


In recent years, Latin America has emerged as a significant player in the global clinical trials landscape, with Colombia taking center stage due to its unique advantages. The region's diverse patient population, favorable regulatory environment, and cost efficiencies make it an attractive destination for pharmaceutical and biotechnology companies seeking to conduct clinical research. As Colombia invests in healthcare infrastructure and fosters collaborations among local institutions, it not only enhances its reputation but also streamlines processes that lead to faster trial execution.
However, navigating the complexities of this evolving environment requires a keen understanding of both the opportunities and challenges present. This article delves into the factors that position Colombia as a prime location for clinical trials and explores the future trajectories of clinical research within the country.
Latin America has emerged as a significant center for research studies, motivated by a blend of elements that increase its appeal for international pharmaceutical and biotechnology firms. The area gains from a varied patient population that frequently mirrors the demographics of numerous diseases, enabling more representative study results.
Furthermore, nations such as this South American country provide a supportive regulatory framework, accelerating the approval process for medical research. The partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a prominent site for medical studies exemplifies this trend, receiving strong backing from the Minister of Health in the country.
Additionally, GlobalCare Clinical Trials has teamed up with bioaccess™ to improve ambulatory services for studies in Colombia, attaining over a 50% decrease in recruitment duration and a retention rate surpassing 95%. Carrying out studies in Latin America is frequently more economical, with estimates indicating savings of 30-40% in comparison to studies in North America and Europe.
The expanding knowledge in research among local institutions and investigators, supported by partnerships such as those with Greenlight Guru, further improves the region's reputation, making it more competitive on the global stage.
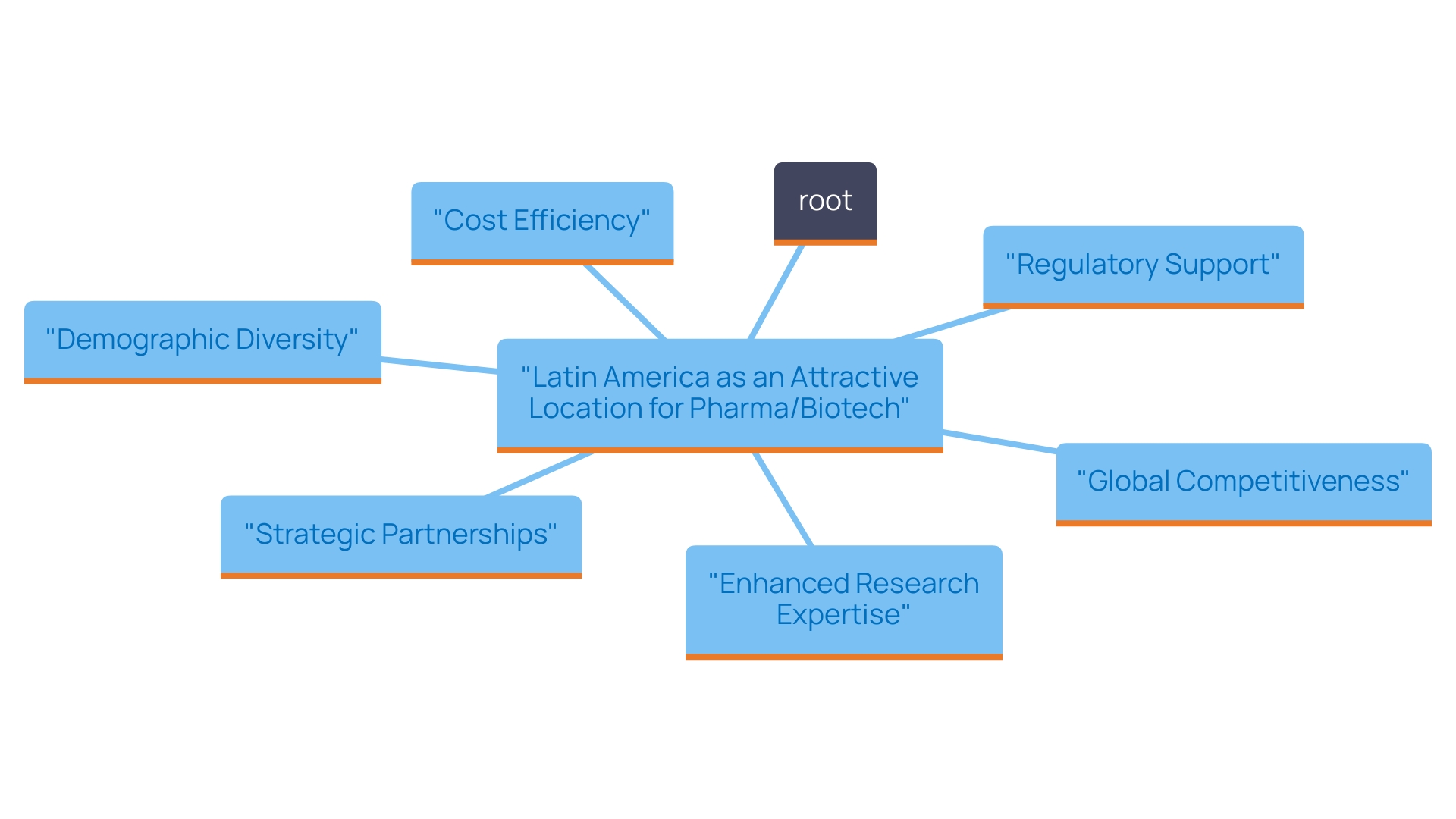
This country stands out as a prime location for clinical studies due to its strategic advantages. The nation provides notable cost efficiency, with savings exceeding 30% in comparison to experiments in North America or Western Europe. Its well-established regulatory framework facilitates compliance and expedites the approval process, with total IRB/EC and INVIMA review times ranging from 90 to 120 days.
