


The primary objective of this article is to delineate essential definitions pertinent to clinical trials, which are indispensable for conducting effective research. It underscores the importance of comprehending these definitions—such as:
as they are crucial for safeguarding participant safety, ensuring study integrity, and achieving regulatory compliance. Mastery of these concepts is imperative for facilitating successful outcomes in medical research.
In the intricate realm of clinical research, grasping key terminology is paramount for successfully navigating the complexities of trial design and execution. This article explores ten fundamental clinical trial definitions that constitute the backbone of effective research, providing insights that can significantly enhance both participant safety and study integrity.
As the landscape of clinical trials continues to evolve, how can researchers guarantee that these definitions are not only comprehended but also applied in ways that promote inclusivity and uphold ethical standards?
By delving into these critical terms, we illuminate the path toward more effective and responsible clinical research.
bioaccess® stands as a leading contract research organization (CRO) specializing in early-phase studies across the Medtech, Biopharma, and Radiopharma sectors. By leveraging the regulatory agility found in Latin America, the diverse patient demographics of the Balkans, and the streamlined processes available in Australia, bioaccess® secures ethical approvals in an impressive timeframe of merely 4-6 weeks. This rapid approval process results in enrollment rates that are 50% faster than those observed in traditional markets. Such efficiency firmly establishes bioaccess® as an indispensable ally for innovators eager to accelerate the journey of medical breakthroughs from concept to market.
Informed consent represents a cornerstone of medical research, ensuring that participants are thoroughly educated about the study's purpose, procedures, risks, and benefits. It is essential for participants to grasp their rights, particularly the right to withdraw from the research at any moment without incurring penalties.
Research indicates that nine out of ten adults in the U.S. encounter challenges with health literacy, resulting in a significant number of participants opting out of research due to inadequate comprehension of the details. By cultivating a clear and transparent informed consent process, researchers not only safeguard participant rights but also bolster the integrity of the research, guaranteeing that participation is both voluntary and informed.
Key principles of informed consent encompass voluntariness, comprehension, and the disclosure of relevant information. Moreover, Institutional Review Boards (IRBs) play a pivotal role in supervising informed consent processes, ensuring that ethical standards are upheld.
Efficient informed consent methods, particularly in Medtech research, demonstrate that when participants are well-informed, they are more likely to engage positively with the research, ultimately leading to enhanced outcomes and confidence in the medical process. Furthermore, the evolving landscape of informed consent practices, such as the Dynamic Consent approach, signifies a steadfast commitment to improving participant engagement and understanding.
A clinical research protocol is an essential document that articulates the project's objectives, design, methodology, and statistical considerations. It provides a structured framework for researchers, detailing how the study will be executed, including criteria for participant selection, treatment regimens, and data collection methods. Adhering to the protocol is vital for preserving the integrity of the research, guaranteeing that results are both valid and reliable.
Notably, studies indicate that approximately 80% of medical studies face delays or cancellations due to recruitment issues, underscoring the necessity of a well-organized protocol to facilitate smooth operations. Furthermore, experts emphasize that a clear and comprehensive protocol is crucial for reproducibility in medical research, as it establishes a consistent framework for research execution.
Successful examples of research protocol designs demonstrate that meticulous planning can lead to enhanced participant engagement and improved outcomes, ultimately contributing to advancements in medical knowledge and patient care.
Bioaccess® offers a wide range of clinical study management services, including:
These services are critical for addressing recruitment challenges. Their expedited regulatory approval process allows studies to be set up and launched in just 6-8 weeks, significantly enhancing patient enrollment for cardiology and neurology groups compared to traditional timelines. This efficiency not only improves testing outcomes but also positively impacts local economies through job creation and advancements in healthcare.
Adverse events (AEs) signify any unwanted experiences occurring during a research study, irrespective of their association with the treatment under examination. The effective monitoring and prompt reporting of AEs are paramount for ensuring participant safety and upholding the ethical standards of clinical research. At bioaccess, we implement robust protocols for the identification, documentation, and reporting of AEs to regulatory bodies, which is crucial for maintaining participant welfare and ensuring regulatory compliance. Our comprehensive services also encompass feasibility assessments and site selection, thereby addressing all facets of trial management.
The significance of monitoring AEs cannot be overstated. Research has demonstrated that thorough adverse event reporting can substantially enhance participant safety by identifying potential risks associated with the medication. Serious adverse events, which may lead to death or hospitalization, necessitate expedited reporting to institutional review boards (IRBs) and regulatory agencies, as mandated by both local and international regulations.
