

The primary objective of this article is to delineate the critical steps necessary for effectively navigating the US FDA medical device approval process. It underscores the significance of comprehending device classifications, pinpointing suitable approval pathways, meticulously preparing documentation, and overseeing post-approval compliance. Each of these elements is vital for ensuring timely market entry and sustaining regulatory adherence.
Navigating the complex landscape of FDA medical device approval presents a significant challenge for manufacturers. In an evolving regulatory environment with varying classification levels, comprehending the intricacies of the approval process is essential for ensuring that innovative medical solutions reach patients efficiently.
What key steps and strategies can enhance the likelihood of successful approval? How can manufacturers adeptly manage the challenges that arise during this journey?
This article explores the essential pathways, documentation requirements, and post-approval compliance measures necessary for success in the FDA medical device approval process.
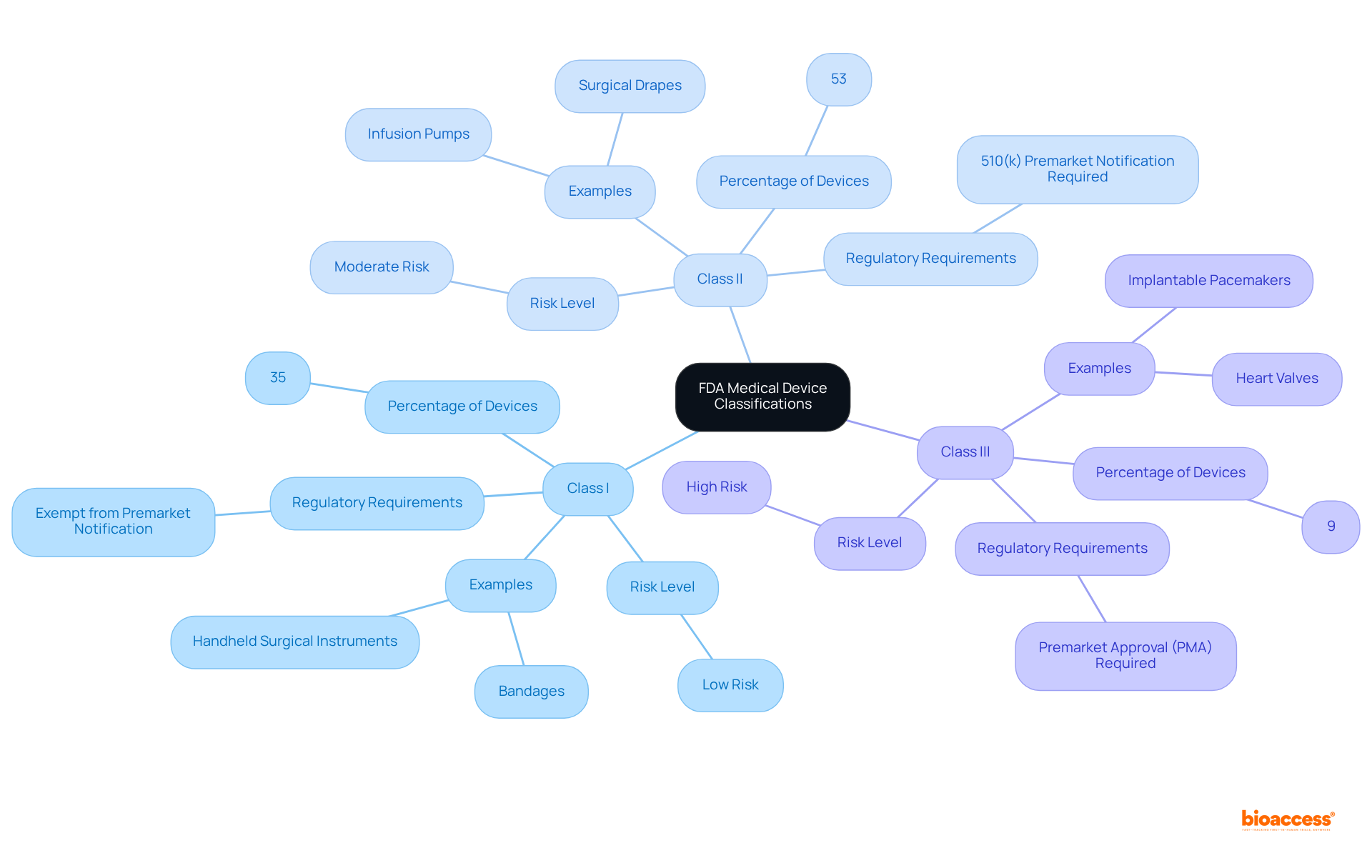
The FDA categorizes medical devices into three classes based on the level of risk associated with their use:
Class I: Low-risk devices that are subject to the least regulatory control. Examples include bandages and handheld surgical instruments. Approximately 35% of all regulated medical instruments fall into this category, and most Type I products are exempt from premarket notification requirements. As stated by the FDA Center for Devices and Radiological Health, 'Category I medical instruments pose the least risk with minimal possibility of harm.'
