


The landscape of clinical trials is experiencing a significant transformation, fueled by the integration of automated data capture technologies. This innovative approach streamlines the data collection process, enhances the accuracy and compliance of research studies, and ultimately accelerates the path to medical breakthroughs. As organizations increasingly embrace these systems, a critical question arises: how can automated data capture revolutionize the efficiency and outcomes of clinical trials, benefiting both researchers and patients alike?
bioaccess® harnesses the power of automated data capture clinical trials to revolutionize clinical study processes, drastically reducing the time required for data collection and analysis. By integrating advanced electronic data capture (EDC) systems, bioaccess® facilitates real-time information gathering, enhancing both accuracy and compliance with regulatory standards. This cutting-edge approach not only accelerates the approval process but also fosters greater patient engagement and retention, resulting in quicker study completions and faster commercialization of innovative medical solutions.
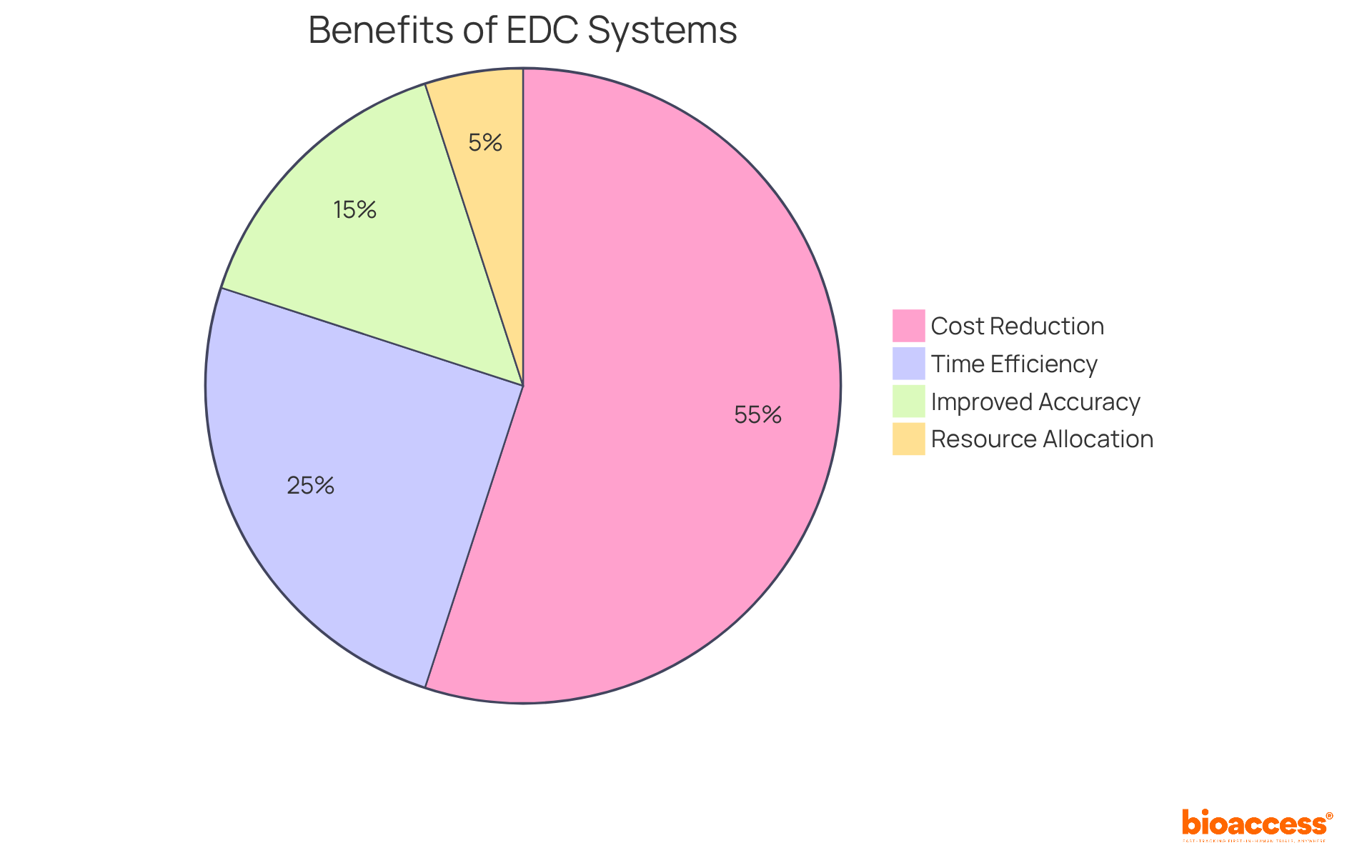
Organizations that have adopted EDC report a staggering reduction in query creation by over 75%, which translates to swifter turnaround times for lab results and streamlined information reconciliation. Healthcare leaders have noted that effective information visualization and automated workflows significantly enhance decision-making, yielding improved outcomes in medical studies. As we look toward 2025, the integration of automated data capture clinical trials is poised to play a pivotal role in boosting the efficiency and timelines of medical studies, with an anticipated market growth rate of 8.3% annually, underscoring its critical importance in the evolving landscape of medical research.