Safety officers underscore the critical importance of transparency in reporting AEs. As highlighted by industry experts, addressing adverse events openly is essential for both patient safety and legal defense. This approach not only fosters trust but also aids in recognizing patterns and sources of risk, ultimately enhancing safety protocols in clinical research.
Moreover, the FDA has instituted specific safety reporting requirements for Investigational New Drugs (INDs) and Bioavailability/Bioequivalence (BA/BE) trials, underscoring the necessity for comprehensive documentation of all observed and spontaneously reported AEs. Each participant should be queried about AEs at every visit to ensure thorough reporting, which is vital for effective safety evaluations.
In summary, the act of monitoring and reporting adverse events is foundational to clinical trial definitions, as it directly influences participant safety and the overall integrity of the study.
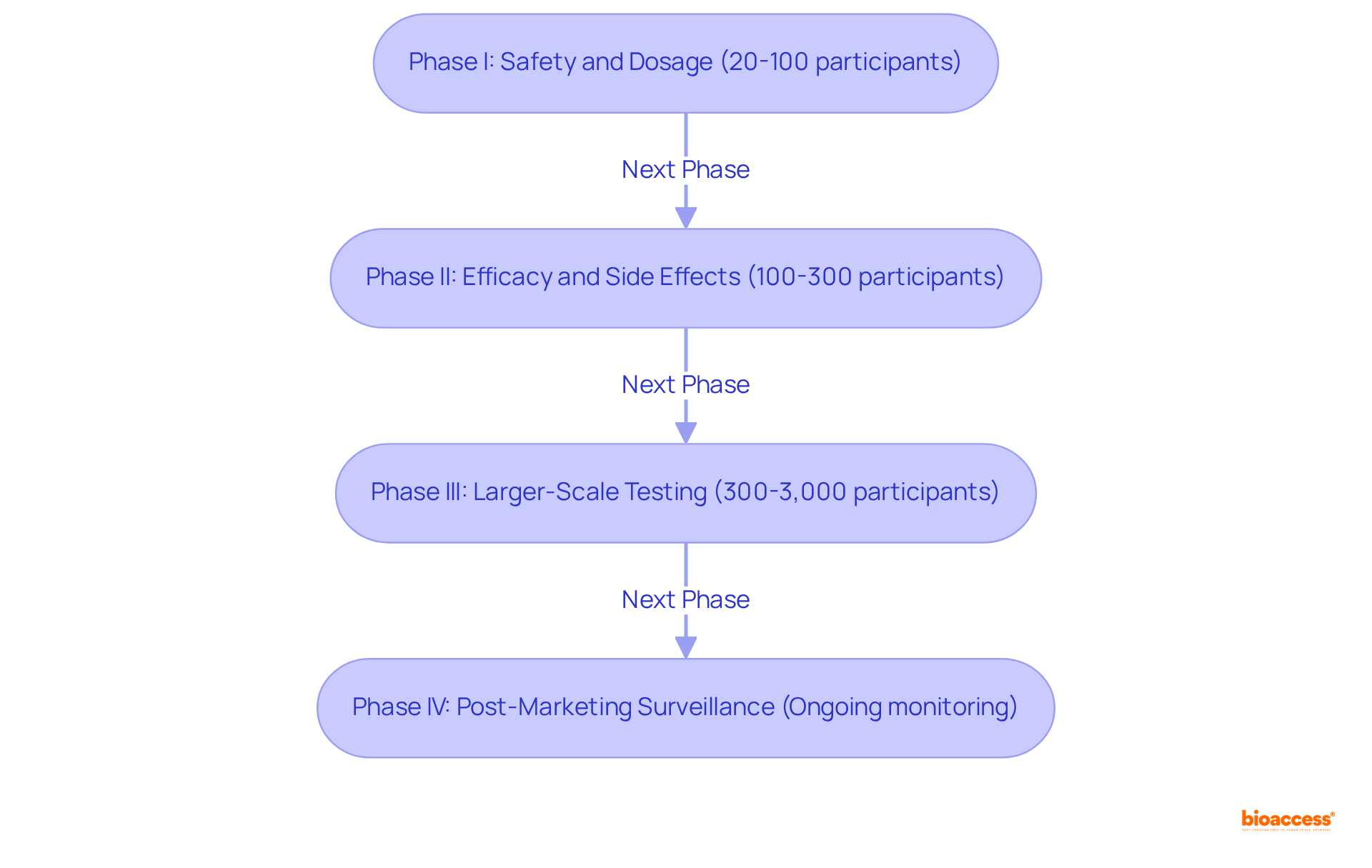
According to clinical trial definitions, clinical studies are systematically categorized into four distinct phases, each serving a critical role in the drug development process.
