


The article delineates the systematic process for conducting feasibility studies in accordance with Colombian regulations, underscoring the critical role of thorough assessments in determining the viability of research initiatives. It identifies key components such as:
This demonstrates that meticulous planning and adherence to local guidelines are vital for achieving successful outcomes in clinical research within Colombia.
In the realm of clinical research, feasibility studies serve as a crucial foundation for success, particularly in the dynamic landscape of Colombia. These systematic evaluations assess the practicality and viability of proposed initiatives, empowering stakeholders to make informed decisions that can significantly mitigate risks and optimize resource allocation.
With a growing medical device market and a regulatory framework designed to facilitate research, Colombia presents unique opportunities for innovative companies. This article delves into the essential components, challenges, and best practices associated with conducting feasibility studies in this region. It highlights how local expertise and stakeholder engagement can dramatically enhance the likelihood of successful clinical trials.
Feasibility analyses serve as crucial systematic assessments that evaluate the practicality and viability of proposed research initiatives. In Colombia, feasibility studies conducted under local regulations play a pivotal role in examining various factors, including market demand, regulatory requirements, and operational capabilities. Their significance is paramount; they empower stakeholders to make informed decisions, mitigate risks, and allocate resources efficiently.
By identifying potential obstacles early in the process, researchers can refine their strategies, ultimately enhancing the likelihood of favorable outcomes in trials.
At bioaccess®, we specialize in comprehensive trial management services, which include studies that assess the availability of suitable patient populations, navigate the regulatory landscape, and address logistical considerations for conducting trials in Colombia. Our services encompass trial setup, obtaining import permits, and project management, ensuring that all critical factors are thoroughly evaluated before progressing to subsequent research stages.
Statistics indicate that involving an average of 8-12 potential researchers during site evaluations can significantly enhance the quality of medical trials. Furthermore, preliminary investigations, a vital component of viability assessments, aid in minimizing risks to validity and guide the planning of adequately powered randomized controlled trials (RCTs).
Expert insights underscore the importance of viability assessments in medical research. Carlson PE, a full-time employee at Pfizer Ltd, India, states, "In a genuine sense, practicality is an investment to guarantee good research." Recognizing practicality as an investment in research quality allows investigators to focus on delivering high-quality trials that meet both scientific and operational standards.
This dual perspective—viewing viability as both an art and a science—ensures that trials are not only scientifically rigorous but also operationally feasible, ultimately leading to higher success rates in trials.
With over 20 years of experience in Medtech, bioaccess® connects innovative companies by providing tailored support in conducting feasibility studies under Colombian regulations. Our expertise in navigating the local regulatory environment, including adherence to INVIMA guidelines, and understanding market dynamics uniquely positions us to assist clients in making informed decisions that enhance the success of their medical studies, contributing to job creation, economic development, and healthcare improvement in the region.
In Colombia, the framework regulating viability assessments for clinical investigations is primarily established by the National Institute for Food and Drug Surveillance (INVIMA) alongside other regulatory agencies. These regulations delineate the essential steps for conducting feasibility studies under Colombian regulations, which encompass the submission of comprehensive protocols, ethical considerations, and strict adherence to local laws. A critical aspect of this process is obtaining ethical approval from Institutional Review Boards (IRBs), which typically takes between four to six weeks for review, underscoring the efficiency of the regulatory process in Colombia.
Additionally, compliance with Good Clinical Practice (GCP) guidelines is mandatory to ensure the integrity and safety of the research.
INVIMA classifies medical devices into various risk categories, significantly influencing their eligibility for first-in-human clinical trials. This classification system is vital for researchers to comprehend, as it directly impacts the regulatory pathway and requirements for conducting feasibility studies under Colombian regulations. For instance, devices classified as higher risk may face more stringent scrutiny and require more extensive documentation.
Furthermore, successful adherence to INVIMA regulations has been demonstrated in various case analyses, particularly those focusing on risk management plans for trials. Sponsors must proactively create these plans to address potential negative occurrences before initiating human trials, thereby enhancing participant safety during first-in-human experiments. As Julio G. Martinez-Clark, CEO of bioaccess, observes, "Colombia’s mix of a large and varied population, experienced research sites, efficient regulatory processes, a cost-effective environment, and a history of successful medtech trials since 2010 render it an appealing option for U.S. medical device firms seeking to carry out research."
