

Clinical data encompasses the comprehensive information amassed during healthcare and research processes, including demographic details, medical histories, lab results, and treatment responses. This data is essential for informed decision-making in medicine.
The evolution of clinical data management is significant, transitioning from rudimentary health records to modern electronic health records (EHRs) and mobile health applications. This transformation emphasizes the crucial role these technologies play in enhancing patient outcomes and fostering evidence-based practices in healthcare.
Understanding the intricacies of clinical data is paramount in an era where healthcare decisions increasingly rely on empirical evidence. These vital pieces of information—ranging from patient demographics to treatment outcomes—form the backbone of medical research and practice, influencing everything from individual care plans to groundbreaking clinical trials. As the landscape of healthcare evolves with advancements like electronic health records and mobile health applications, a pressing question arises: how can healthcare professionals effectively harness the power of clinical data to improve patient outcomes and drive innovation in medical practices?
Health information encompasses a wide array of details related to individual wellness, therapies, and outcomes, which leads to the question of what are clinical data primarily collected during research studies or standard healthcare procedures. What are clinical data? This information includes demographic details, medical histories, laboratory results, and treatment responses, forming a crucial foundation for healthcare and decision-making in medicine. Notably, by 2025, approximately 70% of medical trials are expected to utilize electronic health records (EHRs) for information gathering, underscoring the shift towards more efficient and precise information management systems.
