


In the rapidly evolving landscape of medical research, the integration of regulatory and ethics approval in Australia stands as a pivotal advancement for innovators in the Medtech and Biopharma sectors. By streamlining these processes, organizations can significantly reduce the time required to bring groundbreaking therapies to market, often achieving authorization in as little as 4 to 6 weeks.
However, a crucial question arises: how can companies effectively navigate the complexities of these combined approvals to not only expedite their research but also ensure compliance with both local and international standards?
This article delves into the multifaceted benefits of this approach and highlights its critical role in shaping the future of clinical trials in Australia.
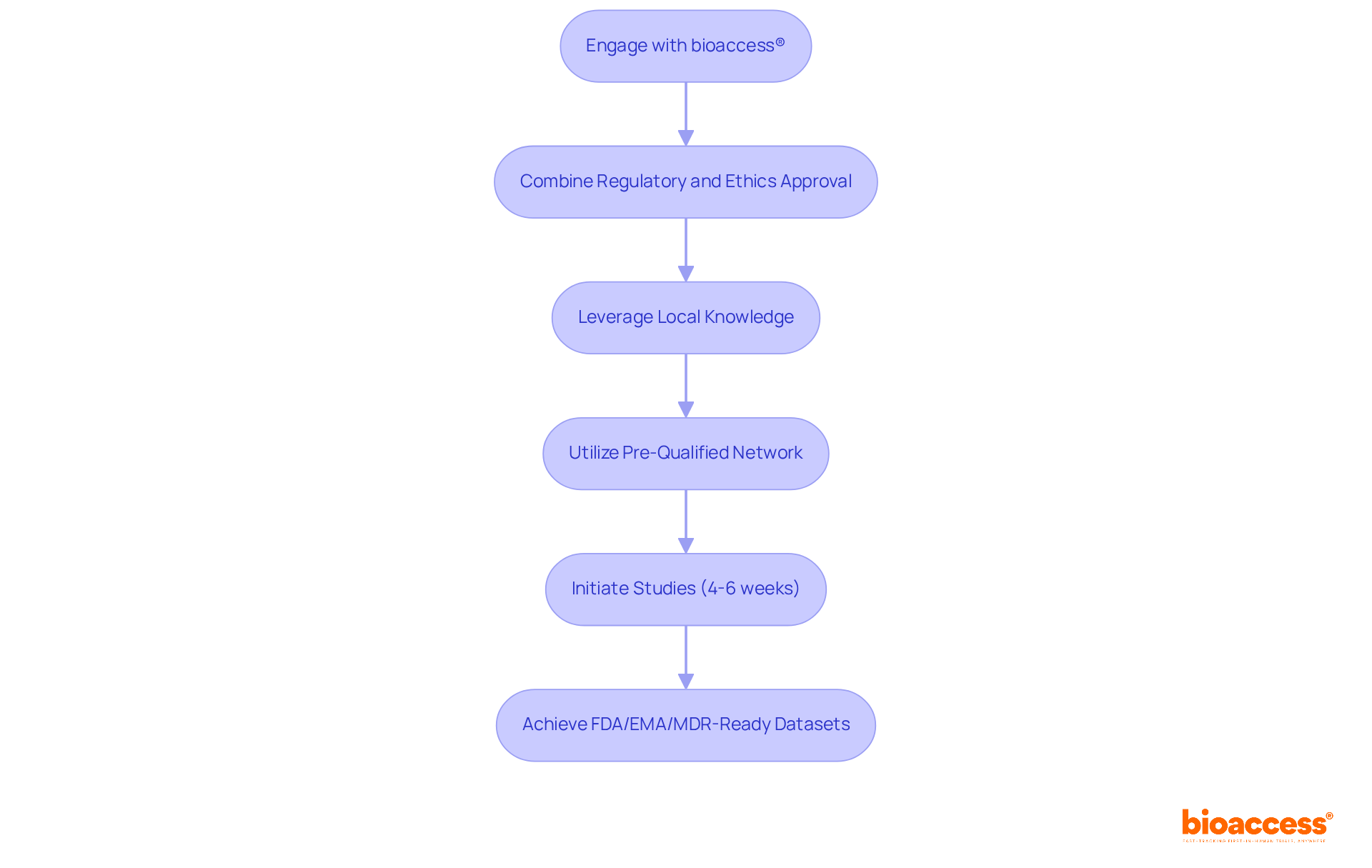
bioaccess® leverages its extensive experience and regional expertise for combining regulatory and ethics approval in Australia to streamline the authorization process. By merging local knowledge with international standards, bioaccess® ensures that studies can commence swiftly-often within just 4 to 6 weeks-thanks to its pre-qualified network of over 50 activated sites. This efficiency is crucial for Medtech, Biopharma, and Radiopharma innovators eager to accelerate their research and development timelines.
