


A Case Report Form (CRF) serves as a pivotal document in clinical research, meticulously designed to systematically collect participant data. This ensures uniformity and precision in information gathering, which is essential for the integrity of study outcomes. CRFs not only enhance the reliability of research findings but also facilitate compliance with regulatory standards. Consequently, they play a significant role in advancing medical knowledge and improving patient care. The importance of CRFs cannot be overstated; they are fundamental to the successful execution of clinical studies.
Understanding the intricacies of clinical research requires a thorough exploration of the tools that facilitate data collection and analysis, with the Case Report Form (CRF) positioned at the forefront. CRFs are not merely documents; they are meticulously crafted instruments that ensure the systematic gathering of critical participant information, thereby safeguarding the integrity of clinical trials. As research methodologies continue to evolve, the challenges associated with CRF design and implementation also transform, raising an essential question: how can researchers effectively balance the need for comprehensive data collection with the dynamic landscape of regulatory requirements and study protocols?
A Case Report Form (CRF) serves as a vital document in research studies, and understanding what is a CRF is essential as it is meticulously designed to systematically gather important information from each participant. Available in both paper and electronic formats, CRFs capture all protocol-required details, including patient demographics, medical history, and treatment outcomes. Understanding what is a CRF is paramount in medical research, as they ensure uniform and precise information collection, which is crucial for maintaining the integrity of study outcomes. By standardizing this process, it becomes clear what is a CRF, as they significantly enhance the evaluation of safety and efficacy for new treatments, ultimately contributing to advancements in medical knowledge and patient care.
With bioaccess®'s expertise in expediting clinical trials, CRFs facilitate a remarkable 50% increase in patient enrollment speed and yield savings of $25K through FDA-ready information, thereby ensuring that the data collection process is not only efficient but also compliant with regulatory standards. bioaccess® excels in managing a diverse array of studies, including:
This positions the company as a frontrunner in Medtech research across Latin America, with a strong focus on innovation and regulatory excellence. Collaboration with bioaccess® means engaging with a leader dedicated to overcoming the challenges inherent in clinical research.

In the early stages of medical research, when information gathering relied predominantly on manual, paper-based methods, it is important to understand what is a CRF, as the development of Case Report Forms (CRFs) is fundamentally linked to this process.
Initially, what is a CRF refers to rudimentary forms designed to capture basic patient information. As research trials became increasingly complex and regulatory scrutiny heightened, the demand for more reliable and standardized information collection methods became apparent.
The introduction of electronic Case Report Forms (eCRFs) in the late 1990s represented a pivotal advancement, facilitating real-time information entry, enhancing accuracy, and ensuring compliance with regulatory standards.
Today, CRFs are crafted with sophisticated functionalities that not only streamline information gathering but also improve administrative processes, reflecting the ongoing evolution of research methodologies in the medical domain.

Understanding what is a CRF is crucial for effective information collection, particularly in specialized medical device clinical trials. It should include several key components:
Furthermore, CRFs must be designed to minimize redundancy, avoid unnecessary information, and promote user-friendly navigation. Clear guidelines and uniform formats are essential to ensure consistent data collection across various locations and participants. By incorporating these elements, understanding what is a CRF can significantly enhance the quality and dependability of research data.