Understanding what are clinical data is crucial, as their importance extends beyond personal care and is vital to evidence-based medicine, which relies on empirical evidence to guide healthcare practices. As experts have pointed out, "Data is indeed everywhere, and those who know how to handle it are in a very powerful position." This statement emphasizes the necessity for healthcare practitioners to effectively utilize medical information to enhance patient outcomes and inform treatment strategies. Furthermore, understanding what are clinical data is crucial for analyzing research data, evaluating the true effects of treatments, distinguishing these effects from random variability, and ensuring that medical practices are grounded in solid evidence.
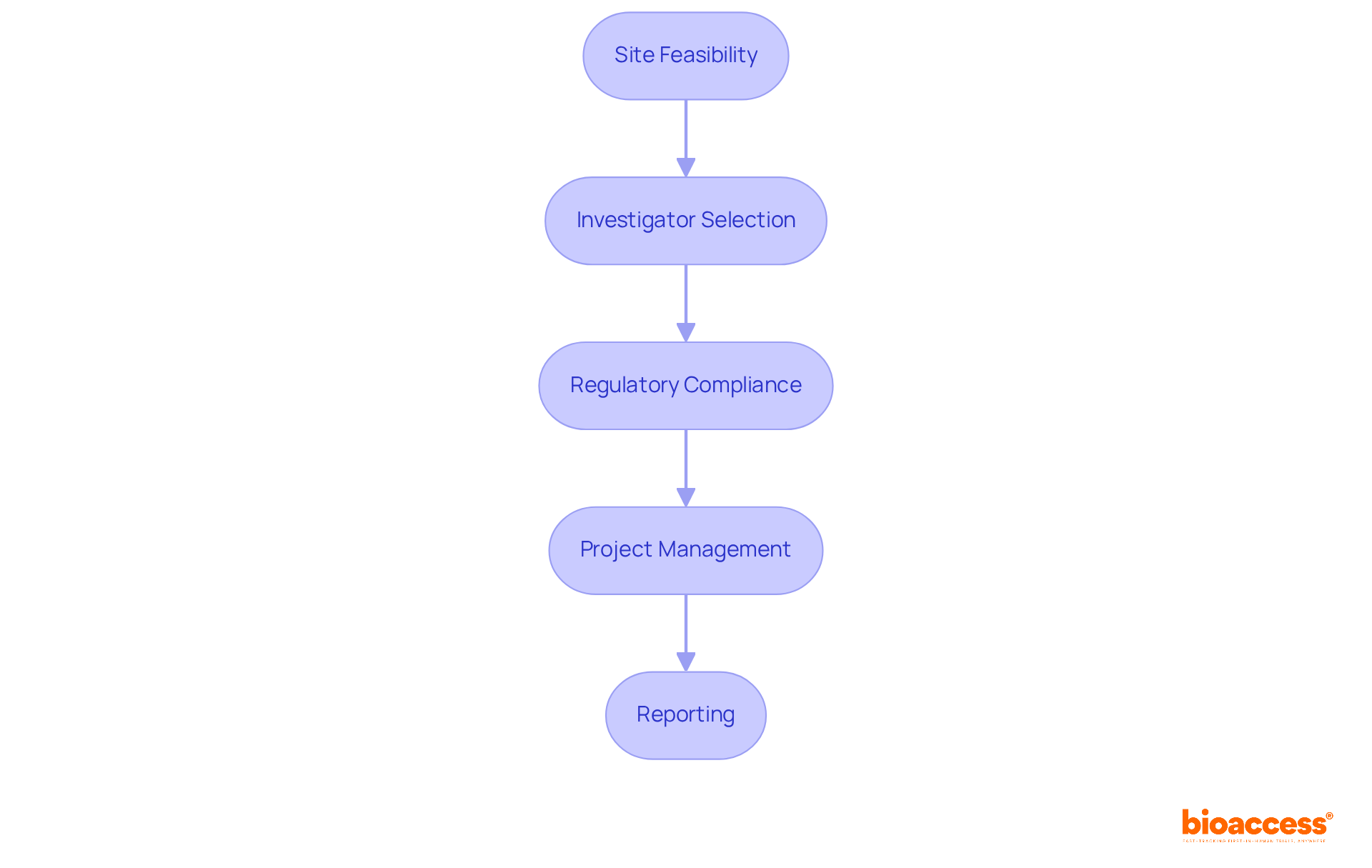
In this landscape, bioaccess® offers comprehensive study management services that include:
These services not only streamline the trial process but also accelerate patient enrollment by as much as 50%, resulting in significant cost savings of $25K per patient with FDA-ready data—no rework, no delays. Additionally, statistical power analysis plays a vital role in this context, as it determines the adequacy of sample sizes in studies, ensuring that findings are both reliable and applicable. Descriptive statistics are also essential in summarizing participant characteristics and treatment outcomes, providing critical insights that inform medical decisions. Therefore, the role of health information in advancing medical research, improving healthcare delivery, and fostering economic growth through trials cannot be overstated.

Information plays a crucial role in medical research and healthcare, enabling the assessment of new therapies and interventions. In medical studies, understanding what clinical data are is essential for gathering information to evaluate the safety and effectiveness of new pharmaceuticals or medical devices. At bioaccess®, we specialize in managing the comprehensive process of advancing medical device trials, including:
Furthermore, clinical data guides healthcare professionals regarding patient demographics, treatment outcomes, and disease prevalence, allowing them to tailor care to specific patient needs. The integration of healthcare information into electronic health records (EHRs) enhances the continuity of care and supports evidence-based decision-making in medical settings. Our expertise in navigating regulatory challenges and optimizing recruitment strategies ensures that trials are executed efficiently, ultimately facilitating the advancement of medical technology.