Moreover, bioaccess® offers a comprehensive suite of research services, including:
This holistic strategy not only addresses the immediate challenges faced in clinical research but also sets the stage for future advancements in the field.
Medidata's electronic information capture (EDC) systems play a pivotal role in enhancing automated data capture clinical trials. These systems facilitate automated data capture in clinical trials by automating information entry and validation, significantly reducing human error and resulting in improved accuracy and reliability. With real-time monitoring capabilities, researchers can quickly obtain crucial information, enabling informed decision-making that is vital for adhering to timelines and preserving information integrity.
This streamlined approach not only accelerates the availability of data for analysis but also enhances the overall efficiency of medical research by utilizing automated data capture clinical trials. In an industry increasingly demanding effective information management solutions, Medidata's EDC systems stand out as essential tools for overcoming key challenges in automated data capture clinical trials.
As the landscape of Medtech evolves, the need for reliable and efficient data management becomes more pressing. By leveraging these advanced systems, researchers can enhance their operational capabilities and ensure that they remain at the forefront of innovation. The collaboration between technology and clinical research is crucial for driving progress and achieving successful outcomes.
OpenClinica's fundamental EDC solutions are designed with a strong emphasis on compliance and information integrity, both of which are crucial for the success of research studies. This platform features comprehensive audit trails, user access controls, and automated validation checks, ensuring that all collected data meets stringent regulatory standards. Such measures not only safeguard patient information but also enhance the overall reliability of research outcomes.
Take, for instance, the I-SPY 2 research study, which has evaluated over 25 agents and amassed more than 20 million data points. This example underscores the effectiveness of robust audit trails in maintaining data integrity. Regulatory bodies like the FDA highlight the importance of accurate and trustworthy data in research, and OpenClinica's capabilities are tailored to support compliance with these standards. By fostering trust among stakeholders, these features contribute to more reliable and acceptable research outcomes.
Prioritizing information integrity through advanced EDC solutions, OpenClinica significantly elevates the quality and transparency of medical research. Furthermore, the integration of bioaccess's capabilities in feasibility studies, site selection, and project management streamlines the process of automated data capture clinical trials, ensuring meticulous handling of all trial aspects. The acceptance criteria for error rates - such as 50 errors per 10,000 fields and 10 errors per 10,000 fields - demonstrate the platform's unwavering commitment to maintaining high-quality standards.
Viedoc's solutions for automated data capture clinical trials are designed with a clear purpose: to enhance the patient experience in clinical research. By providing intuitive interfaces and mobile access, these solutions allow patients to effortlessly enter information and track their involvement in studies. This not only significantly improves data collection efficiency but also fosters a sense of involvement and satisfaction among participants, which is crucial for maintaining retention and compliance throughout the study.
In today's Medtech landscape, the role of mobile technology cannot be overstated. Researchers can communicate with patients remotely, sending timely reminders for clinic visits and medication intake. This proactive approach has been shown to improve patient retention rates. In fact, studies indicate that mobile service usage can lead to a 5% increase in visit compliance and a 4.5% reduction in early patient withdrawal. By prioritizing the patient experience, Viedoc effectively addresses logistical challenges, which is vital for automated data capture clinical trials, while cultivating a more engaged participant group that ultimately aids the success of research studies.
The importance of collaboration in clinical research cannot be overlooked. As Viedoc continues to optimize the patient experience, it not only tackles operational hurdles but also enhances the overall quality of research outcomes. This commitment to patient engagement is essential for fostering a robust and compliant participant base, paving the way for more successful studies.
