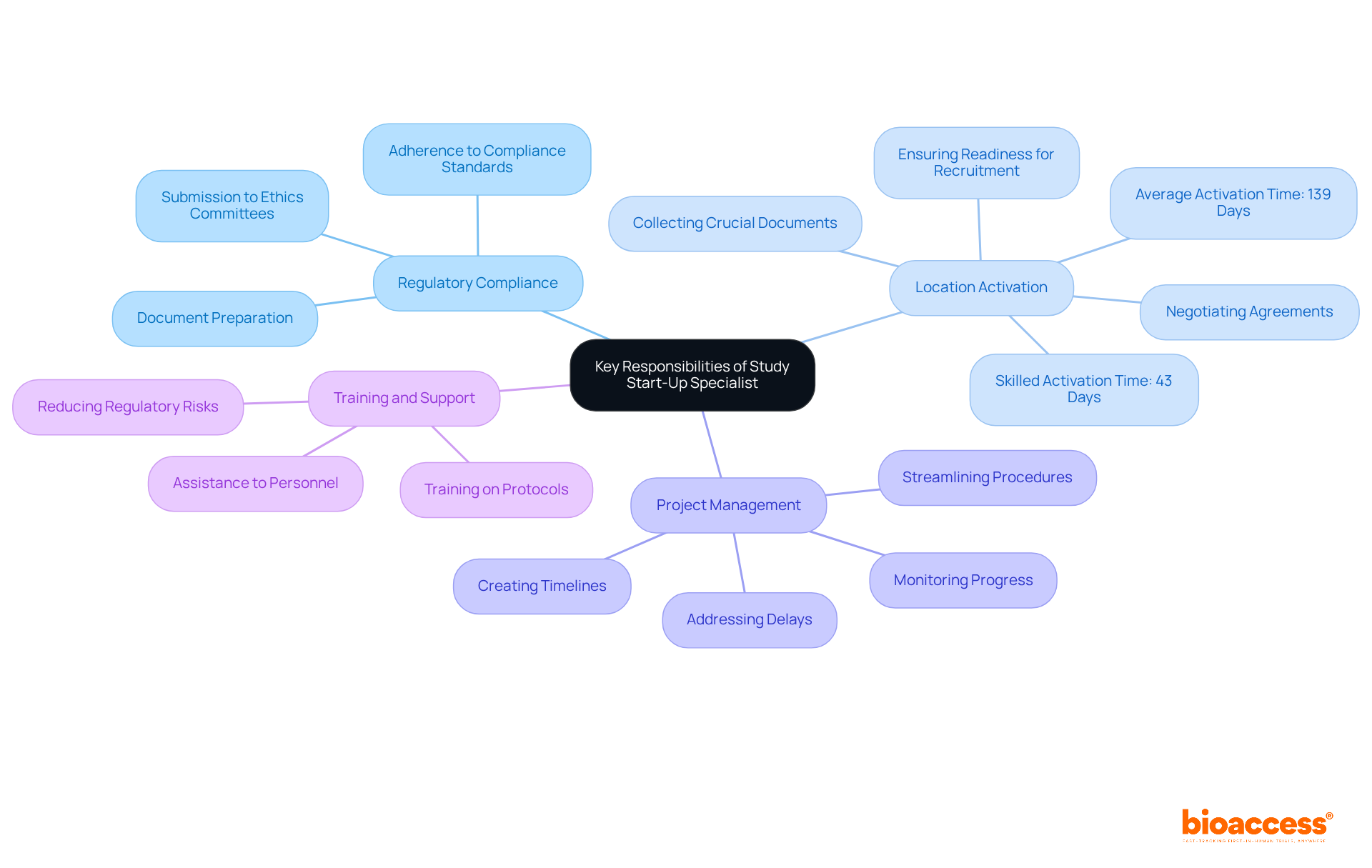
Regulatory Compliance: Specialists ensure that all necessary regulatory submissions and approvals are secured before trial commencement. This involves meticulous preparation and submission of documents to ethics committees and regulatory authorities, adhering to the latest compliance standards in 2025.
Location Activation: Coordinating the initiation of clinical trial locations is pivotal. This entails collecting crucial documents, negotiating agreements, and ensuring that locations are fully ready for patient recruitment. The industry average for activation is roughly 139 days, but skilled professionals can significantly shorten this timeframe, accomplishing activation in as few as 43 days.
Study Start-Up Specialists act as the main intermediary for investigators and site personnel, facilitating smooth stakeholder communication among all parties engaged in the research. This role is essential for maintaining alignment and addressing any concerns that may arise during the start-up phase.
Project Management: Creating and upholding comprehensive timelines for research start-up activities is vital. Specialists meticulously monitor progress, addressing any delays or issues promptly to ensure that the research remains on schedule. Streamlined procedures can shorten activation timelines by as much as 76%.
Training and Support: Providing extensive training and assistance to personnel on research protocols and procedures is essential for ensuring adherence and a complete understanding of trial requirements. This training helps reduce risks linked to regulatory non-compliance and improves the overall efficiency of the initiation process.
Successful site activation examples by study start-up specialists illustrate the impact of their expertise. A recent case study highlighted how a specialist's involvement decreased the activation time from the industry average of 20 weeks to just 6.5 weeks, showcasing the significant value they contribute to research initiatives.
Key skills and qualifications for a study start-up specialist encompass a range of competencies vital for navigating the complexities of research.

The role of a study start-up specialist is fundamental to the successful initiation of clinical trials, acting as the linchpin that connects various stakeholders and ensures compliance with regulatory requirements. By efficiently managing the critical path to activation, these specialists not only streamline processes but also significantly enhance the overall efficiency of clinical research, paving the way for timely advancements in healthcare.
Key points throughout the article highlight the multifaceted responsibilities of study start-up specialists, including:
Their expertise is crucial in navigating the complexities of the clinical research landscape, particularly in addressing delays caused by recruitment challenges and ensuring that studies commence on schedule. With a growing demand for these specialists, their role has never been more critical in overcoming initiation challenges and driving research forward.
As the clinical research environment continues to evolve, the importance of study start-up specialists cannot be overstated. Their ability to adapt to new methodologies and streamline processes is vital for accelerating the delivery of innovative therapies to patients. Emphasizing the value of this role can inspire organizations to invest in developing skilled professionals who can navigate the complexities of clinical trials, ultimately leading to improved health outcomes and advancements in medical science.
What is the role of a study start-up specialist in clinical research?
A study start-up specialist oversees the initiation phase of clinical trials, managing the critical path to activation and ensuring that all necessary regulatory approvals, preparations, and documentation are completed efficiently and in compliance with regulations.
Why is the study start-up specialist important in clinical trials?
Their role is vital in minimizing delays and enhancing overall study efficiency, as approximately 31% of research locations identify slow study initiation timelines as a significant obstacle.
What stakeholders do study start-up specialists coordinate with?
Study start-up specialists coordinate with various stakeholders, including investigators, regulatory bodies, and ethics committees.
How do study start-up specialists contribute to the efficiency of clinical research?
They streamline processes and facilitate a seamless transition from planning to execution, significantly impacting the timely delivery of medical studies and contributing to advancements in healthcare.
What challenges do study start-up specialists help to overcome?
They help overcome initiation challenges in clinical research, ensuring that studies progress smoothly and efficiently.