In today’s competitive landscape, minimizing bureaucratic delays is essential. bioaccess® not only facilitates rapid study initiation but also guarantees FDA/EMA/MDR-ready datasets. This capability allows organizations to respond more agilely to market demands and regulatory requirements, positioning them for success in a fast-paced environment.
Collaboration is key in navigating the complexities of clinical research. By partnering with bioaccess®, innovators can focus on their core objectives while benefiting from a streamlined process that enhances their operational efficiency. The next step is clear: engage with bioaccess® to unlock the full potential of your research initiatives.
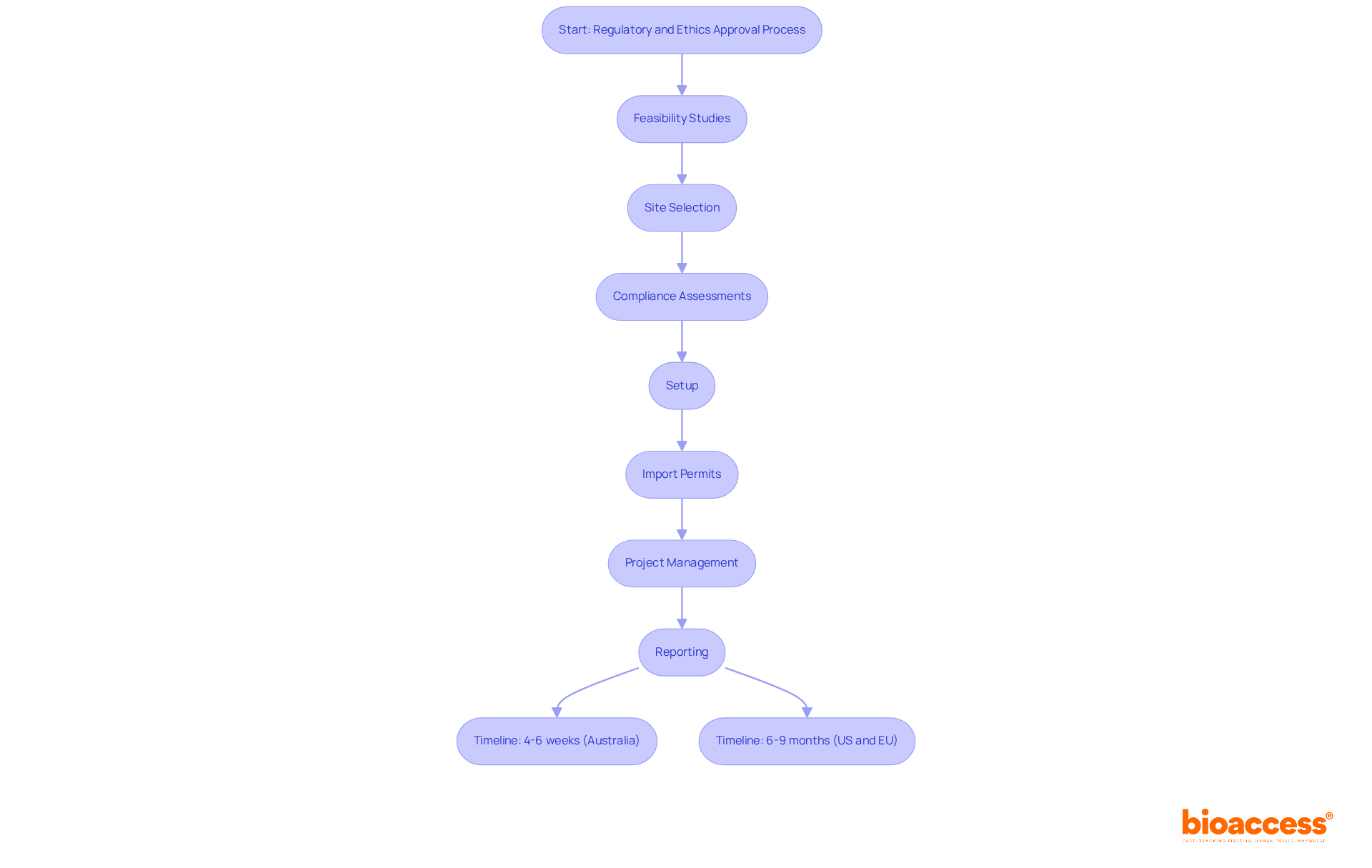
In Australia, combining regulatory and ethics approval in Australia is crucial for expediting the time to market for new medical products. By leveraging bioaccess®'s efficient methods and comprehensive research management services - including feasibility studies, site selection, compliance assessments, setup, import permits, project management, and reporting - organizations can secure authorizations in just 4-6 weeks. This timeline is significantly shorter than the typical 6-9 months seen in traditional markets like the US and EU.