Furthermore, the nation has a diverse and accessible patient population—over 50 million individuals, with around 95% covered by universal healthcare—giving researchers the chance to carry out studies that produce trustworthy and widely applicable results.
Additionally, investments in science and technology are incentivized through substantial R&D tax benefits, including a 100% tax deduction and various credits. The presence of skilled research organizations and a network of highly qualified investigators boosts the country's attractiveness.
Industry leaders such as Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, and Dr. John B. Simpson, CEO of Avinger, have shared their favorable experiences with studies carried out in the South American nation, further validating its potential.
To commence a research study in the nation, researchers must secure study approval from their site's institutional review board (IRB) or ethics committee (EC), followed by authorization from the country's regulatory agency (INVIMA) and the Ministry of Health (MoH). Additionally, an import permit from the Ministry of Industry and Commerce (MinCIT) is required to ship investigational devices to the site.
The nation's commitment to enhancing its healthcare infrastructure and investing in research and development highlights its dedication to becoming a leader in medical research. These factors, along with comparatively reduced operational expenses, place this country as an appealing location for research studies, attracting both domestic and foreign sponsors.
While this country provides numerous benefits as a clinical research location, including comprehensive clinical research management services such as:
it is essential to be aware of the challenges that may arise. For instance, regulatory complexities overseen by INVIMA (Colombia's National Food and Drug Surveillance Institute) can sometimes slow down the approval process, despite the overall favorable environment. INVIMA's role includes inspecting and supervising health products and ensuring compliance with health standards, which is vital for integrity of the process.
Additionally, cultural differences and varying levels of healthcare access among different regions can impact patient recruitment and retention. Logistical issues, such as transportation and communication barriers, may also pose challenges for execution of the test.
Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics, emphasizes the importance of navigating these obstacles effectively. Her insights indicate that sponsors and researchers should implement localized strategies, such as collaborating with local healthcare providers and utilizing regional data, to reduce risks and improve the success of their studies. By understanding these challenges and leveraging expert guidance, stakeholders can better prepare for the unique environment that the country presents.
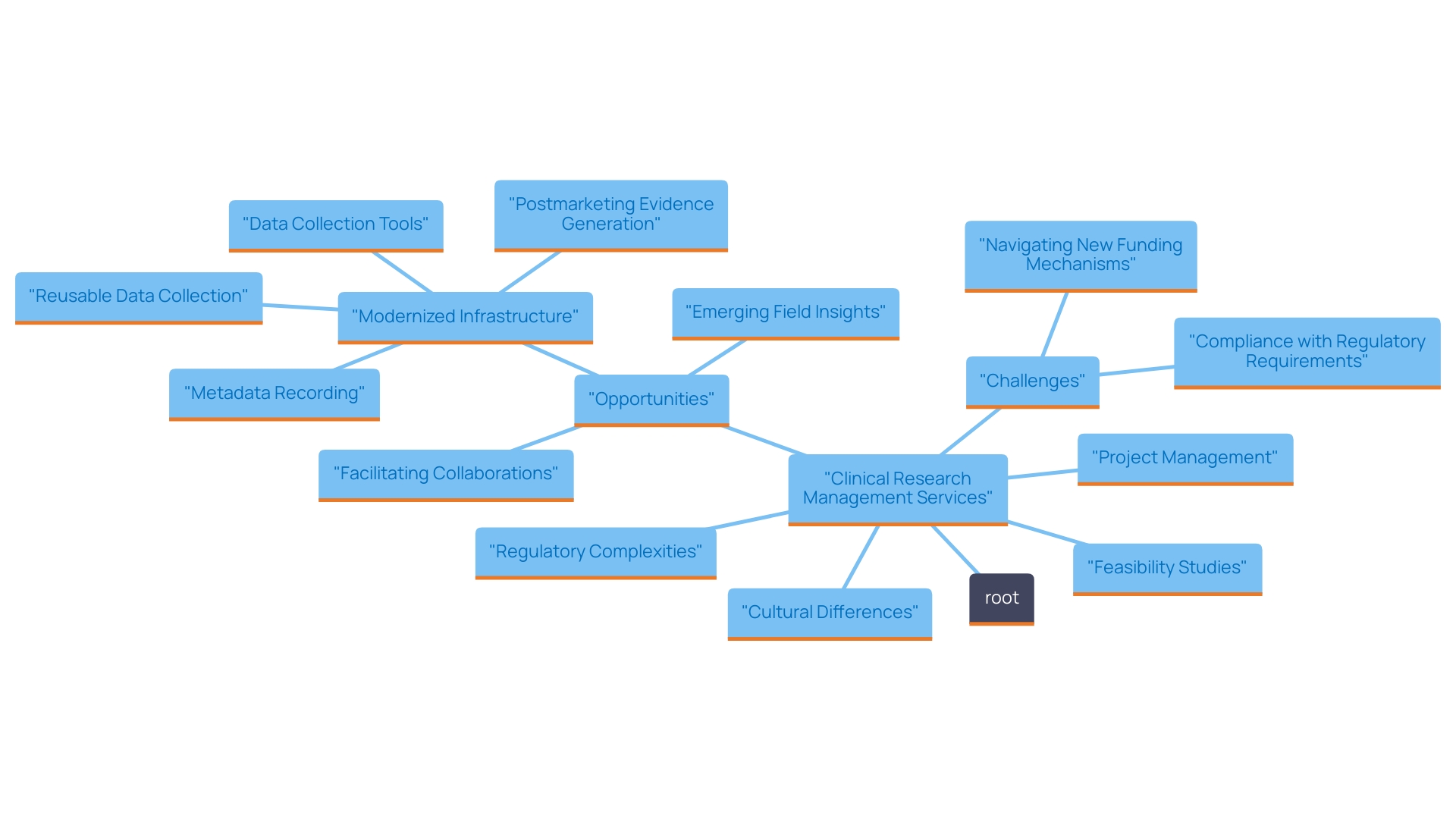
The outlook for medical studies in the nation appears encouraging, with various trends suggesting ongoing expansion and creativity. Our comprehensive trial management services encompass:
This ensures a thorough approach to trial execution. The Colombian government is actively investing in healthcare infrastructure and regulatory improvements, with INVIMA (Colombia National Food and Drug Surveillance Institute) playing a crucial role in overseeing medical device regulation and compliance as a Level 4 health authority recognized by PAHO/WHO. These advancements are anticipated to boost the country's attractiveness for clinical studies.
Furthermore, the rise of digital health solutions and telemedicine is streamlining research processes and enhancing patient engagement. For instance, recent partnerships between academic institutions and the pharmaceutical sector have resulted in innovative projects, fostering a culture of exploration and development.
As Colombia continues to strengthen its position in the global clinical trials landscape, it holds the potential to attract more international sponsors, create jobs, promote economic growth, improve healthcare, and contribute to advancements in medical research through international collaboration.
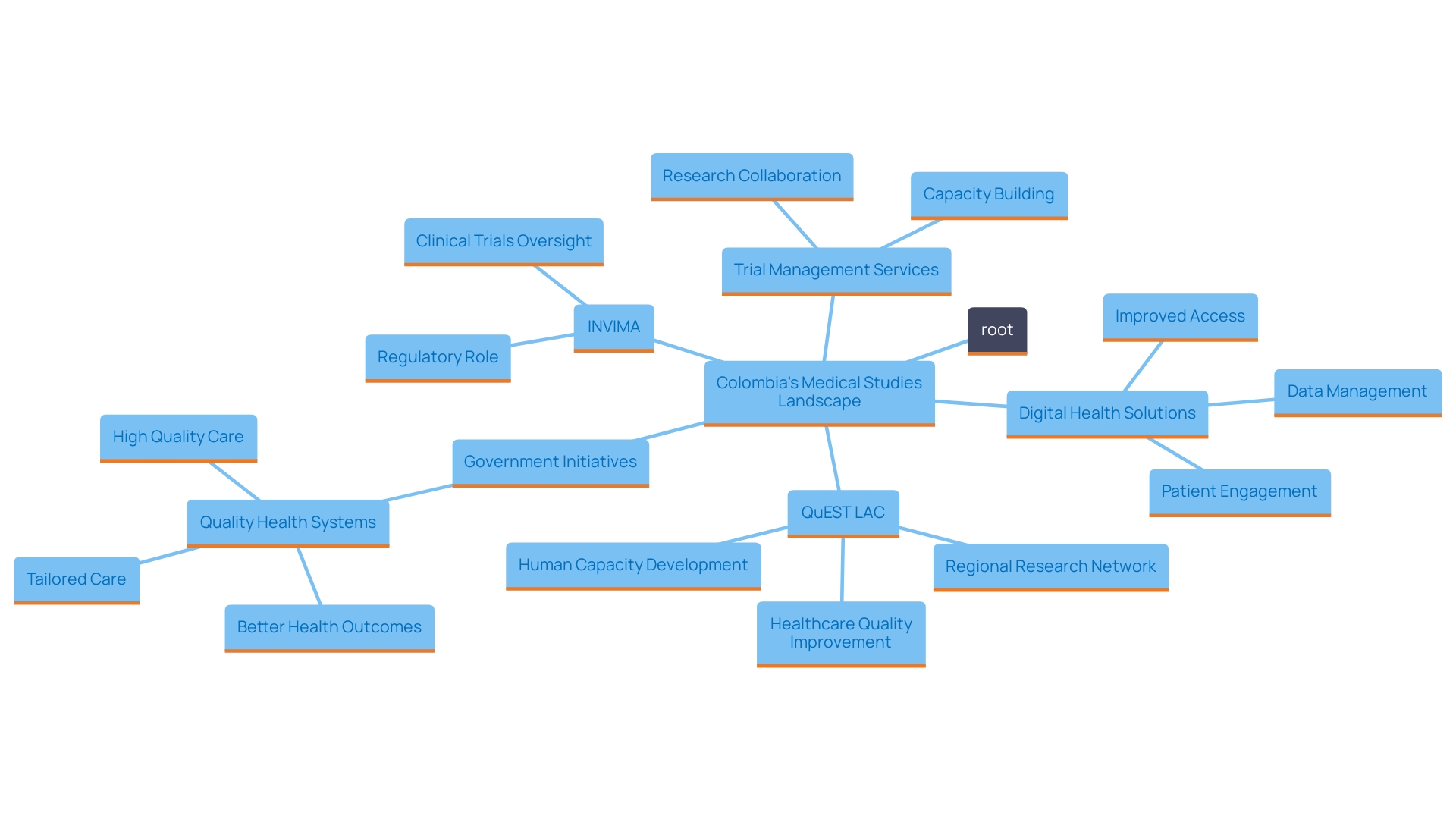
The emergence of Colombia as a pivotal hub for clinical trials is underscored by its diverse patient population, favorable regulatory framework, and significant cost efficiencies. The strategic collaborations between local institutions and industry leaders not only enhance the country's capabilities but also streamline the processes necessary for successful trial execution. With a commitment to improving healthcare infrastructure and investing in research and development, Colombia is well-positioned to continue attracting both local and international sponsors.
Despite the advantages, navigating the complexities of Colombia's clinical trials landscape requires careful consideration of potential challenges, such as regulatory intricacies and regional disparities in healthcare access. By adopting localized strategies and leveraging expert insights, stakeholders can effectively mitigate these challenges, paving the way for successful clinical research endeavors.