In this context, bioaccess™ plays a crucial role in facilitating these processes, as exemplified by its partnership with Avantec Vascular for their first-in-human trial of an innovative vascular device in Latin America. This collaboration highlights bioaccess's extensive management services for trials, which encompass viability assessments, site selection, compliance evaluations, trial preparation, import permits, project oversight, and reporting.
Colombia's regulatory framework, characterized by its efficient processes and a large, diverse population, positions it as an attractive location for conducting research trials. The combination of these elements, along with a history of successful clinical trials since 2010, emphasizes the significance of understanding and adhering to feasibility studies under Colombian regulations for researchers intending to carry out assessments in this region.
Conducting a feasibility study is a systematic process that encompasses several critical steps to ensure its effectiveness and alignment with regulatory standards, particularly in the context of Colombian regulations and the oversight of INVIMA, the National Food and Drug Surveillance Institute.
By following these steps, researchers can ensure that their assessments are thorough, well-structured, and aligned with both regulatory standards and the strategic objectives of their organizations. Optimal methods, including ongoing stakeholder involvement and repetitive feedback cycles, can further improve the quality and influence of the research. As mentioned by Divya Sampark, "I’d have appreciated more comments on projects, but overall, it was a wonderful experience," highlighting the significance of feedback in the evaluation process.
Furthermore, bioaccess provides thorough trial management services, encompassing trial setup, project oversight, and reporting, which are crucial for managing the intricacies of executing assessments in accordance with INVIMA regulations. The effect of Medtech clinical research on local economies, including job creation and healthcare enhancement, further emphasizes the significance of these investigations in promoting international cooperation and economic development.

A comprehensive feasibility study in Colombia must encompass several essential components to ensure thorough evaluation and compliance with local regulations:
Integrating these elements into feasibility studies under Colombian regulations not only ensures compliance but also enhances the likelihood of successful market entry and product acceptance. By leveraging local resources and understanding the demographics of target populations, researchers can conduct effective market analyses that inform their strategies and decision-making processes. As Alvaro Enrique Rincon Mautner from SPI Americas notes, comprehending the local regulatory landscape is crucial for success in the Colombian market.

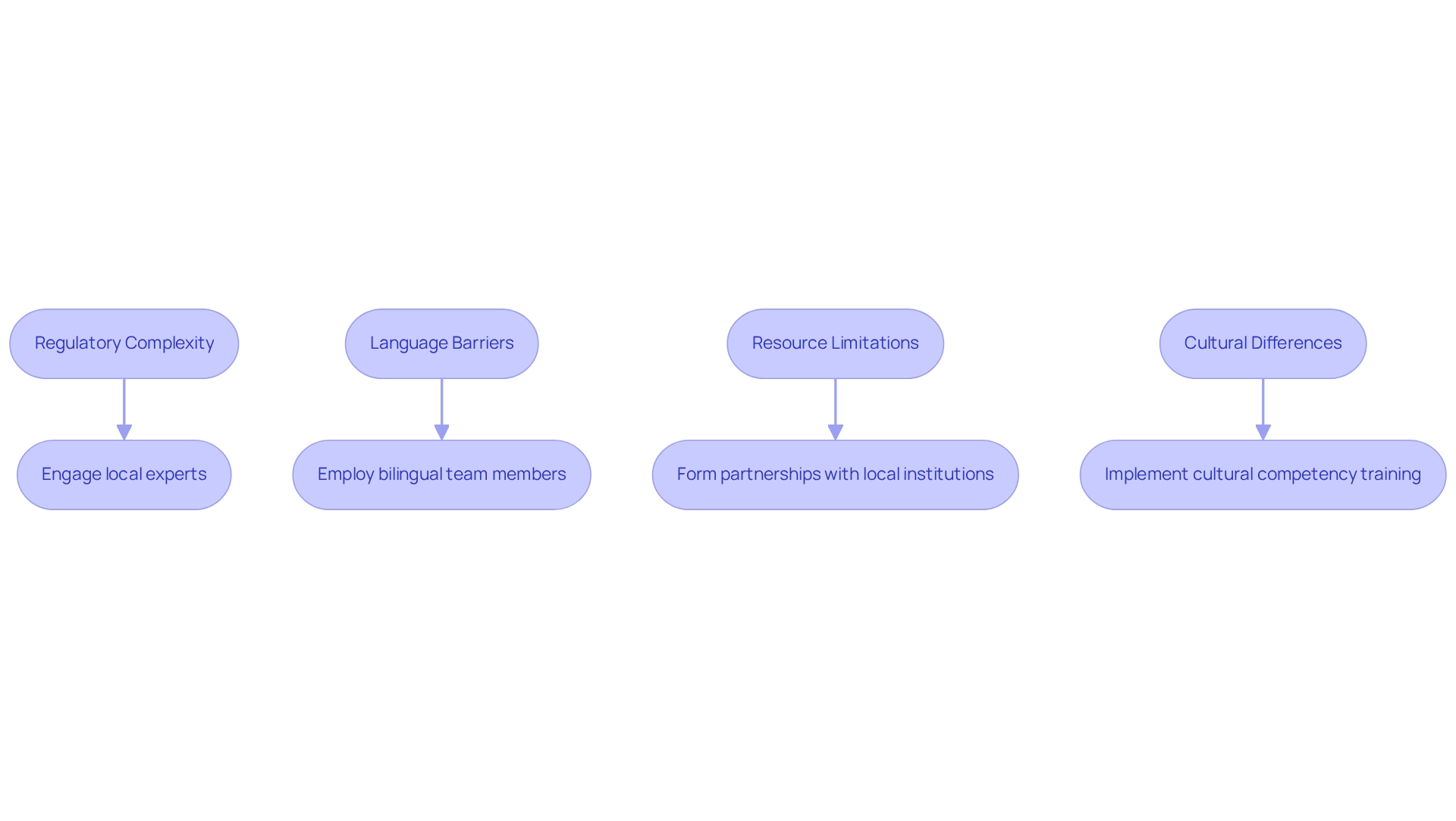
Conducting feasibility studies under Colombian regulations presents various obstacles that can significantly impact the success of medical investigation initiatives. Key challenges include:
Regulatory Complexity: The intricate regulatory landscape in Colombia can overwhelm researchers. To effectively navigate this complexity, engaging local experts or consultants with a deep understanding of feasibility studies under Colombian regulations is advisable. Their expertise in site selection, compliance reviews, trial setup, import permits, project management, and reporting can streamline the approval process, ensuring adherence to all necessary guidelines. As Ville-Veikko Pulkka observed, government dedication is essential in promoting medical studies, underscoring the significance of comprehending the regulatory landscape.