This accelerated process not only enables the rapid launch of innovative therapies but also offers a competitive edge in the fast-paced healthcare landscape. Organizations embracing this streamlined approach report improved operational agility and reduced costs, empowering them to seize emerging market opportunities more effectively. Industry leaders emphasize that the process of combining regulatory and ethics approval in Australia is vital for hastening the development of new therapies, particularly in addressing unmet medical needs and rare diseases.
As Australia continues to enhance its regulatory framework, including the TGA's tightening requirements for electronic Common Technical Documents (eCTD), the potential for quicker market access is set to grow. This evolution positions Australia as an increasingly attractive destination for Medtech and Biopharma innovators.
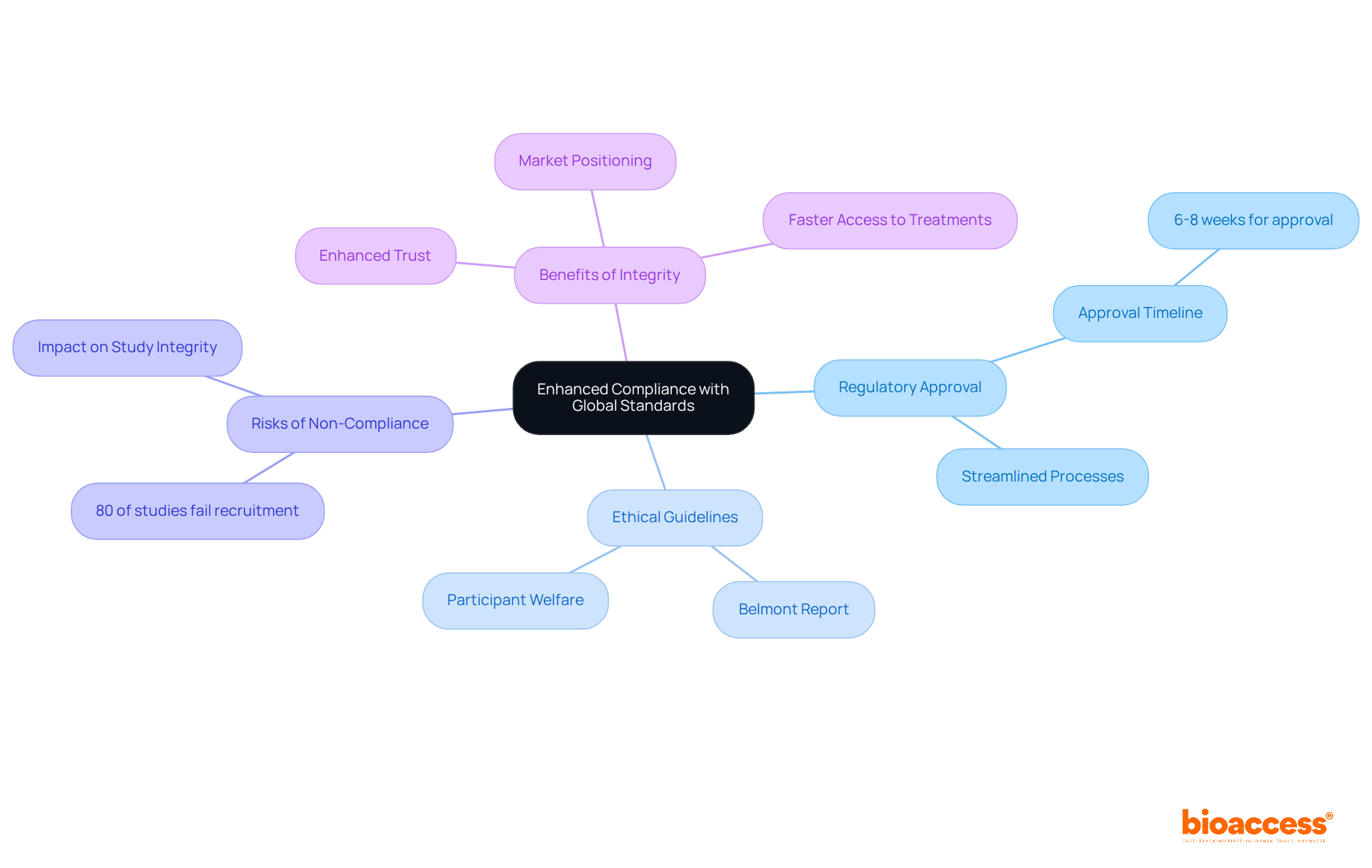
bioaccess® places a strong emphasis on adherence to international regulations and moral standards, ensuring that all trials meet the highest scientific and ethical benchmarks. This dedication streamlines the approval process, achieving regulatory approval in just 6-8 weeks, while enhancing clients' positions in global markets. By following established moral guidelines, such as the Belmont Report, bioaccess® significantly reduces the risks linked to regulatory non-compliance - a major issue in medical research where studies indicate that nearly 80% fail to achieve their recruitment goals due to compliance problems.
