


The article defines clinical studies as systematic examinations, predominantly clinical trials, designed to evaluate the safety and effectiveness of medical interventions. These studies are essential for advancing medical knowledge and enhancing patient outcomes. They play a pivotal role in regulatory approval processes and evidence-based medicine. The article highlights the evolution, types, and key characteristics of clinical studies, including rigorous methodologies and ethical considerations, which are fundamental to ensuring the integrity and validity of the research.
Clinical studies play a pivotal role in shaping the future of healthcare, serving as the backbone of evidence-based medicine. By systematically evaluating the safety and efficacy of new medical interventions, these studies not only enhance patient outcomes but also ensure that clinical practices are grounded in scientific rigor.
As the landscape of medical research evolves, questions arise about the methodologies employed and the ethical considerations involved.
What are the key types of clinical studies, and how do they contribute to the advancement of medical knowledge? This inquiry is essential for understanding the ongoing developments in the Medtech landscape and the role of bioaccess in addressing key challenges.
The clinical studies definition encompasses clinical trials, which serve as systematic examinations designed to evaluate the safety and effectiveness of medical interventions, including medications, devices, and therapeutic protocols. They are pivotal in advancing medical knowledge by providing evidence-based information that informs clinical practice. The primary objective of these medical trials is to determine whether new therapies are safe and effective for human application, ultimately leading to improved patient outcomes. Such investigations are essential for regulatory approval and constitute a cornerstone of evidence-based medicine, ensuring that healthcare practices are firmly grounded in scientific research.