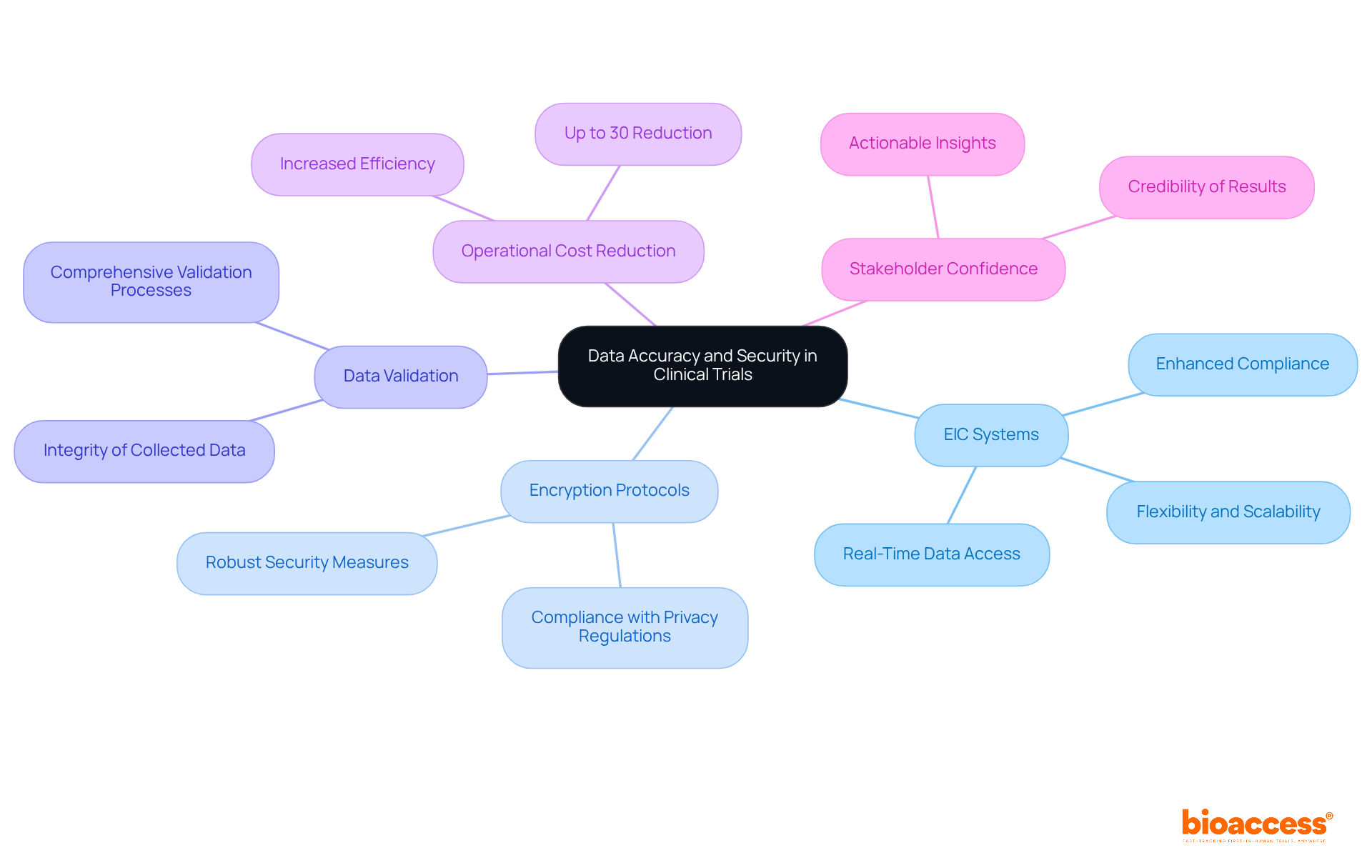
The importance of information accuracy and security in clinical trials cannot be overstated. Advanced Electronic Information Capture (EIC) systems are pivotal in the context of automated data capture clinical trials to achieve these objectives. By employing robust encryption protocols that comply with stringent privacy regulations, these systems safeguard sensitive patient information from unauthorized access. Additionally, comprehensive data validation processes are essential for maintaining the integrity of the collected data.
Automated data capture clinical trials can significantly reduce operational expenses by up to 30%, thereby enhancing the overall effectiveness of the research process. This dual focus on encryption and validation not only bolsters the reliability of outcomes but also instills confidence among stakeholders, making the results more credible and actionable. As W. Edwards Deming famously stated, 'In God we trust, all others bring evidence,' highlighting the critical nature of information integrity.
As automated data capture clinical trials increasingly rely on digital solutions, addressing current information security challenges is vital for achieving successful research outcomes. The collaboration between technology and clinical research is essential in navigating these complexities, ensuring that the integrity of data remains intact while fostering innovation in the Medtech landscape.