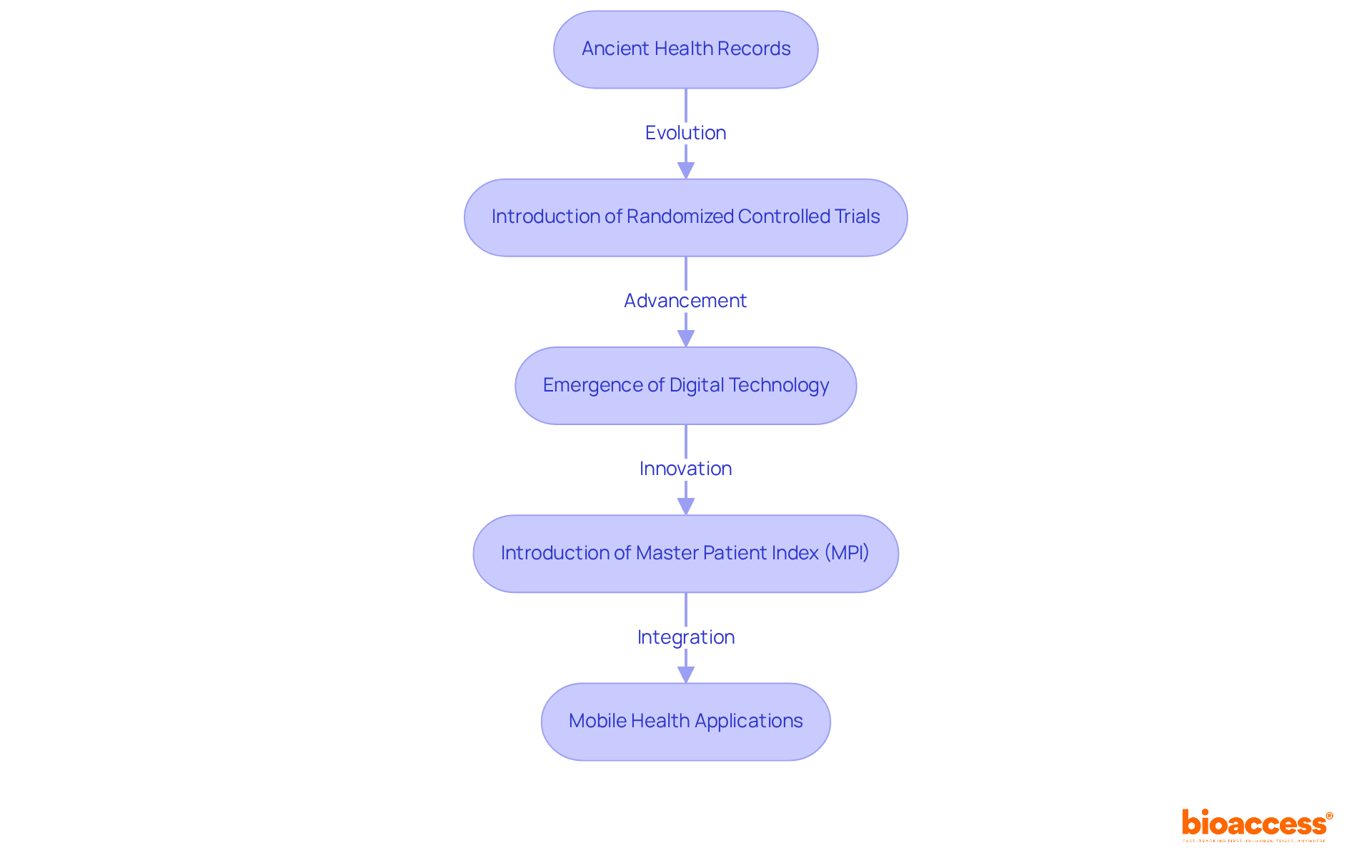
The evolution of clinical information traces back to ancient civilizations, where rudimentary forms of health records were maintained. Over the centuries, the practice of documenting patient information has transformed significantly. The introduction of randomized controlled trials in the 20th century marked a pivotal moment, establishing rigorous methodologies for information collection. Furthermore, the emergence of digital technology in the late 20th century revolutionized healthcare information management, enabling real-time information capture and analysis. Notably, the introduction of the Master Patient Index (MPI) in the 1980s enhanced information storage and retrieval efficiency.
Today, mobile health (mHealth) applications, alongside the incorporation of electronic health records (EHRs) and wearable devices, are shaping the future of healthcare information, facilitating advanced analyses and tailored medicine strategies. The synergy of extensive information and artificial intelligence is driving advanced processing, ultimately improving patient outcomes. This ongoing evolution underscores the essential role of what are clinical data in advancing healthcare and improving treatment effectiveness.