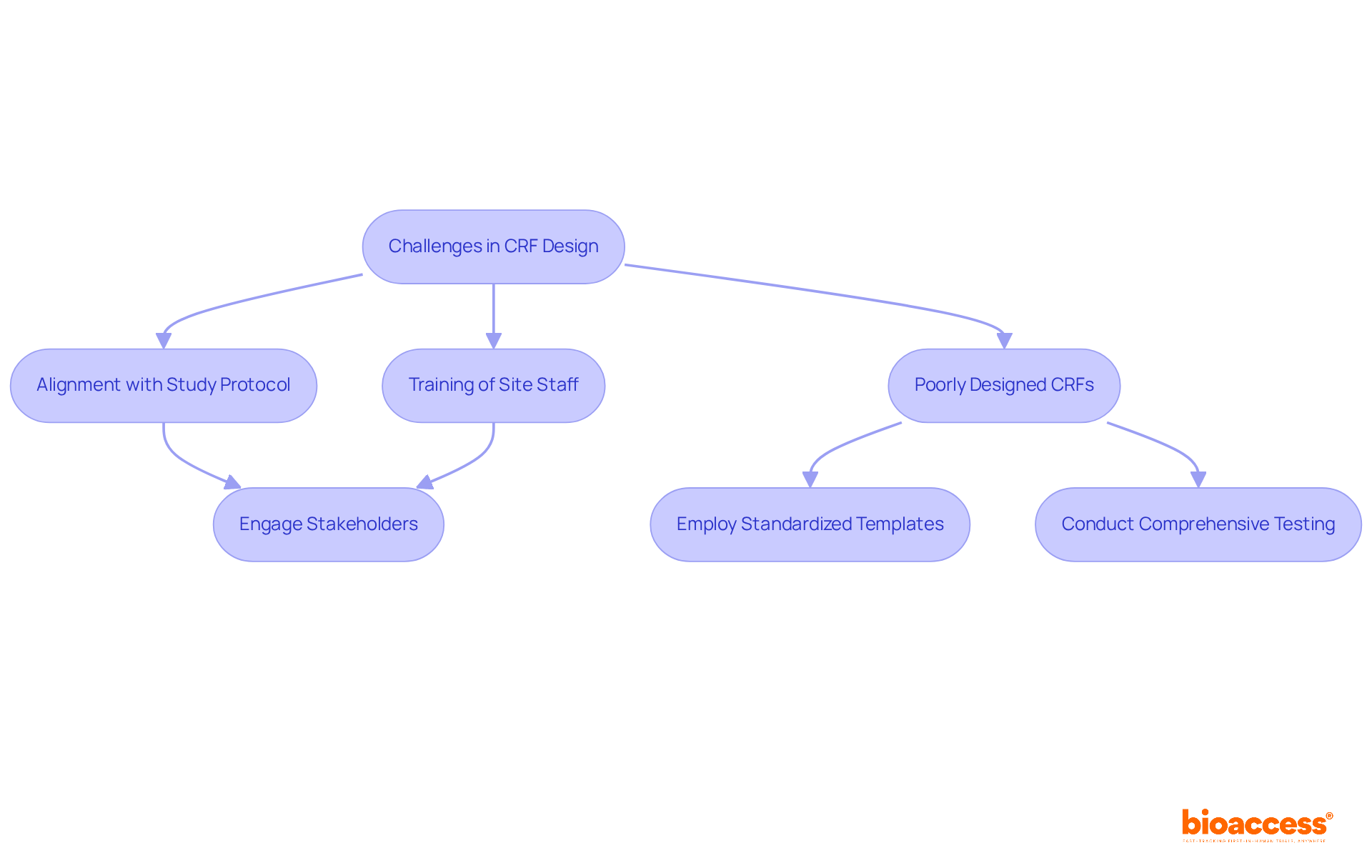
Creating and executing Case Report Forms (CRFs), which raises questions about what is a CRF, presents significant challenges that can greatly impact the success of clinical studies. A primary concern is ensuring that what is a CRF aligns with the study protocol while also being adaptable enough to accommodate necessary changes throughout the trial.
Furthermore, understanding what is a CRF is crucial, as poorly designed CRFs can lead to entry errors, missing information, and compliance issues, all of which pose risks to the study's integrity. Another critical challenge lies in the effective training of site staff to understand what is a CRF and complete them accurately; misunderstandings in this area can result in inconsistent data collection.
To effectively tackle these challenges, it is crucial to:
Understanding the role of a Case Report Form (CRF) is essential for anyone involved in clinical research. CRFs are pivotal documents that standardize the collection of vital participant data, ensuring that studies maintain accuracy and integrity. By grasping the significance of CRFs, researchers can enhance the reliability of their findings and contribute to the advancement of medical knowledge and patient care.
The evolution of CRFs has transitioned from basic paper forms to sophisticated electronic versions, streamlining data collection and improving compliance. Key components of an effective CRF include clarity and user-friendliness, which minimize errors and promote consistent data entry. Furthermore, the challenges faced in CRF design and implementation underscore the necessity for stakeholder engagement and thorough training to ensure successful outcomes.
As clinical research continues to evolve, the importance of well-designed CRFs cannot be overstated. Engaging with experts and utilizing best practices in CRF development will enhance the efficiency of clinical trials and safeguard the integrity of the data collected. Embracing these principles is crucial for advancing research efforts and ultimately improving patient care in the ever-changing landscape of medical science.
What is a Case Report Form (CRF)?
A Case Report Form (CRF) is a vital document in clinical research designed to systematically gather important information from each study participant. It captures protocol-required details such as patient demographics, medical history, and treatment outcomes.
Why is a CRF important in clinical research?
CRFs are essential because they ensure uniform and precise information collection, which is crucial for maintaining the integrity of study outcomes. They enhance the evaluation of safety and efficacy for new treatments, contributing to advancements in medical knowledge and patient care.
In what formats are CRFs available?
CRFs are available in both paper and electronic formats.
How do CRFs impact patient enrollment in clinical trials?
Utilizing CRFs can facilitate a remarkable 50% increase in patient enrollment speed.
What financial benefits do CRFs provide in clinical research?
CRFs can yield savings of $25,000 through FDA-ready information, ensuring that the data collection process is efficient and compliant with regulatory standards.
What types of studies does bioaccess® manage using CRFs?
bioaccess® manages a diverse array of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
What is the role of bioaccess® in Medtech research?
bioaccess® is positioned as a frontrunner in Medtech research across Latin America, focusing on innovation and regulatory excellence while overcoming challenges in clinical research.