Category II: Moderate-risk instruments that necessitate enhanced regulatory measures to guarantee safety and effectiveness. Common examples include infusion pumps and surgical drapes. About 53% of authorized products are categorized as Category II, and these generally require a 510(k) premarket notification to show substantial equivalence to current products. The FDA declares, 'Category II medical instruments are at moderate risk, presenting a greater risk than Category I instruments.'
Category III: High-risk products that require premarket approval (PMA) due to their potential to cause significant harm. Examples include implantable pacemakers and heart valves. Category III instruments undergo the most stringent evaluation procedure, representing only 9% of all authorized medical instruments. The FDA emphasizes, 'Class III medical products present the highest risk, encompassing items that sustain or support life.'
Recent changes in FDA medical product classification have introduced new pathways and requirements, making it essential for manufacturers to stay informed. Understanding these classifications is crucial for navigating the FDA approval process effectively. Manufacturers should consult the FDA's product classification database, taking into account their product's intended use and indications for use. This knowledge not only aids in compliance but also streamlines the path to market, ensuring that innovative medical solutions can reach patients in a timely manner.
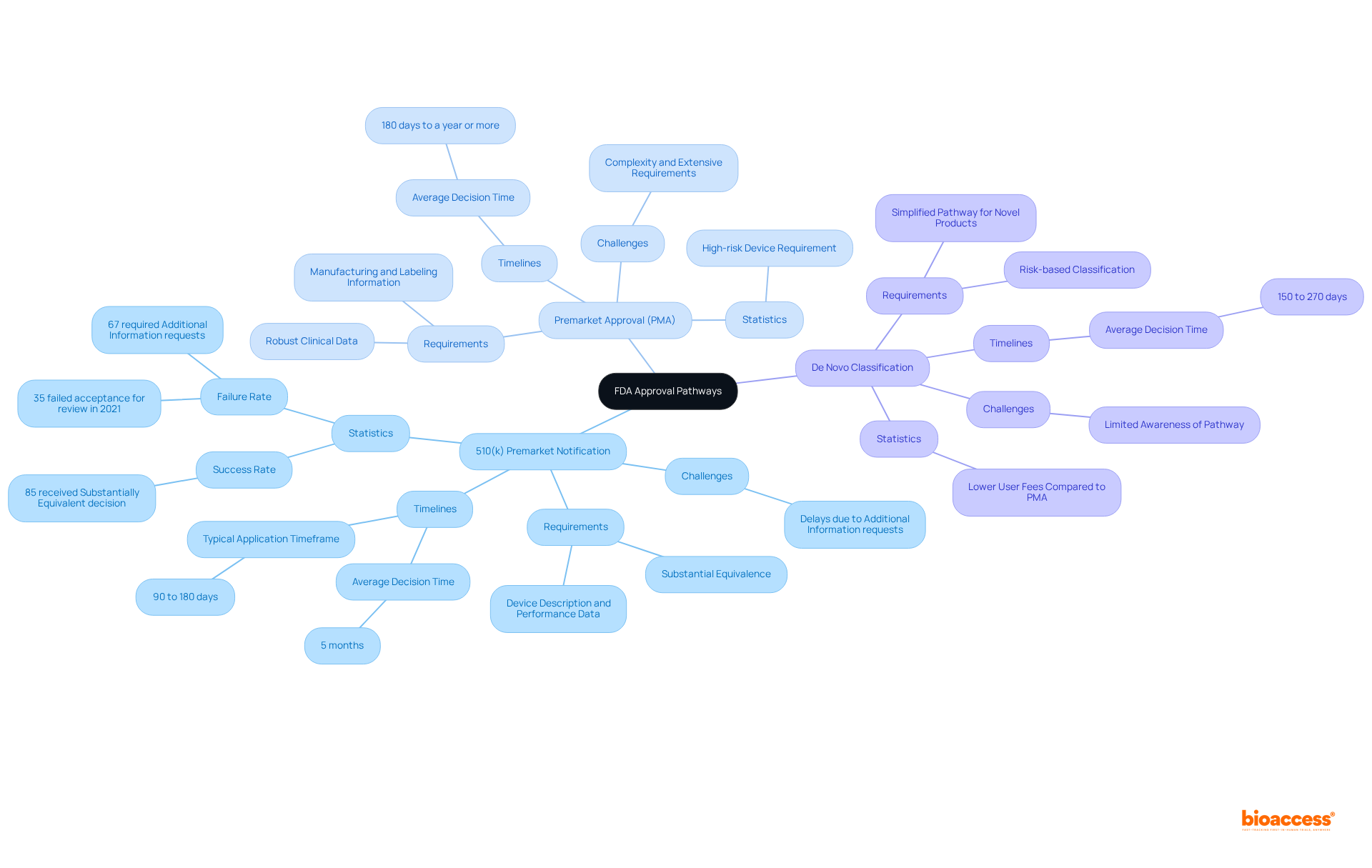
Identifying the appropriate FDA approval pathway is crucial for the success of your medical product. Here’s a breakdown of the main pathways:
510(k) Premarket Notification: This pathway is primarily for Class II devices. To gain clearance, you must demonstrate that your apparatus is substantially equivalent to an existing legally marketed item. A comprehensive proposal should include a detailed device description, intended use, and performance data. Notably, as