Expert views underscore the importance of upholding integrity, as it fosters trust and credibility in research results. Organizations that prioritize moral standards not only protect participant well-being but also enhance their marketability. This ultimately leads to more successful studies and quicker access to innovative treatments. In a landscape where the stakes are high, collaboration and adherence to ethical practices are paramount for success.
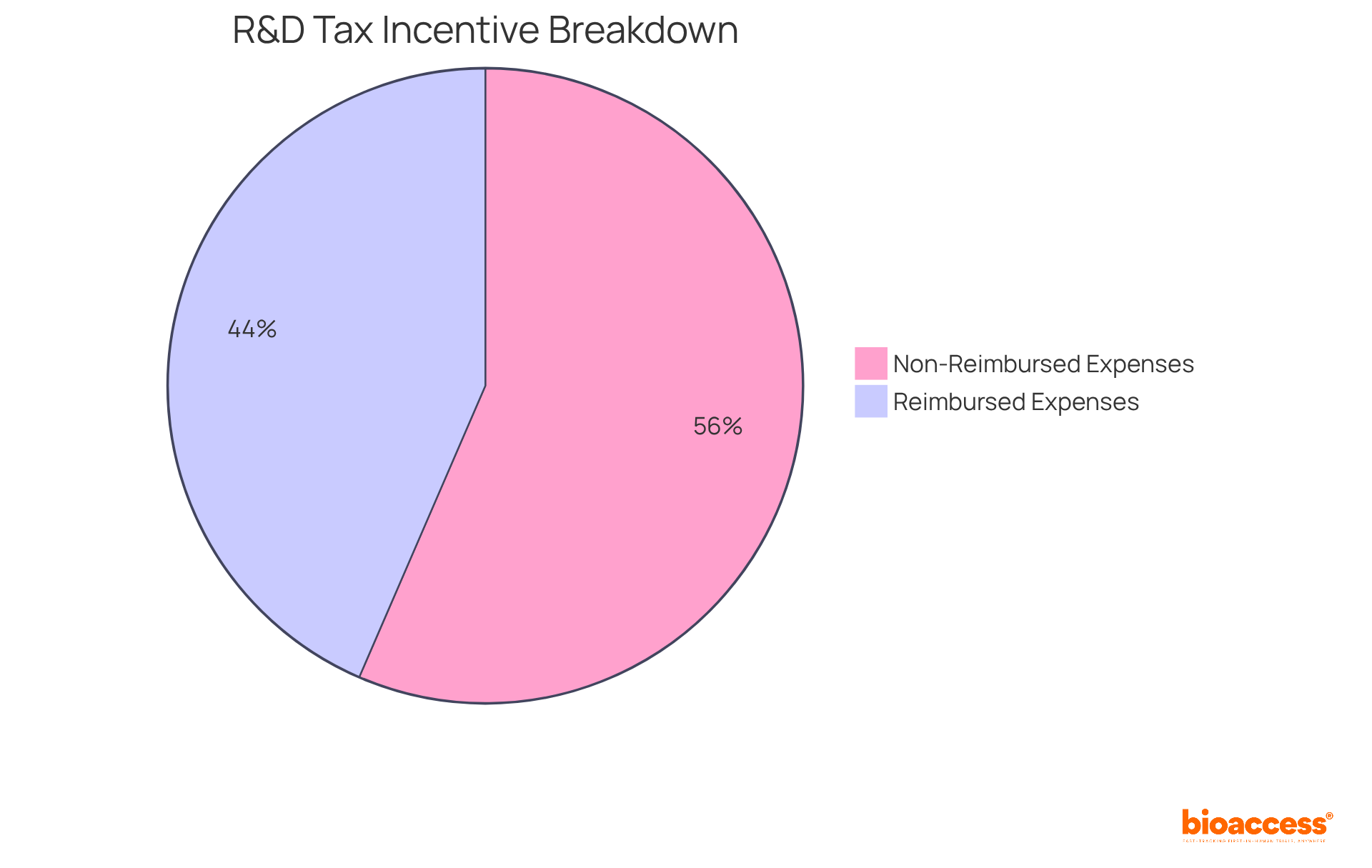
Australia offers substantial financial incentives for research and development, particularly through an R&D tax rebate that can reimburse up to 43.5% of eligible expenses. This support is crucial for startups and small to medium enterprises in the Medtech and Biopharma sectors, allowing them to redirect funds towards innovation and product development. By effectively utilizing these incentives, companies can significantly lower their research expenses, making medical studies more feasible.
For example, numerous startups have successfully decreased their research costs by leveraging these financial incentives, enabling them to concentrate on advancing their groundbreaking technologies.