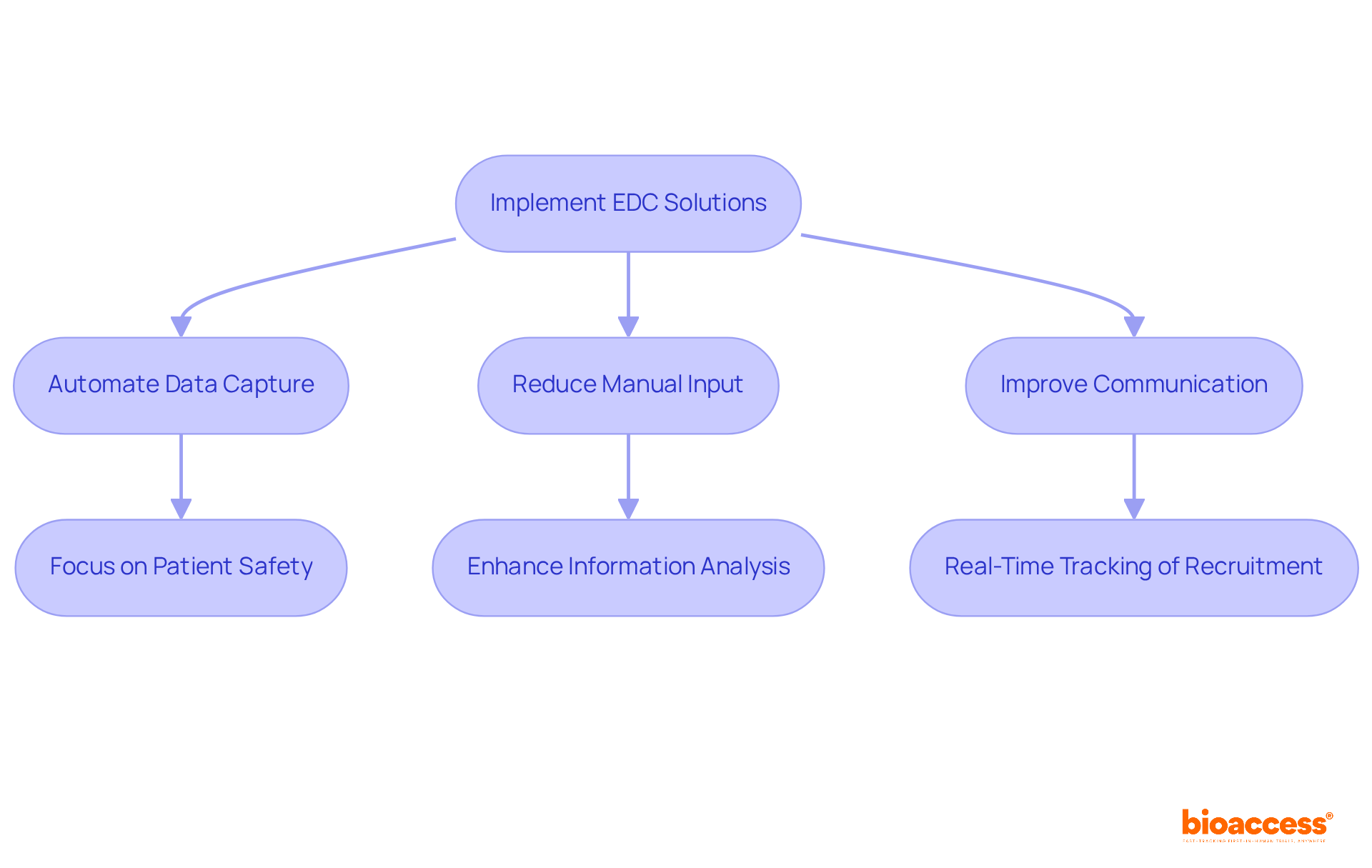
CCRPS effectively addresses various challenges in clinical studies through the implementation of automated data capture clinical trials using advanced Electronic Data Capture (EDC) solutions. These systems greatly simplify information gathering procedures in automated data capture clinical trials, reducing the load of manual input and improving communication among research teams. By automating standard tasks, CCRPS enables researchers to focus on crucial elements of the study, such as patient safety and information analysis in automated data capture clinical trials.
This change not only results in more efficient studies but also enhances overall results. For instance, with 45% of information entered on the same day as the visit date, the efficiency of information management is significantly improved. Furthermore, the integration of EDC systems facilitates real-time tracking of participant recruitment, which is crucial for meeting enrollment timelines.
Consequently, medical studies can reach their goals more quickly and efficiently, ultimately aiding both researchers and participants. This streamlined approach not only boosts productivity but also fosters a collaborative environment that is essential for successful automated data capture clinical trials.