bioaccess® enhances this phase by leveraging its global network of fast-track clinical trial sites, significantly reducing the time required for approvals and patient recruitment, while ensuring compliance with country requirements.bioaccess®'s comprehensive clinical trial management services, including trial setup and compliance reviews, facilitate smoother transitions through this phase.bioaccess® in site selection and project management plays a crucial role in optimizing this phase, ensuring efficient execution and reporting.Each phase is carefully crafted to address specific research inquiries while prioritizing participant safety and collecting extensive data on the intervention's effectiveness based on clinical trial definitions. The structured progression through these phases is critical for ensuring that only the most promising therapies reach the market, ultimately enhancing patient care. As highlighted by research specialists, ongoing safety surveillance in Phase IV is crucial for detecting uncommon side effects and ensuring the intervention's sustained effectiveness in varied populations.
The eligibility criteria outlined in clinical trial definitions represent the specific requirements that individuals must fulfill to participate in a clinical study, including factors such as age, gender, health status, and prior treatment history. Well-defined eligibility standards are crucial for ensuring that the study group adheres to the clinical trial definitions, thereby enhancing the reliability of results and safeguarding participant well-being.
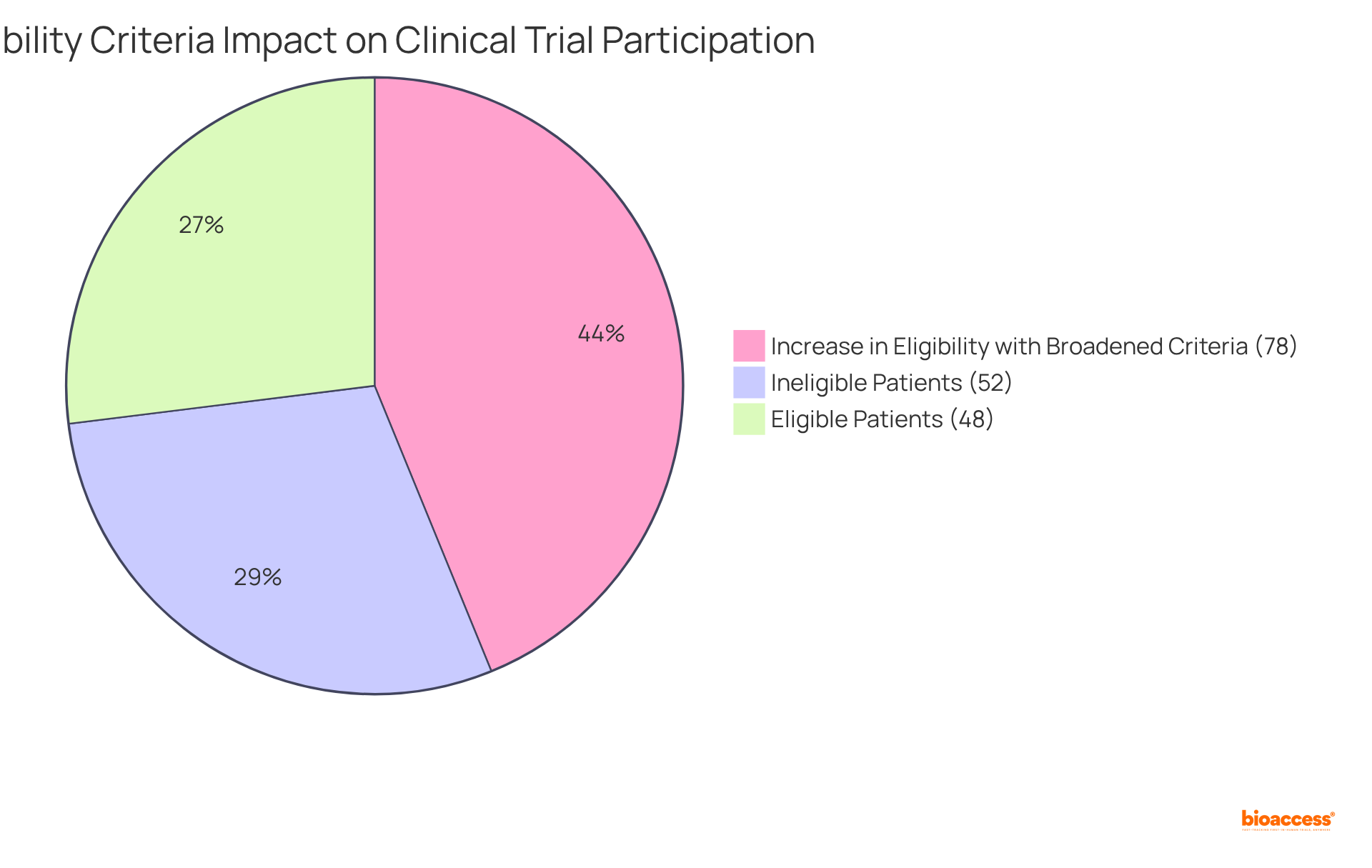
The significance of these criteria is underscored by recent findings revealing that under stringent eligibility conditions, only 48% of identified cancer patients qualified for studies, with even higher exclusion rates among women, the elderly, and individuals from lower socioeconomic backgrounds. This scenario highlights an urgent need for more inclusive approaches that can foster diversity in clinical research.