At bioaccess®, our commitment to ethical considerations is paramount, particularly in patient research where safety is non-negotiable. We adhere strictly to moral standards throughout the research process, conducting comprehensive risk evaluations and ensuring informed consent from all participants. This robust ethical framework not only safeguards participants but also enhances the credibility of our research. By cultivating trust among stakeholders and the public, bioaccess® demonstrates that prioritizing patient safety can harmoniously coexist with the pursuit of innovation.
In Australia, the integration of ethical standards into research studies is increasingly recognized as vital, particularly when it involves combining regulatory and ethics approval in Australia to maintain the integrity of investigations. Various organizations are leading the charge in establishing best practices that balance innovation with the essential need for patient safety. Notably, the ACP's ethics education initiatives underscore the significance of professionalism and moral integrity among physicians, aligning seamlessly with bioaccess®'s mission.
Moreover, our extensive management services for studies - including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting - illustrate our dedication to facilitating effective medical device research. Case studies such as 'Providing Care to Undocumented Immigrants' and 'Pandemic Treatment Resource Allocation Ethics' highlight the tangible impacts of ethical considerations in medical practice, reinforcing the necessity for a strong ethical framework in research.
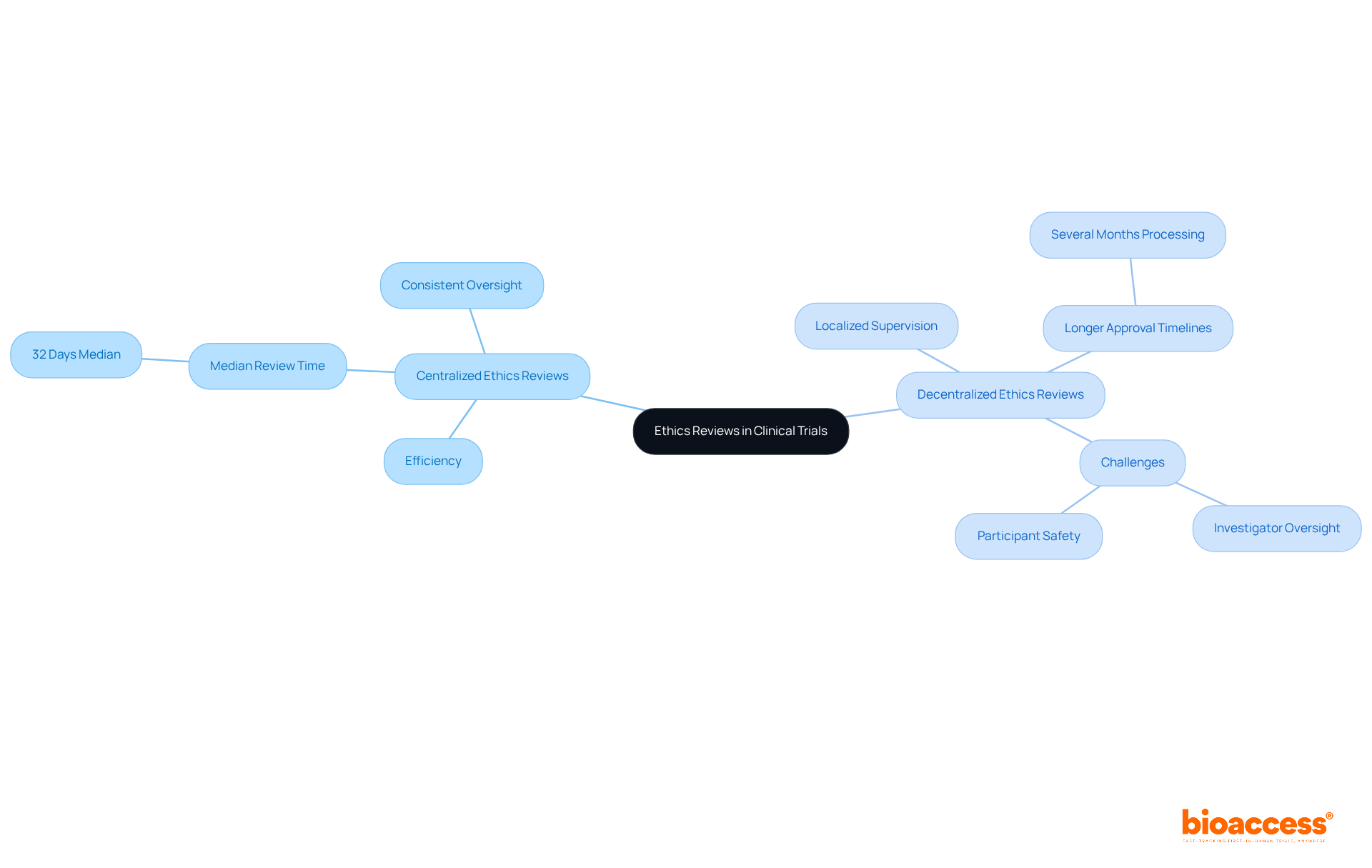
The choice between centralized and decentralized ethics reviews plays a crucial role in the validation process for clinical trials. Centralized assessments, where a single ethics committee oversees multiple locations, can streamline permissions and minimize redundancy. For example, studies show that centralized ethics clearances can achieve median review times as short as 32 days. In contrast, decentralized processes often extend processing times to several months due to the need for multiple committee evaluations.
Regulatory experts assert that centralized systems not only boost efficiency but also provide consistent oversight across sites, which is essential for ensuring participant safety and maintaining research integrity. While decentralized evaluations offer localized supervision, they frequently result in longer approval timelines, complicating the initiation process.
bioaccess® advocates for a strategic approach, recommending the selection of the most effective review process tailored to the unique needs of each study. This method optimizes the balance between oversight and efficiency, addressing key challenges in the Medtech landscape. By fostering collaboration and understanding the intricacies of ethics reviews, stakeholders can enhance the clinical research process.