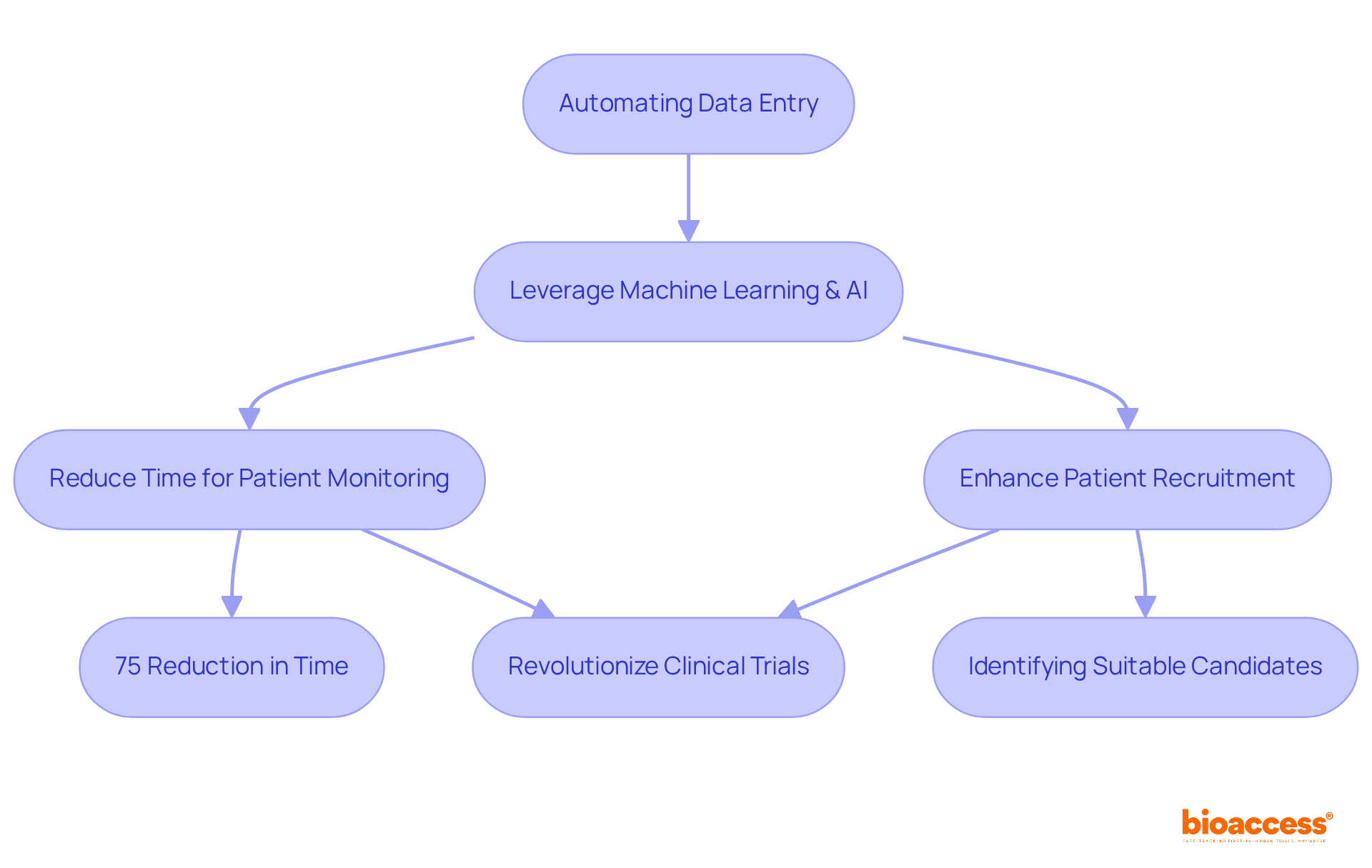
Automating information entry processes is crucial for enhancing efficiency in automated data capture clinical trials. By leveraging advanced technologies such as machine learning and artificial intelligence, organizations can significantly reduce the time and effort required for information gathering. This automation not only minimizes human error but also accelerates the overall process timeline, facilitating quicker data analysis and informed decision-making.
For example, machine learning algorithms can streamline adverse event coding and query management, resulting in an estimated 75% reduction in time for patient monitoring tasks. Furthermore, AI-powered tools enhance patient recruitment by identifying suitable candidates more effectively. This capability is vital, especially considering that the global AI in healthcare studies market is projected to grow from USD 2.60 billion in 2025 to approximately USD 22.36 billion by 2034.
As these technologies continue to evolve, they are poised to revolutionize automated data capture clinical trials, making them more efficient and responsive to the needs of both researchers and participants. The importance of collaboration in this landscape cannot be overstated, as it paves the way for innovative solutions that address key challenges in clinical research.