of 2025, approximately 85 percent of FDA 510(k) applications received a Substantially Equivalent decision, highlighting the pathway's effectiveness. However, it is important to note that 35 percent of FDA 510(k) applications failed the acceptance for review check in 2021, indicating potential challenges in the application process. Furthermore, 67 percent of 510(k) applications resulted in an Additional Information request during the initial regulatory review cycle, which can cause delays. The typical timeframe for 510(k) applications is between 90 to 180 days, with the average duration to reach a decision being around five months.
Premarket Approval (PMA): Required for Class III devices, the PMA process is more rigorous and necessitates robust clinical data to substantiate the safety and effectiveness of your device. Your application must include clinical study results, manufacturing details, and labeling information. The PMA pathway is often perceived as daunting due to its complexity; however, it offers intrinsic value and protections akin to patent rights. In 2025, the success rate for PMA applications remains a critical consideration, as manufacturers navigate the extensive requirements. It is also important to acknowledge that PMA applications may take 180 days to a year or more, which can significantly affect your timeline.
De Novo Classification: If your product is novel and lacks a predicate, you can pursue a De Novo classification. This pathway allows for a risk-based classification and can facilitate a subsequent 510(k) pathway if granted. The De Novo process is beneficial for novel products, offering a simplified pathway to market with possibly reduced user fees in comparison to PMA submissions.
When choosing the most suitable pathway, assess your equipment's features and intended application carefully. This decision will significantly influence your timeline and resource allocation, ultimately impacting your device's market entry and success.