Language Barriers: Communication with stakeholders can be significantly hindered by language differences. To mitigate this issue, employing bilingual team members or utilizing professional translation services is essential. This approach ensures that all parties are aligned, facilitating smoother interactions and a clearer understanding of project requirements.
Resource Limitations: Access to funding and skilled personnel can be restricted, presenting a significant obstacle to advancing feasibility assessments. The GINI index, which has shown a gradual rise in inequality, underscores the socioeconomic factors that may influence feasibility studies under Colombian regulations. To address this, researchers should consider forming partnerships with local institutions or organizations that can provide additional resources and support. Such collaborations can enhance the ability to conduct thorough and effective research, leveraging the comprehensive services offered by bioaccess.
Cultural Differences: Understanding and respecting local customs and practices is crucial for successful stakeholder engagement. To cultivate improved connections with local stakeholders, implementing cultural competency training for the project team is suggested. This training prepares team members with the understanding and abilities required to navigate cultural subtleties, ultimately resulting in more efficient collaboration.
Furthermore, a case analysis on acceptability and effectiveness outcomes highlights the significance of addressing mental health results in healthcare examination. Participants were evaluated before and after the intervention, demonstrating notable advancements in mental health results such as anxiety and depression. This aligns with the necessity for comprehending local contexts in practical assessments. By proactively tackling these challenges and incorporating insights from outside sources, including the knowledge of experts like Katherine Ruiz and Juan Cuya, MD, researchers can enhance the practicality and effectiveness of their efforts in Colombia, paving the way for successful clinical research outcomes.
Involving stakeholders is crucial for the success of feasibility assessments, particularly in the evolving environment of Latin American Medtech. Key strategies for effective engagement include:
Effective stakeholder engagement strategies have been shown to greatly enhance feasibility studies under Colombian regulations and project quality. A recent research titled 'Impact of Partner Engagement on Study Feasibility and Quality' highlighted that 38 partners specified two or more types of impact within their projects, demonstrating the value of collaborative input in enhancing trial design and implementation. As Professor Philip Kotler noted, the contributions of experts in the field are invaluable in shaping effective strategies.
By prioritizing effective communication and stakeholder involvement, investigators can navigate the complexities of medical studies more successfully. At bioaccess®, our knowledge and tailored method in fostering stakeholder involvement are essential in progressing medical devices through efficient clinical methodologies, especially with regard to feasibility studies under Colombian regulations for early viability assessments.