Looking ahead, the outlook for clinical trials in Colombia remains bright. With ongoing government investments, advancements in digital health solutions, and a growing culture of collaboration between academia and the pharmaceutical industry, the country is poised for continued growth and innovation in the clinical research sector. As Colombia solidifies its role on the global stage, it not only enhances its reputation but also contributes significantly to the advancement of medical research and healthcare improvements worldwide.
Why has Latin America become a significant center for research studies in pharmaceuticals and biotechnology?
Latin America has become a significant center due to its diverse patient population, supportive regulatory framework, successful partnerships, cost efficiency, and improving research capabilities.
What are the advantages of conducting clinical studies in Colombia?
Advantages include cost savings of over 30% compared to North America and Western Europe, a well-established regulatory framework, a diverse patient population with universal healthcare coverage, and significant R&D tax benefits.
What is the process for commencing a research study in Colombia?
Researchers must secure approval from the site’s institutional review board (IRB) or ethics committee (EC), obtain authorization from the regulatory agency INVIMA, and acquire an import permit from the Ministry of Industry and Commerce (MinCIT).
What services are included in clinical research management in Colombia?
Services include feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
What challenges might researchers face when conducting studies in Colombia?
Challenges may include regulatory complexities, cultural differences, varying healthcare access, and logistical issues such as transportation and communication barriers.
How is the Colombian government supporting the growth of clinical research?
The government is investing in healthcare infrastructure and regulatory improvements, which are expected to enhance the country’s attractiveness for clinical studies.
What role does digital health play in the future of medical studies in Colombia?
Digital health solutions and telemedicine are streamlining research processes and enhancing patient engagement, contributing to the growth of clinical trials in the country.
What are the anticipated outcomes of strengthening Colombia's position in the global clinical trials landscape?
The anticipated outcomes include attracting more international sponsors, creating jobs, promoting economic growth, improving healthcare, and advancing medical research through collaboration.