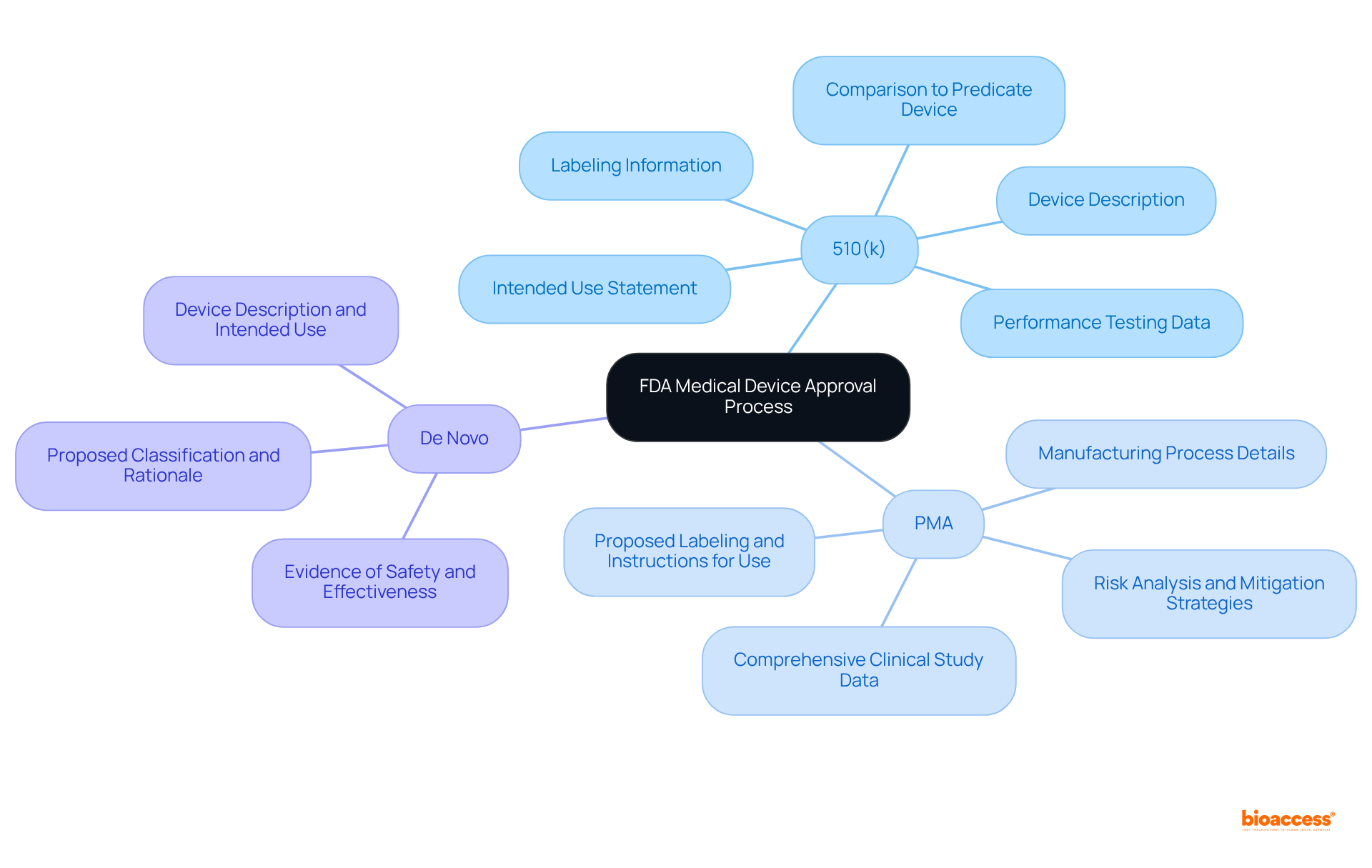
Preparing the necessary documentation is a critical step in the US FDA medical device approval process, with requirements varying significantly based on the selected pathway. A well-organized and comprehensive entry can greatly enhance the chances of approval and expedite the process.
For 510(k) submissions, the essential documentation includes:
Statistics indicate that nearly 32% of 510(k) submissions failed the initial acceptance check in the year leading up to September 2022, often due to incomplete documentation. This underscores the importance of ensuring that all required elements are thoroughly addressed. Additionally, 67% of US FDA medical device 510(k) applications resulted in requests for additional information during the review process, further emphasizing the need for meticulous preparation.