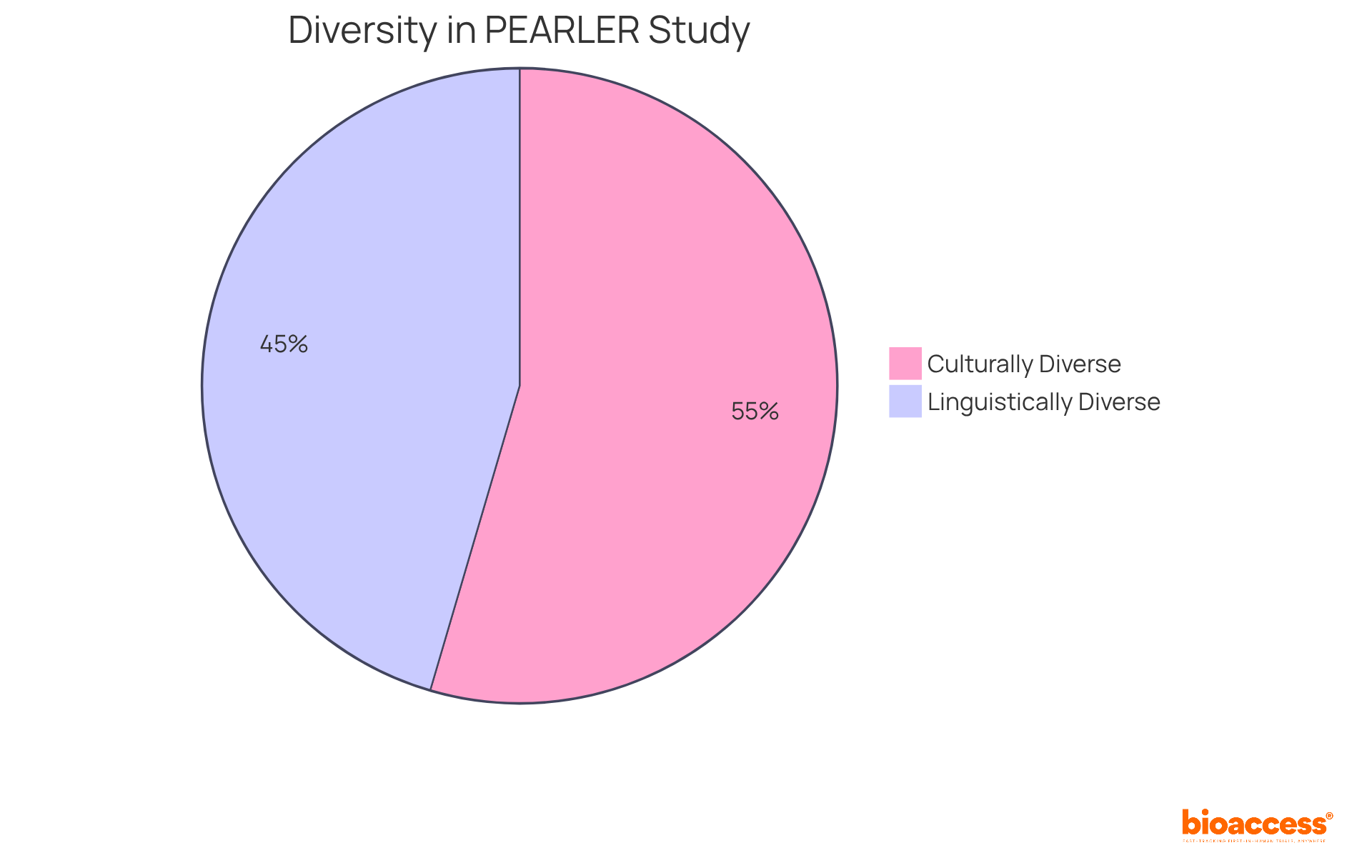
Access to varied patient groups is a crucial advantage of conducting studies in Australia. bioaccess® effectively leverages its extensive network to recruit participants from diverse backgrounds, ensuring that studies accurately reflect the population's diversity. This inclusivity not only strengthens the validity of research findings but also plays a pivotal role in developing treatments that are effective across various demographic groups.
For instance, the PEARLER study revealed that:
This underscores the importance of representation in research. Engaging diverse populations can lead to improved patient outcomes, as treatments developed with a broad spectrum of participants are more likely to meet the needs of the entire community.
Researchers emphasize that enhancing inclusivity in medical studies is not merely an ethical obligation but a scientific necessity, as it ensures that research outcomes are generalizable and relevant to real-world populations. By promoting diversity in recruitment, bioaccess® significantly contributes to advancing medical research that is reflective of and responsive to the needs of all patients.
