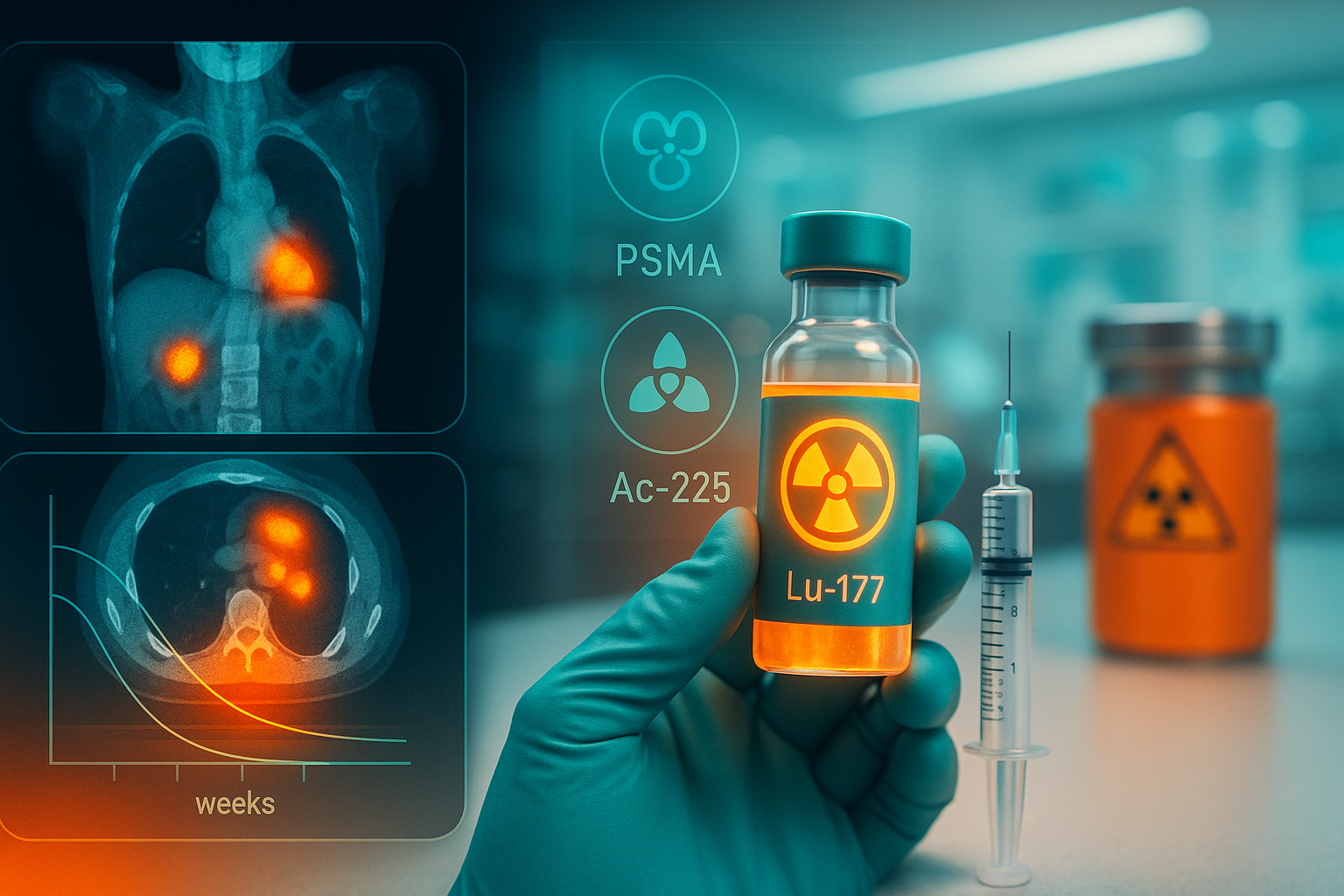
Transform Cancer Care Through Targeted Radiopharmaceuticals
The global radiopharmaceutical market is projected to reach $26.5 billion by 2031, driven bybreakthroughs in theranostics and alpha-emitting isotopes like actinium-225.
At bioaccess®, we specialize in accelerating radiopharmaceutical trials across Latin America,leveraging the region’s 650M+ population and streamlined regulatory frameworks to deliver results 40% faster than traditional pathways.