Clinical researchers assert that excessively strict eligibility criteria defined in clinical trial definitions can hinder patient participation and undermine the generalizability of results. For example, broadening eligibility criteria has demonstrated a 78% increase in the number of eligible patients, particularly benefiting historically underrepresented groups such as women and the elderly. This adjustment not only enhances study inclusivity but also improves the external validity of the findings, making them more applicable to the wider patient population.
In the realm of Medtech research overseen by bioaccess in Latin America, establishing eligibility criteria is vital for ensuring that innovative medical devices are tested on a representative sample of the population. Comprehensive trial management services, including feasibility analyses and site selection, are instrumental in this process. For instance, studies focusing on devices for chronic conditions must account for a diverse range of patient profiles to accurately evaluate efficacy and safety.
Ultimately, the importance of eligibility criteria in clinical trial definitions in medical research cannot be overstated. They serve as a foundational component that influences legal outcomes, participant safety, and the overall success of medical innovations. As the landscape of healthcare evolves, so too must the standards that govern clinical studies, ensuring they reflect the realities of patient demographics and medical advancements.
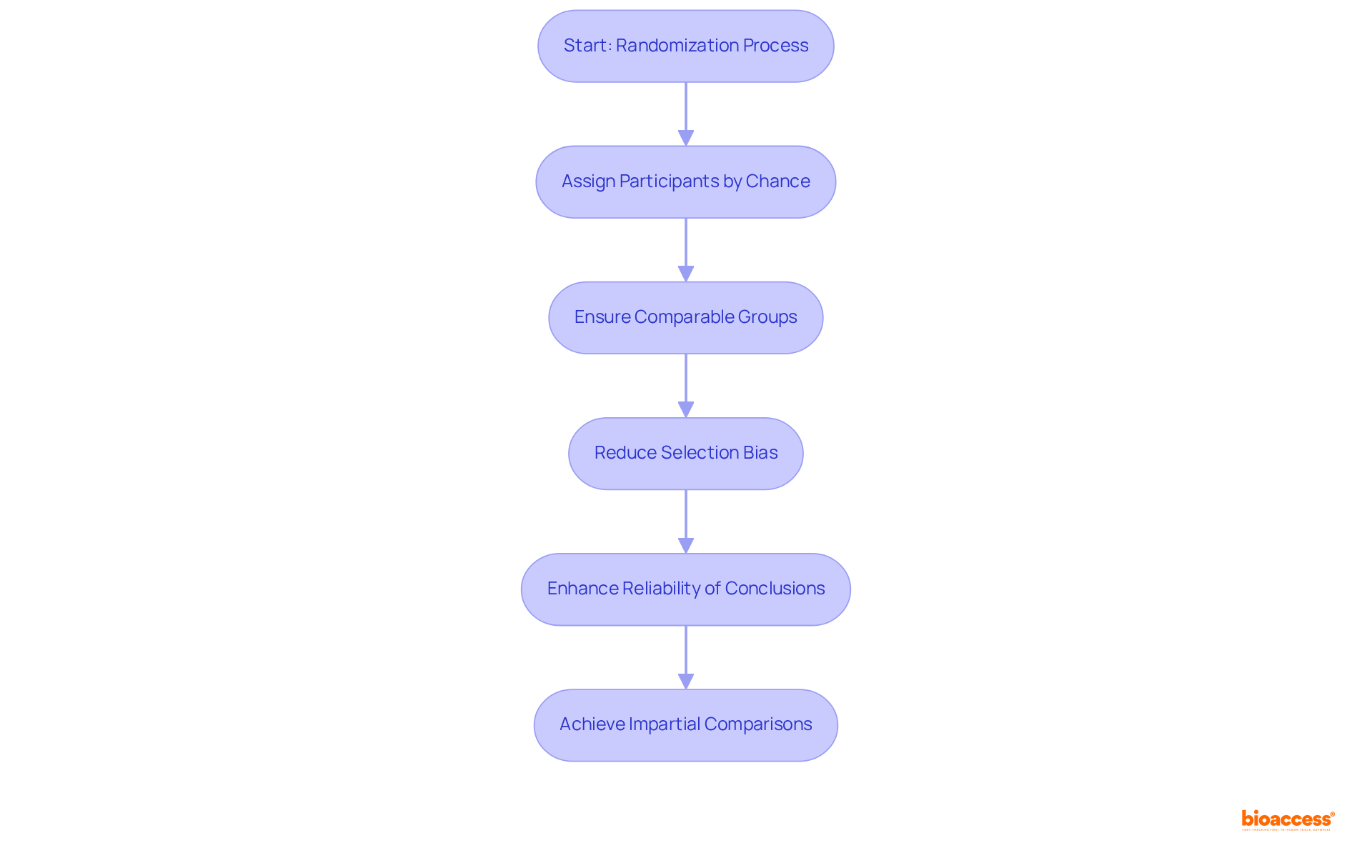
Randomization serves as a pivotal process in assigning participants to different intervention groups by chance rather than by choice. This method is essential for establishing that the groups are comparable at the outset of the study, thereby reducing selection bias and enhancing the reliability of conclusions regarding the treatment's effectiveness. Indeed, randomized controlled trials (RCTs) are regarded as the gold standard in medical research, providing robust evidence for cause-effect relationships between interventions and outcomes.
The significance of randomization cannot be overstated; it stands as a cornerstone of rigorous clinical study design that substantially bolsters the credibility of results. For instance, research reveals that the likelihood of significant imbalance in complete randomization with only 10 subjects is 0.0216, underscoring the risk of bias in smaller studies. Conversely, employing stratified randomization can effectively mitigate this risk by grouping participants based on key characteristics, thus ensuring a balanced distribution across intervention groups.
Biostatisticians stress the necessity of minimizing bias through randomization. As one expert articulated, "Adjustment for baseline variables should generally be considered when stratified randomization is used or when there is a known or anticipated strong association between baseline characteristics and the primary outcome." This statement emphasizes the importance of meticulous preparation in study design to maintain integrity and validity.