Clinical information encompasses both structured and unstructured elements, each playing a vital role in healthcare research. Structured information includes quantifiable elements such as lab results, vital signs, and medication dosages, typically organized in databases for efficient retrieval and analysis. In contrast, unorganized information comprises free-text notes, imaging reports, and patient narratives, often necessitating advanced processing techniques, such as natural language processing (NLP), to extract meaningful insights.
Essential elements of healthcare information management involve robust information collection techniques, rigorous quality assurance procedures, and adherence to regulatory standards. Quality assurance (QA) is critical in research involving human subjects, as it ensures the precision and thoroughness of information, thereby protecting participant welfare and enhancing the trustworthiness of study results. Efficient QA practices can significantly reduce the risk of adverse drug events (ADEs) by ensuring that both structured and unstructured information is accurately captured and analyzed.
Current quality assurance methods in research studies include systematic oversight and verification of information throughout the research process. This encompasses the implementation of standard operating procedures (SOPs) that govern various aspects of trial management, ensuring compliance with guidelines established by organizations such as the International Council for Harmonisation (ICH). By focusing on these elements, healthcare management not only supports high-quality research but also contributes to improved outcomes for individuals, as evidenced by studies indicating that evidence-based practices enhance healthcare system return on investment.
In summary, understanding what are clinical data is essential for effective clinical data management, which transforms raw data into actionable insights, ultimately driving advancements in patient care and clinical research.

Understanding clinical data is fundamental to advancing healthcare and improving patient outcomes. This information encompasses a wide range of details, including demographics, medical histories, and treatment responses, serving as the backbone of evidence-based medicine. As the healthcare landscape evolves, the integration of electronic health records and advanced data management techniques is becoming increasingly vital. This highlights the need for healthcare professionals to harness this information effectively.
The article delves into the multifaceted nature of clinical data, illustrating its critical role in medical research and healthcare delivery. Key insights include:
Furthermore, the emphasis on the shift towards digital tools and technologies enhances the efficiency and accuracy of data collection, ultimately driving better decision-making in clinical settings.
In light of these insights, it is clear that understanding and effectively managing clinical data is not merely an operational necessity but a pivotal element in the quest for improved healthcare. As the industry moves towards 2025 and beyond, embracing these changes and leveraging the power of data will be essential for healthcare professionals aiming to optimize patient care and foster innovation in medical research. Engaging with these advancements will not only enhance individual practices but also contribute to a more robust healthcare system overall.
What are clinical data?
Clinical data refers to information collected during research studies or standard healthcare procedures, including demographic details, medical histories, laboratory results, and treatment responses. This data is essential for healthcare decision-making and evidence-based medicine.
Why are clinical data important?
Clinical data are crucial for evidence-based medicine as they help guide healthcare practices through empirical evidence. They are also vital for analyzing research data, evaluating treatment effects, and ensuring medical practices are based on solid evidence.
How is the use of electronic health records (EHRs) expected to change by 2025?
By 2025, it is expected that approximately 70% of medical trials will utilize electronic health records (EHRs) for information gathering, indicating a shift towards more efficient and precise information management systems.
What services does bioaccess® provide in relation to clinical data?
Bioaccess® offers comprehensive study management services including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. These services aim to streamline the trial process and enhance patient enrollment.
How does bioaccess® impact patient enrollment and costs?
Bioaccess® services can accelerate patient enrollment by as much as 50%, resulting in significant cost savings of $25,000 per patient with FDA-ready data, eliminating rework and delays.
What role does statistical power analysis play in clinical studies?
Statistical power analysis is essential for determining the adequacy of sample sizes in studies, ensuring that findings are reliable and applicable.
Why are descriptive statistics important in clinical research?
Descriptive statistics are important as they summarize participant characteristics and treatment outcomes, providing critical insights that inform medical decisions.