For PMA submissions, the documentation requirements are more extensive and include:
Real-world examples demonstrate the success of meticulous PMA documentation. Companies that have adhered to these requirements have experienced smoother approval processes and quicker market entry. As regulatory affairs consultant Trey Thorsen notes, "A successful PMA is the result of several requirements being met."
For De Novo submissions, the necessary documents consist of:
It is crucial to ensure that all documents are meticulously prepared, as incomplete or inaccurate submissions can lead to significant delays or outright denials. Utilizing templates and checklists can help organize your documentation effectively, reducing the risk of common errors that can hinder the approval process. Expert regulatory support from organizations like bioaccess® and Arrotek can also provide valuable guidance in navigating these complexities, ensuring that your submission meets all necessary criteria for success.
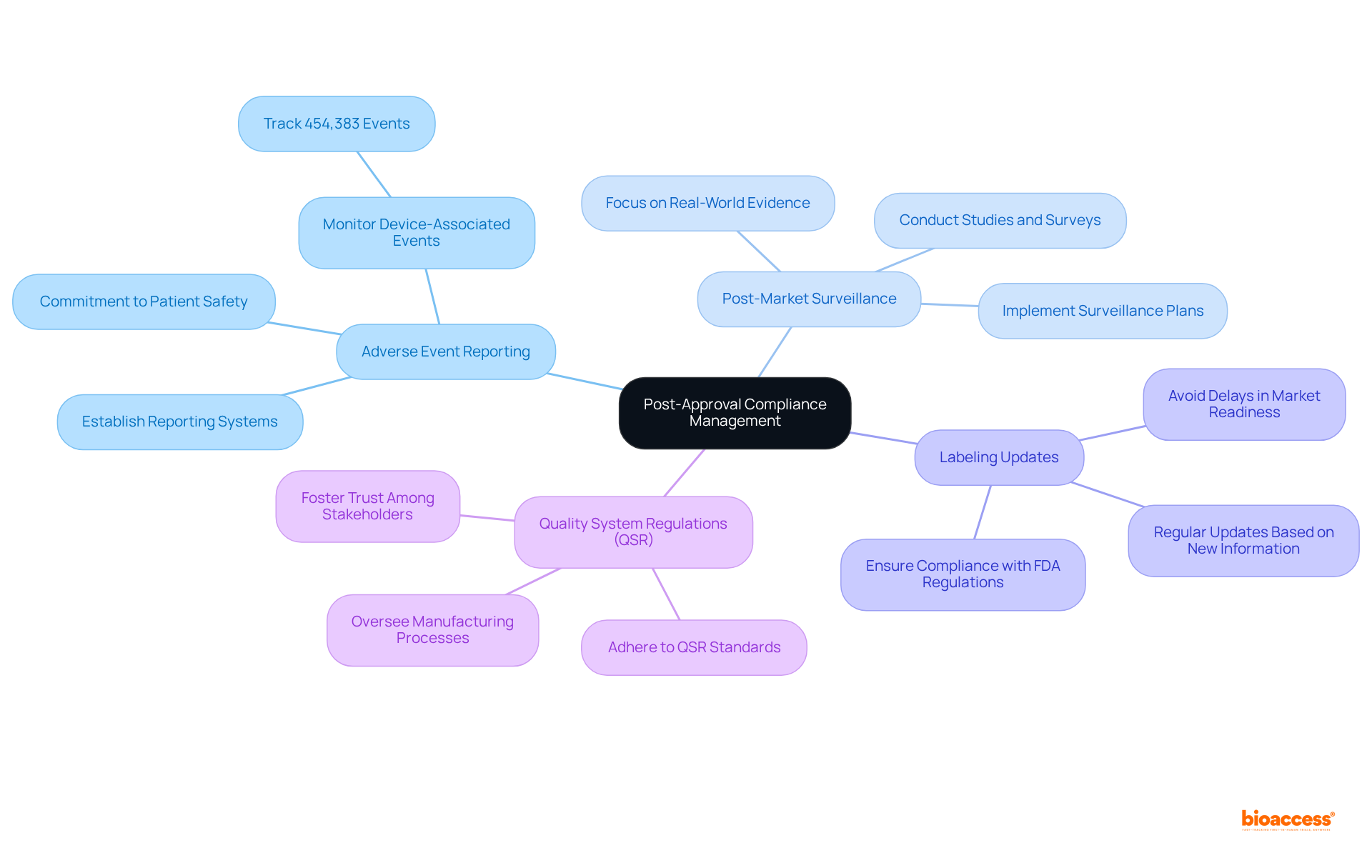
After receiving FDA approval, managing post-approval compliance effectively is essential:
Adverse Event Reporting: Monitoring and reporting any adverse events associated with your device to the FDA is imperative. Establishing a robust system for tracking and documenting complaints is crucial, as approximately 454,383 device-associated adverse events have been reported in recent years. This statistic underscores the critical need for effective reporting systems to ensure patient safety and equipment integrity. Prompt adverse event reporting is not just a regulatory requirement; it is a commitment to patient safety and equipment integrity.