Moreover, data indicates that non-randomized trials are vulnerable to selection bias, which can lead to systematic differences between intervention groups. Such bias can distort observed intervention effects, making randomization a critical strategy for achieving impartial comparisons. By utilizing robust randomization methods, researchers can ensure equitable distribution of patients to study groups, thereby enhancing the overall quality and reliability of medical research.
A placebo is defined as an inactive substance or intervention devoid of therapeutic effects, primarily serving as a control in clinical studies. By juxtaposing the outcomes of an active intervention against those of a placebo, researchers can ascertain whether the intervention produces genuine effects that extend beyond mere participant expectations. Placebo-controlled trials are widely regarded as the gold standard for evaluating the efficacy of new treatments, as they effectively eliminate bias and provide a clearer understanding of a treatment's true impact.
Experts underscore the significance of placebo controls in medical research. For instance, a meta-analysis revealed that approximately 40% of prescriptions may function as placebos, highlighting their pervasive role in clinical practice. Furthermore, research indicates that the placebo effect can yield notable therapeutic responses, particularly in subjective issues such as pain and anxiety, where it can offer relief comparable to active interventions. Benedetti noted that the placebo effect in pain ranges from 39% to 56%, emphasizing its relevance in pain management.
The mechanics of placebo controls involve establishing a comparison group that receives the placebo while another group receives the active intervention. This design empowers researchers to assess the intervention's effectiveness with precision. Importantly, the placebo effect is not merely a consequence of optimistic thinking; it encompasses intricate interactions between the mind and body, influenced by factors such as patient expectations and the care setting.
In research trials, the utilization of placebos enhances scientific rigor, enabling researchers to draw more reliable conclusions about treatment efficacy. The ethical implications of employing placebos, particularly in vulnerable populations, remain a topic of debate. For example, 77% of surveyed physicians reported prescribing a placebo at least once a week, illustrating the prevalence of placebo use in medical practice. Additionally, the nocebo effect—characterized by negative reactions following placebo administration—serves as an important consideration in comprehending the full context of placebo use. As the landscape of medical research evolves, grasping the dynamics of placebo-controlled studies will be essential for developing effective therapeutic interventions.