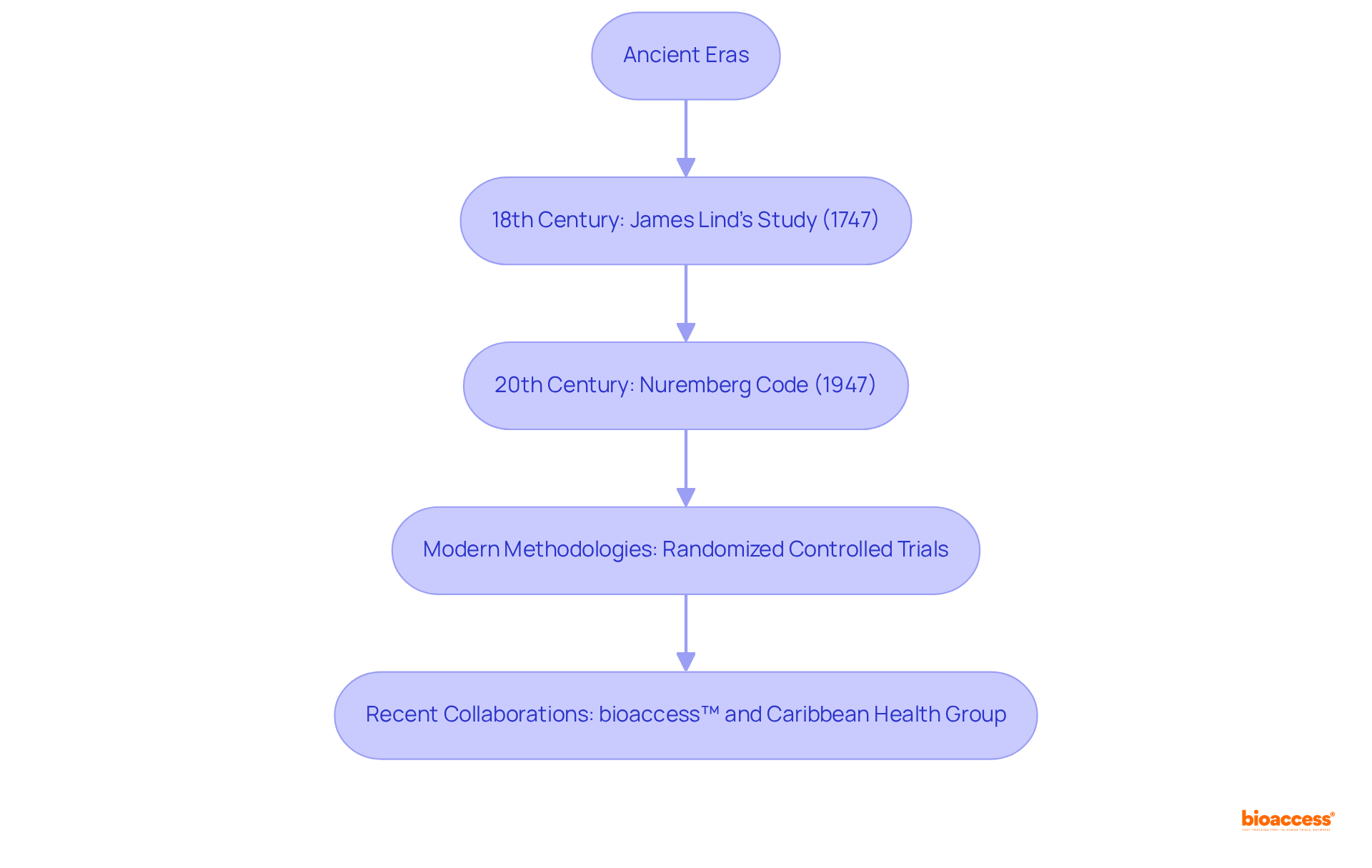
The history of medical research traces back to ancient eras, with early instances found in biblical writings. However, the modern concept of medical experimentation began to take shape in the 18th century, notably through James Lind's systematic study of scurvy therapies in 1747. The 20th century marked a pivotal period of advancement, highlighted by the establishment of ethical guidelines following the Nuremberg Code in 1947, which underscored the importance of informed consent and the protection of human subjects.
Over the years, the clinical studies definition has evolved to incorporate rigorous methodologies, such as randomized controlled trials, now considered the gold standard in medical research. In recent times, collaborations like that between bioaccess™ and Caribbean Health Group have emerged, aiming to position Barranquilla as a leading site for clinical trials in Latin America. Supported by Colombia's Minister of Health, this initiative has made remarkable strides, achieving over a 50% reduction in recruitment time and impressive 95% retention rates, underscoring the ongoing evolution and significance of research within today's healthcare landscape.
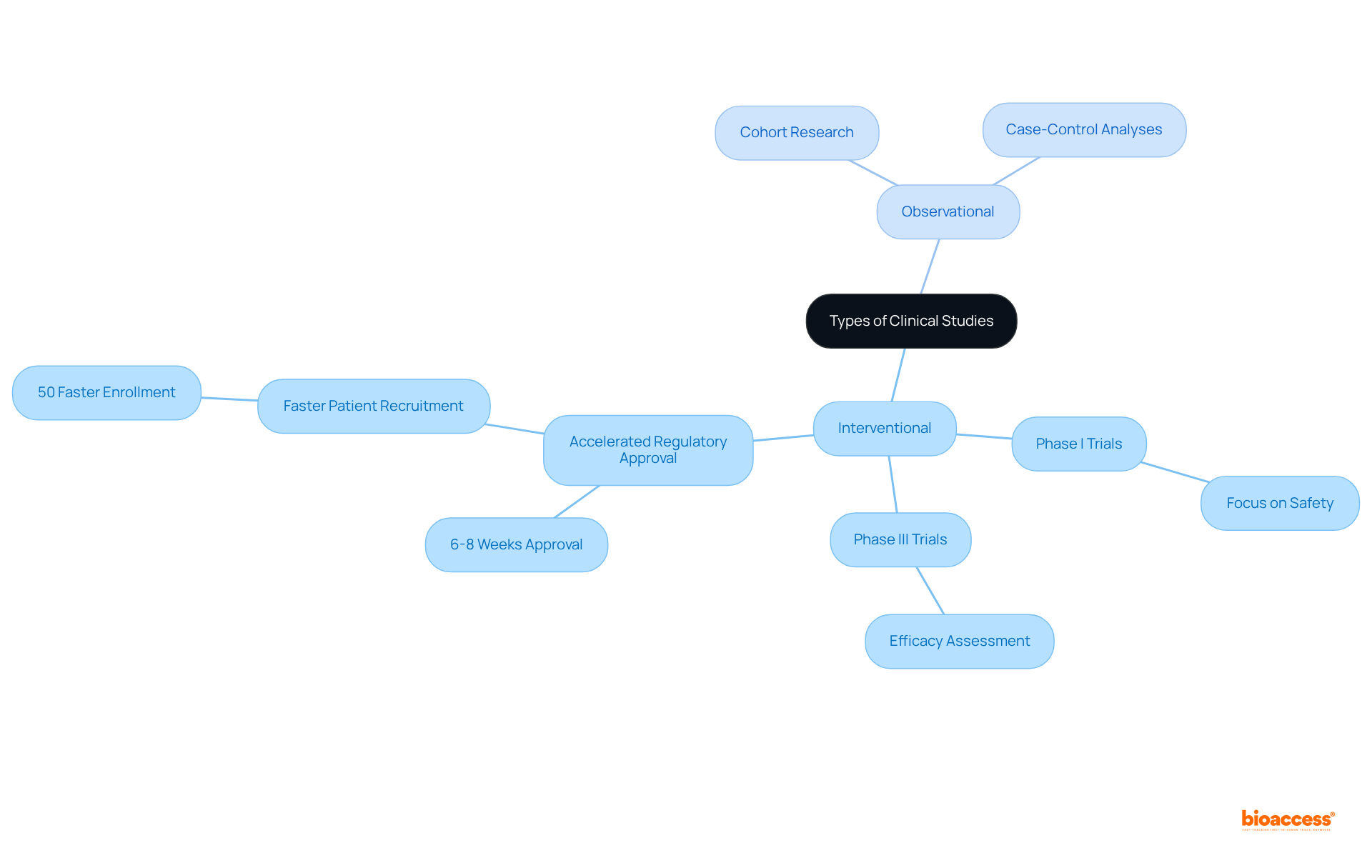
According to the clinical studies definition, clinical research can be broadly categorized into two primary types: interventional and observational.
Interventional experiments, which are often referred to in the clinical studies definition, involve the active manipulation of variables. This includes administering a new drug or treatment to evaluate its effects. For instance:
Notably, Bioaccess® offers an accelerated regulatory approval process, achieving this in just 6-8 weeks compared to the typical 6-12 months in the US/EU. This rapid approval not only facilitates faster entry into the market but also enables the enrollment of treatment-naive cardiology or neurology cohorts 50% faster than traditional Western sites, directly addressing common patient recruitment challenges.