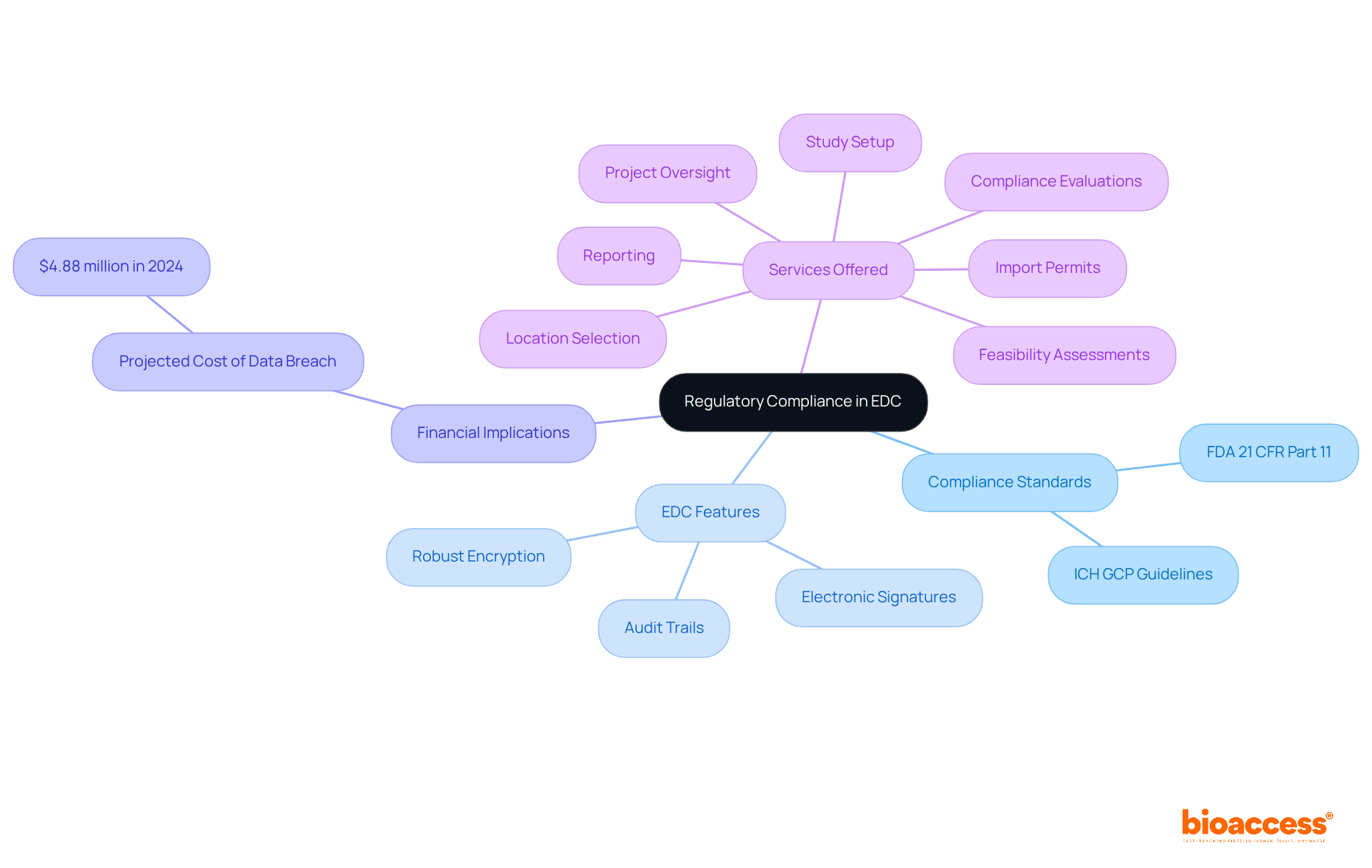
Regulatory compliance stands as a cornerstone in electronic record capture (EDC) systems, essential for adhering to stringent standards like FDA 21 CFR Part 11 and ICH GCP guidelines. Notably, compliance with 21 CFR Part 11 among leading organizations has reached approximately 85%, a testament to the industry's unwavering commitment to high standards. EDC systems are equipped with vital features such as:
These elements not only enhance the reliability of research findings but also cultivate trust among stakeholders and regulatory authorities.
Moreover, the financial implications of non-compliance are stark, with the average cost of a data breach projected to reach $4.88 million in 2024. This statistic underscores the critical need for organizations to adhere to FDA regulations. By doing so, entities like bioaccess can optimize their research studies while protecting sensitive information. Bioaccess offers a comprehensive suite of research study management services, including:
These services are specifically designed to help organizations navigate the complexities of compliance effectively.