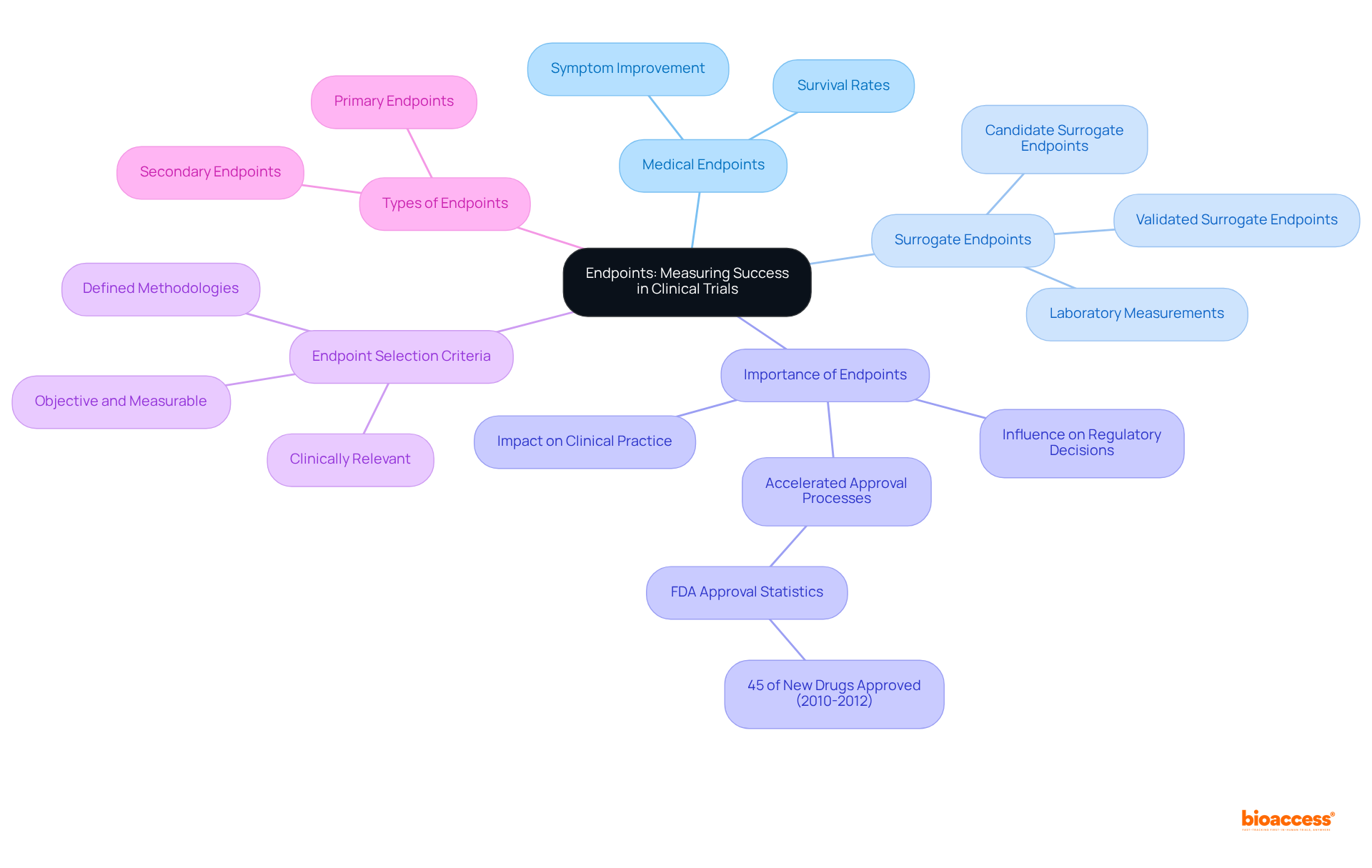
Endpoints are predefined outcomes that act as critical indicators of a research trial's success. They can be categorized into:
The selection of appropriate endpoints is vital according to clinical trial definitions, as they not only evaluate the effectiveness and safety of the intervention but also influence regulatory decisions and clinical practice. For example, between 2010 and 2012, the FDA approved 45% of new drugs based on surrogate endpoints, underscoring their significance in accelerating the approval process.
Successful measurement of endpoints has been evidenced in various Medtech studies, particularly in Latin America, where bioaccess® excels in:
For instance, a cancer medication study might utilize the rate of tumor reduction as a primary endpoint, providing a clear and quantifiable outcome that reflects the treatment's effectiveness. Clinical researchers emphasize that well-defined endpoints, as outlined in clinical trial definitions, are essential for study success, as they must accurately represent the intervention's effects and be meaningful to patients.