Conversely, according to the clinical studies definition, observational research entails observing participants without intervention, allowing researchers to collect data on results in real-world environments. Examples include:
These provide valuable insights into disease progression and treatment effects.
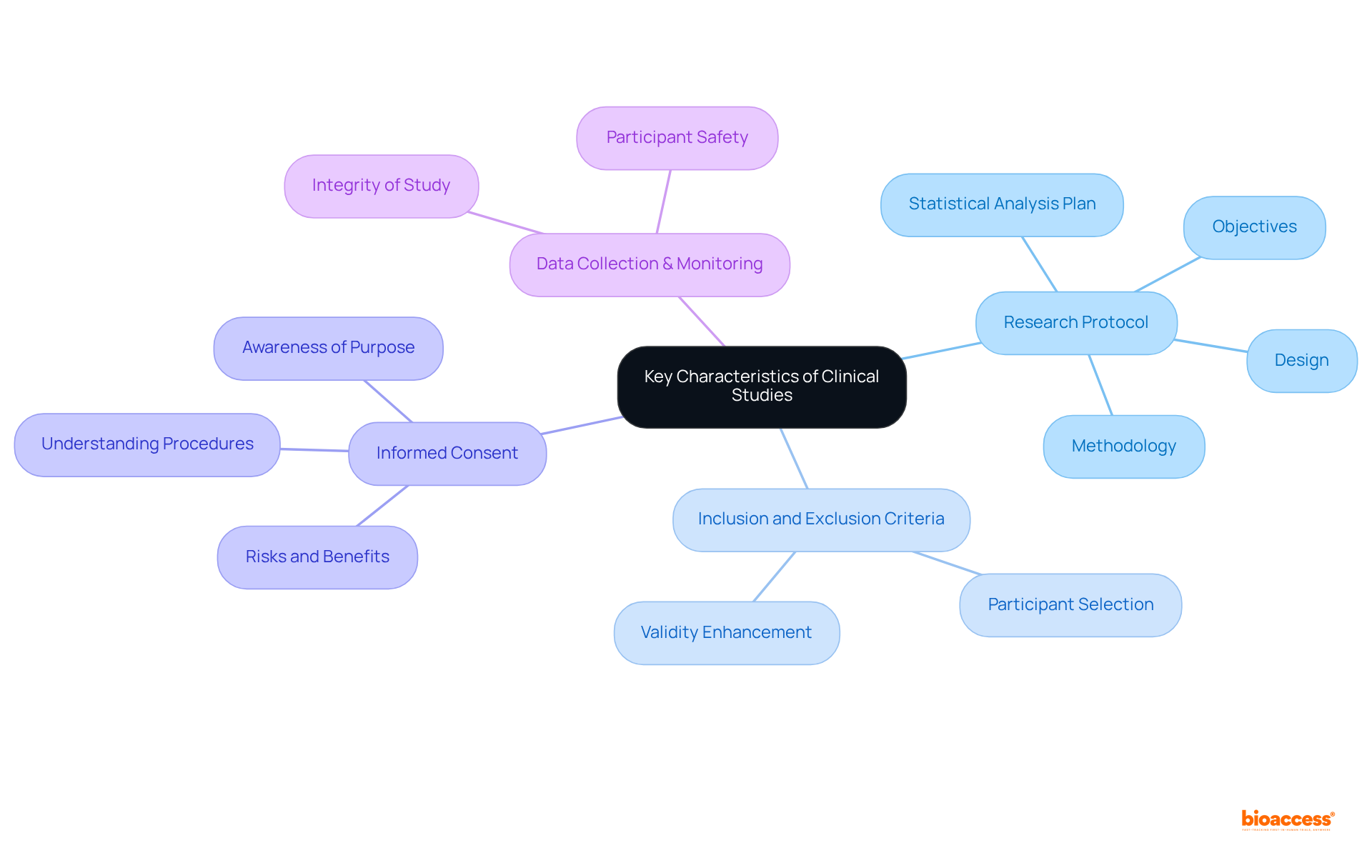
Key characteristics of clinical research encompass a well-defined protocol, clear inclusion and exclusion criteria, and informed consent from participants. The research protocol delineates the objectives, design, methodology, and statistical analysis plan, ensuring a systematic approach to conducting the research. Inclusion and exclusion criteria are pivotal in selecting appropriate participants, thereby enhancing the validity of the results. Informed consent stands as a vital ethical necessity, ensuring that participants are fully aware of the research's purpose, procedures, risks, and benefits prior to their agreement to participate. Furthermore, robust data collection and monitoring processes are essential to uphold the integrity of the study and safeguard participant safety.
Clinical studies are pivotal in the healthcare landscape, systematically evaluating medical interventions to ensure their safety and efficacy. This foundational aspect of clinical research not only advances medical knowledge but also underpins evidence-based practices that significantly enhance patient care. By understanding the multifaceted nature of clinical studies, from their historical evolution to contemporary applications, we can appreciate their indispensable value in the medical field.
The article presented key insights, including:
The evolution of clinical studies—particularly the establishment of ethical guidelines and the emergence of innovative research methodologies—illustrates a commitment to safeguarding participant welfare while striving for scientific advancement. Furthermore, the emphasis on informed consent and robust data collection underscores the integrity of the research process.
The significance of clinical studies extends beyond academic circles; they are vital for regulatory approvals, the development of new therapies, and the overall improvement of healthcare outcomes. As the landscape of medical research continues to evolve, it is imperative to support and participate in clinical studies. They are the backbone of medical innovation and ultimately lead to better health solutions for all. Engaging with clinical research not only empowers individuals to contribute to scientific progress but also enhances the collective understanding necessary for advancing healthcare practices.
What are clinical studies?
Clinical studies encompass clinical trials, which are systematic examinations aimed at evaluating the safety and effectiveness of medical interventions such as medications, devices, and therapeutic protocols.
What is the purpose of clinical trials?
The purpose of clinical trials is to determine whether new therapies are safe and effective for human application, ultimately leading to improved patient outcomes.
Why are clinical studies important?
Clinical studies are important because they advance medical knowledge by providing evidence-based information that informs clinical practice and ensures healthcare practices are grounded in scientific research.
How do clinical studies contribute to regulatory approval?
Clinical studies are essential for regulatory approval as they provide the necessary evidence regarding the safety and efficacy of medical interventions, which is required for their use in healthcare.
What role do clinical studies play in evidence-based medicine?
Clinical studies constitute a cornerstone of evidence-based medicine, ensuring that healthcare practices are based on solid scientific research and data.