Directors of Clinical Research are strongly encouraged to evaluate their current EDC systems to ensure they meet these essential compliance standards. Are your systems equipped to handle the challenges of regulatory compliance? Taking proactive steps now can safeguard your organization’s integrity and enhance the quality of your research.
Electronic Data Capture (EDC) systems are revolutionizing clinical research by delivering substantial cost and time efficiency benefits. When paired with the comprehensive clinical research management services offered by bioaccess, these systems become even more powerful. Bioaccess provides essential services such as:
By implementing automated data capture clinical trials and minimizing manual entry, EDC systems significantly cut operational costs and speed up data processing.
Consider this: the transition to EDC can slash expenses related to paper documentation by an impressive 49 to 62%, while also shortening study timelines by weeks or even months. This remarkable efficiency not only accelerates the overall testing process but also empowers sponsors to allocate resources more strategically, enhancing the potential for a higher return on investment. Moreover, EDC systems facilitate real-time information entry and validation, boosting accuracy and reducing the likelihood of costly errors. Features like edit checks and automated prompts further enhance the tracking and reviewing of trial progress.
As a result, organizations can achieve quicker study completion and improved information quality by utilizing automated data capture clinical trials, ultimately leading to more effective clinical research outcomes. The global EDC system market, valued at $1.25 billion in 2022, highlights the increasing importance of these systems in the industry. Additionally, EDC systems comply with regulations such as FDA 21 CFR Part 11, ensuring the security and integrity of information. The integration of EDC systems with digital tools and devices marks a significant trend, enhancing information collection and monitoring capabilities, which is crucial for advancing global health through international cooperation and innovation in Medtech.
The influence of automated data capture clinical trials on healthcare studies is profound, fundamentally transforming how information is collected, managed, and analyzed. By enhancing accuracy, improving compliance, and accelerating timelines, automated systems empower researchers to focus on what truly matters: advancing medical knowledge and improving patient outcomes.
For instance, bioaccess's innovative approach enables medical studies to achieve patient enrollment 50% faster, particularly within cardiology and neurology groups. This efficiency translates to significant cost savings of $25K per patient, thanks to FDA-ready data that eliminates rework and delays.
Moreover, partnerships like that of bioaccess with GlobalCare Clinical Trials exemplify the effectiveness of these solutions. They have achieved over a 50% reduction in recruitment time and a retention rate exceeding 95% in Colombia.
As the clinical trial landscape evolves, the integration of automated data capture clinical trials will be crucial in shaping the future of research. The collaboration between technology and clinical research not only enhances operational efficiency but also paves the way for groundbreaking advancements in patient care.
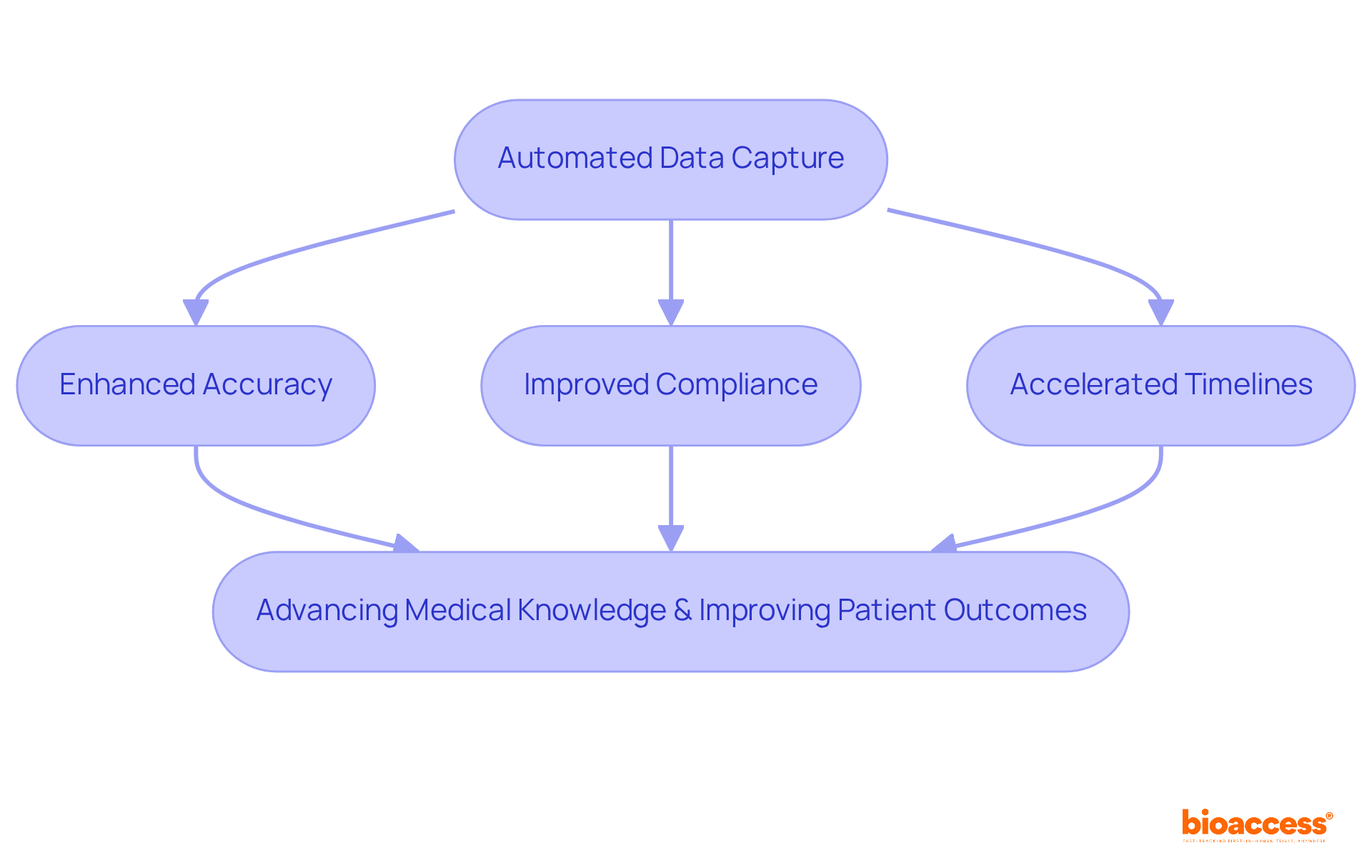