Furthermore, the correlation between endpoints and regulatory approval rates is noteworthy. Validated surrogate endpoints, supported by robust evidence of their predictive ability for specific health benefits, are crucial in the context of clinical trial definitions to expedite approval processes. This relevance is particularly pronounced in discussions surrounding Type C meetings, where sponsors engage with the FDA to evaluate the feasibility of employing novel surrogate endpoints as primary efficacy measures.
In conclusion, the importance of endpoints in evaluating success cannot be overstated. They must be objective, measurable, and relevant to the research objectives, ensuring that clinical investigations produce meaningful data that ultimately benefits patients. Bioaccess® achieves 50% quicker patient enrollment and $25K savings with FDA-ready data, demonstrating how optimized endpoint strategies can enhance study efficiency.
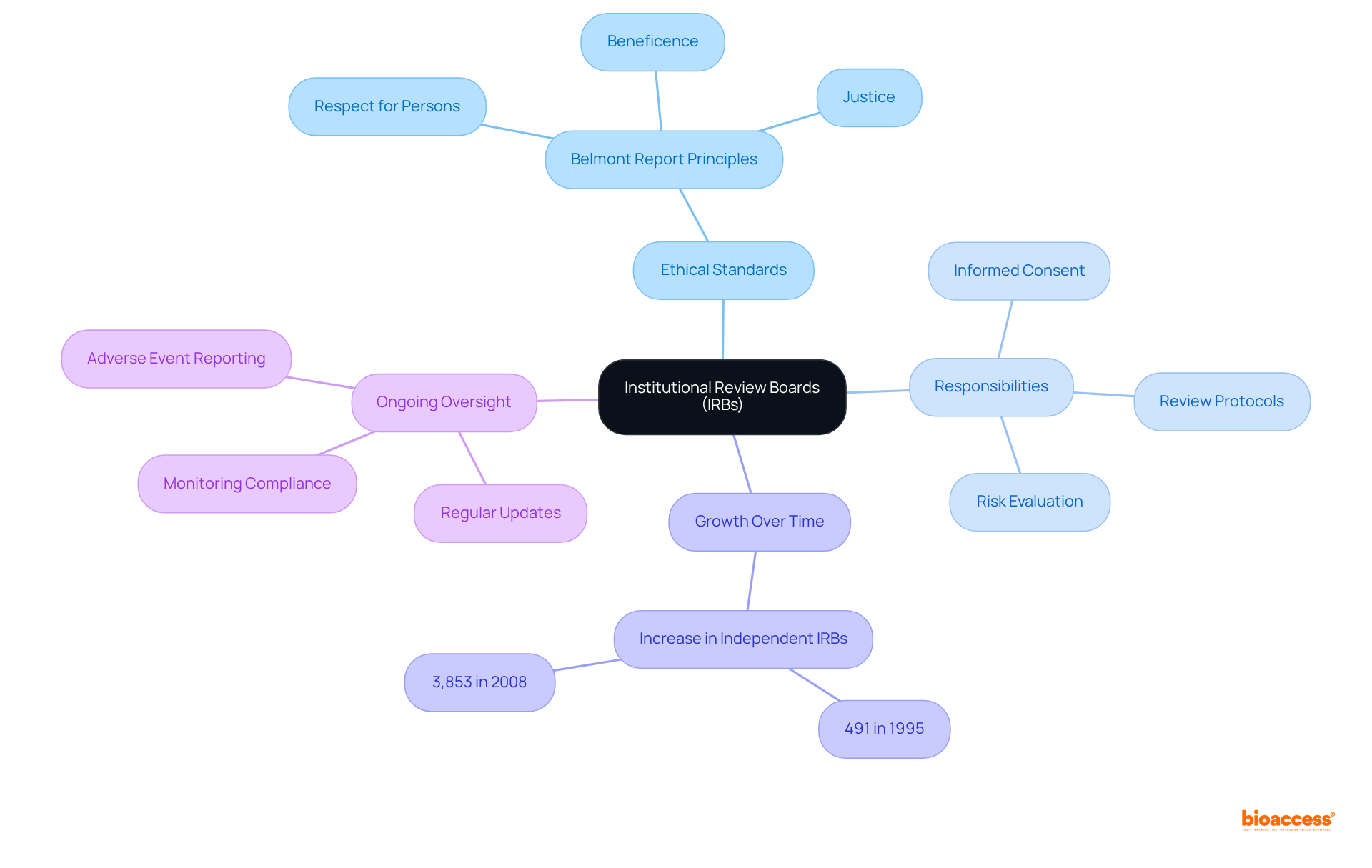
Institutional Review Boards (IRBs) are essential committees responsible for the review and approval of research involving human subjects, ensuring that ethical standards are rigorously upheld. They meticulously evaluate research designs, informed consent procedures, and potential risks to participants, striking a balance between the advantages of investigation and any possible harm. The significant increase in independent IRBs—from 491 in 1995 to 3,853 in 2008—highlights their critical role in safeguarding participant rights and promoting ethical conduct as outlined in clinical trial definitions.