Post-Market Surveillance: Implementing a comprehensive post-market surveillance plan is essential for gathering data on your product's performance in real-world settings. This may involve conducting studies or surveys to assess long-term safety and effectiveness. Current trends indicate a shift towards more rigorous post-market monitoring, emphasizing real-world evidence and data-informed decision-making to enhance patient outcomes.
Labeling Updates: Ensure that your labeling remains compliant with FDA regulations. Regular updates are necessary based on new information or changes in indications, as non-compliance can lead to significant delays in market readiness.
Quality System Regulations (QSR): Adherence to QSR is crucial, overseeing the manufacturing processes and quality assurance for medical products. A proactive approach to compliance not only mitigates risks but also fosters trust among stakeholders.
By addressing these critical areas, manufacturers can ensure ongoing compliance, enhance patient safety, and ultimately contribute to the sustained success of their medical devices in the competitive healthcare landscape.
Understanding the intricacies of the FDA medical device approval process is paramount for manufacturers aiming to bring innovative healthcare solutions to market. By navigating the classifications, approval pathways, documentation requirements, and post-approval compliance measures, companies can significantly enhance their chances of success and ensure patient safety.
The article highlights the importance of recognizing the three classes of medical devices—Class I, II, and III—each with its unique regulatory requirements. It emphasizes the need to choose the appropriate approval pathway, whether it’s:
Furthermore, meticulous preparation of documentation and adherence to post-approval compliance are critical steps that cannot be overlooked.
Ultimately, staying informed about the evolving landscape of FDA regulations and maintaining a proactive approach to compliance will not only facilitate smoother approvals but also foster trust within the healthcare community. As the medical device industry continues to evolve, manufacturers are encouraged to leverage the insights shared in this guide to navigate the FDA approval process with confidence and efficiency.
What are the three classes of medical devices according to the FDA?
The three classes of medical devices are Class I (low-risk), Class II (moderate-risk), and Class III (high-risk).
What characteristics define Class I medical devices?
Class I medical devices are low-risk devices with minimal regulatory control. Examples include bandages and handheld surgical instruments, and about 35% of all regulated medical instruments fall into this category. Most Class I products are exempt from premarket notification requirements.
What are some examples of Class II medical devices?
Class II medical devices are moderate-risk instruments that require enhanced regulatory measures. Common examples include infusion pumps and surgical drapes, and approximately 53% of authorized products are categorized as Class II.
What is the requirement for Class II medical devices before they can be marketed?
Class II medical devices generally require a 510(k) premarket notification to demonstrate substantial equivalence to current products.
What distinguishes Class III medical devices from the other classes?
Class III medical devices are high-risk products that require premarket approval (PMA) due to their potential to cause significant harm. Examples include implantable pacemakers and heart valves, and they undergo the most stringent evaluation process.
What percentage of authorized medical instruments are classified as Class III?
Only 9% of all authorized medical instruments are classified as Class III.
Why is it important for manufacturers to understand FDA medical product classifications?
Understanding FDA medical product classifications is crucial for navigating the FDA approval process effectively. It aids in compliance and streamlines the path to market, ensuring that innovative medical solutions can reach patients in a timely manner.
Where can manufacturers find more information about product classifications?
Manufacturers should consult the FDA's product classification database, considering their product's intended use and indications for use.