To ensure the success of feasibility studies in Colombia, researchers must adhere to best practices that are foundational to effective clinical research.
By adhering to these best practices, researchers can improve the chances of successful assessments, including feasibility studies under Colombian regulations, ultimately aiding the progress of medical technologies in the region. For instance, consider a consumer goods company conducting a feasibility assessment for a new product line, evaluating market interest, technological requirements, financial forecasts, organizational preparedness, and legal conformity, ultimately opting to move forward with the project. Additionally, monitoring participant adherence and involvement in intervention components is crucial for assessing program effectiveness, ensuring that the research meets its objectives.
At bioaccess, we are committed to facilitating these processes through our comprehensive clinical trial management services.
Post-examination assessments are pivotal in comprehending the effects of viability analyses, such as ReGelTec's Early Viability Analysis on HYDRAFIL™ for treating chronic low back pain in Colombia. Researchers must undertake the following steps to ensure thorough evaluation and improvement:
Conducting feasibility studies in Colombia represents a multifaceted process that demands meticulous attention to various components, ranging from regulatory compliance to stakeholder engagement. This article has underscored the essential steps involved, including:
All critical for ensuring the study's success. By emphasizing local expertise and comprehending market dynamics, the likelihood of favorable outcomes in clinical trials is further enhanced.
Beyond the practical steps, the significance of perceiving feasibility studies as an investment in quality cannot be overstated. By acknowledging their role in risk mitigation and resource optimization, researchers can adeptly navigate the complexities inherent in the Colombian clinical research landscape. The regulatory framework established by INVIMA, in conjunction with the burgeoning medical device market, positions Colombia as an appealing destination for innovative companies intent on conducting clinical research.
Ultimately, the insights derived from feasibility studies not only bolster the chances of successful trials but also yield broader benefits, including job creation and advancements in healthcare within the region. As the clinical research landscape continues to evolve, embracing best practices and nurturing collaborative relationships will be pivotal in unlocking the full potential of medical innovation in Colombia. By prioritizing these elements, stakeholders can ensure that their initiatives are not only viable but also impactful in propelling progress within the healthcare sector.
What is the purpose of feasibility analyses in research?
Feasibility analyses evaluate the practicality and viability of proposed research initiatives, helping stakeholders make informed decisions, mitigate risks, and allocate resources efficiently.
Why are feasibility studies important in Colombia?
In Colombia, feasibility studies are crucial for examining market demand, regulatory requirements, and operational capabilities, which ultimately enhance the likelihood of favorable outcomes in trials.
What services does bioaccess® provide in relation to trial management?
Bioaccess® offers comprehensive trial management services, including feasibility studies, trial setup, obtaining import permits, and project management, ensuring thorough evaluation of critical factors before progressing to subsequent research stages.
How does involving multiple researchers during site evaluations impact medical trials?
Involving an average of 8-12 potential researchers during site evaluations can significantly enhance the quality of medical trials.
What role do preliminary investigations play in viability assessments?
Preliminary investigations help minimize risks to validity and guide the planning of adequately powered randomized controlled trials (RCTs).
What is the significance of practicality in research, according to expert insights?
Practicality is viewed as an investment in research quality, allowing investigators to focus on delivering high-quality trials that meet both scientific and operational standards.
How does Colombia's regulatory framework support feasibility assessments?
The framework, primarily established by INVIMA and other regulatory agencies, outlines essential steps for conducting feasibility studies, including ethical approvals and compliance with Good Clinical Practice (GCP) guidelines.
What is the typical duration for obtaining ethical approval from Institutional Review Boards (IRBs) in Colombia?
The review process for obtaining ethical approval typically takes between four to six weeks.
How does INVIMA classify medical devices, and why is this important?
INVIMA classifies medical devices into various risk categories, which significantly influences their eligibility for first-in-human clinical trials, impacting the regulatory pathway and requirements for conducting feasibility studies.
What is the significance of risk management plans in clinical trials?
Sponsors must proactively create risk management plans to address potential negative occurrences before initiating human trials, which enhances participant safety during first-in-human experiments.
Why is Colombia considered an attractive location for conducting research trials?
Colombia offers a large and varied population, experienced research sites, efficient regulatory processes, a cost-effective environment, and a history of successful medtech trials, making it appealing for U.S. medical device firms.
What specific role does bioaccess® play in facilitating clinical trials in Colombia?
Bioaccess® facilitates the trial process by providing management services that include viability assessments, site selection, compliance evaluations, trial preparation, import permits, project oversight, and reporting.