IRBs are dedicated to protecting human subjects by adhering to the principles outlined in the Belmont Report, which emphasizes respect for persons, beneficence, and justice. They conduct comprehensive reviews of research protocols to ensure that risks are minimized and that potential benefits outweigh any risks. This oversight is particularly vital in Medtech research, where innovative technologies are tested for the first time on human participants.
Furthermore, IRBs require regular updates on research status, participant enrollment, and safety data, ensuring ongoing adherence to ethical standards. Their authority extends to imposing conditions on research activities, including specific informed consent procedures and data protection measures. As guardians of research ethics, IRBs play a crucial role in maintaining public trust in scientific inquiry, facilitating valuable advancements while ensuring that studies are conducted responsibly and with respect for human dignity.
Understanding the essential definitions and concepts surrounding clinical trials is crucial for effective research and innovation in the medical field. By grasping key terms such as informed consent, clinical trial protocols, and the role of Institutional Review Boards (IRBs), stakeholders can navigate the complexities of clinical research with greater confidence and integrity. These foundational elements not only enhance the quality of research but also ensure that participant rights and safety remain a top priority throughout the trial process.
The article has highlighted several critical aspects of clinical trials, including:
Additionally, the role of randomization and the use of placebos are underscored as vital strategies to ensure objectivity and credibility in research findings. Each of these components plays a significant role in the overall success and ethical conduct of clinical studies.
In light of these insights, it is imperative for researchers, sponsors, and regulatory bodies to commit to continuous improvement in clinical trial practices. Emphasizing inclusivity in eligibility criteria and maintaining transparent communication about participant safety can foster trust and enhance the quality of research outcomes. As the field of clinical research evolves, staying informed about these essential definitions and adopting best practices will not only advance medical knowledge but ultimately lead to better patient care and outcomes.
What does bioaccess® specialize in?
bioaccess® is a leading contract research organization (CRO) that specializes in early-phase studies across the Medtech, Biopharma, and Radiopharma sectors.
How does bioaccess® expedite the clinical research process?
bioaccess® leverages regulatory agility in Latin America, diverse patient demographics in the Balkans, and streamlined processes in Australia to secure ethical approvals in 4-6 weeks, resulting in enrollment rates that are 50% faster than traditional markets.
What is informed consent in medical research?
Informed consent is a fundamental process that ensures participants are fully educated about a study's purpose, procedures, risks, and benefits, and understand their rights, including the right to withdraw from the study at any time without penalties.
Why is health literacy important in the context of informed consent?
Health literacy is crucial because research shows that nine out of ten adults in the U.S. face challenges in understanding health information, which can lead to participants opting out of research due to inadequate comprehension of study details.
What are the key principles of informed consent?
The key principles of informed consent include voluntariness, comprehension, and the disclosure of relevant information.
What role do Institutional Review Boards (IRBs) play in informed consent?
IRBs supervise informed consent processes to ensure that ethical standards are maintained throughout medical research.
What is a clinical research protocol?
A clinical research protocol is a detailed document that outlines a study's objectives, design, methodology, and statistical considerations, serving as a structured framework for how the research will be executed.
Why is adherence to the clinical trial protocol important?
Adhering to the protocol is vital for preserving the integrity of the research, ensuring that results are valid and reliable.
What challenges do medical studies face regarding recruitment?
Approximately 80% of medical studies experience delays or cancellations due to recruitment issues, highlighting the need for a well-organized protocol.
What services does bioaccess® offer for clinical study management?
bioaccess® offers services such as feasibility assessments, site selection, compliance reviews, and project management to address recruitment challenges and enhance study execution.
How quickly can bioaccess® set up and launch studies?
bioaccess® can set up and launch studies in just 6-8 weeks, significantly improving patient enrollment for cardiology and neurology groups compared to traditional timelines.