The integration of automated data capture in clinical trials marks a significant shift in medical research practices. By utilizing advanced electronic data capture (EDC) systems, organizations can greatly improve the accuracy, efficiency, and compliance of their studies. This advancement not only accelerates patient enrollment but also enhances outcomes, streamlining the data collection process and fostering greater participant engagement. As a result, the path to quicker commercialization of innovative medical solutions is paved.
Key benefits of automated data capture include:
Companies such as bioaccess, Medidata, OpenClinica, and Viedoc demonstrate how these systems effectively tackle various challenges in clinical trials, from ensuring data integrity to enhancing patient experiences. Furthermore, the financial advantages - significant cost savings and reduced operational expenses - underscore the necessity of adopting these technologies in the research landscape.
Looking ahead, the reliance on automated data capture will be essential for advancing medical research and enhancing patient care. Organizations are urged to embrace these innovative solutions, not only to bolster their operational capabilities but also to contribute to the ongoing evolution of healthcare. By prioritizing automation and collaboration, groundbreaking advancements in clinical outcomes become achievable, ultimately benefiting both researchers and patients.
What is bioaccess® and how does it improve clinical trials?
bioaccess® is a platform that utilizes automated data capture in clinical trials to streamline study processes, significantly reducing the time needed for data collection and analysis. It integrates advanced electronic data capture (EDC) systems for real-time information gathering, enhancing accuracy and compliance with regulatory standards.
What are the benefits of using automated data capture in clinical trials?
The use of automated data capture in clinical trials leads to faster approval processes, improved patient engagement and retention, quicker study completions, and faster commercialization of medical solutions. Organizations report over a 75% reduction in query creation, resulting in faster lab results and streamlined information reconciliation.
What types of studies does bioaccess® support?
bioaccess® offers a comprehensive suite of research services including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies.
How does Medidata enhance data collection in clinical trials?
Medidata's electronic information capture (EDC) systems automate data entry and validation, reducing human error and improving accuracy. They provide real-time monitoring capabilities, enabling quick access to crucial information for informed decision-making.
What challenges do Medidata's EDC systems help overcome in clinical trials?
Medidata's EDC systems address key challenges in automated data capture clinical trials, particularly in effective information management, which is essential for maintaining timelines and data integrity.
What compliance measures does OpenClinica implement in its EDC solutions?
OpenClinica emphasizes compliance and data integrity through features like comprehensive audit trails, user access controls, and automated validation checks, ensuring that collected data meets regulatory standards.
Can you provide an example of OpenClinica's effectiveness in maintaining data integrity?
The I-SPY 2 research study, which evaluated over 25 agents and gathered more than 20 million data points, exemplifies OpenClinica's effectiveness in maintaining data integrity through robust audit trails.
What are the acceptance criteria for error rates in OpenClinica's platform?
OpenClinica has established acceptance criteria for error rates, including 50 errors per 10,000 fields and 10 errors per 10,000 fields, demonstrating its commitment to high-quality standards in data handling.