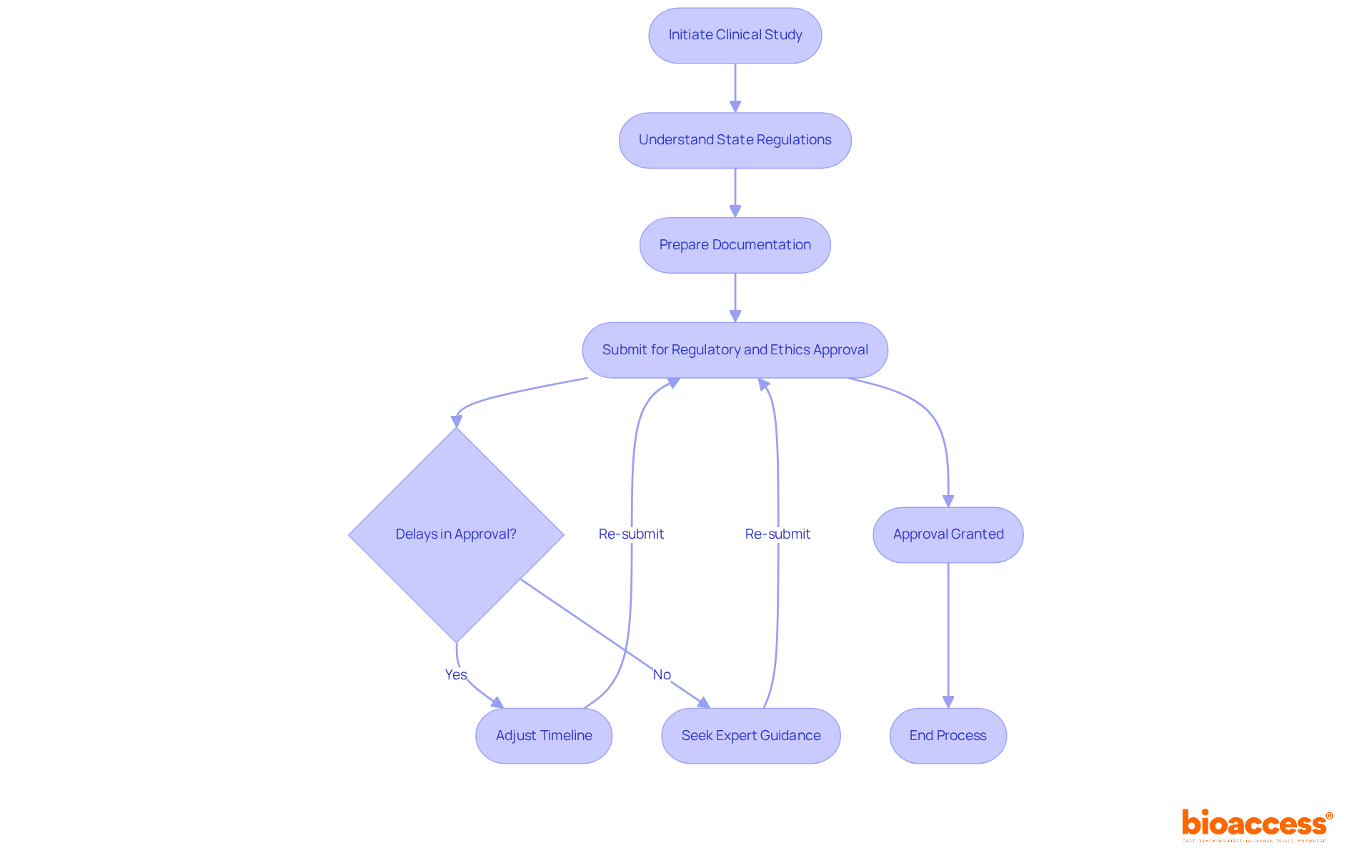
Conducting clinical studies in Australia, especially when considering the challenges of combining regulatory and ethics approval in Australia, presents a wealth of benefits. Companies often face varying state regulations that complicate the validation process. For instance, statistics reveal that delays in combining regulatory and ethics approval in Australia can significantly extend timelines, with some studies experiencing delays of up to 12 weeks. Such setbacks can impede the momentum of innovative therapies eager to enter the market swiftly. Additionally, the necessity for thorough documentation introduces another layer of complexity, requiring meticulous preparation to meet the stringent standards established by the Therapeutic Goods Administration (TGA).
To effectively navigate these challenges, bioaccess® offers expert guidance that emphasizes combining regulatory and ethics approval in Australia’s unique regulatory landscape. By leveraging our extensive experience, we assist clients in anticipating potential roadblocks and streamlining the approval process. As one regulatory expert noted, "Understanding the nuances of state regulations is crucial for successful execution in Australia." With our support, clients can ensure that their evaluations progress smoothly and efficiently, ultimately accelerating the journey to market for groundbreaking medical innovations.
Accelerating the authorization process for clinical trials in Australia requires combining regulatory and ethics approval in Australia. At bioaccess®, we prioritize forging strong connections with these entities, fostering open dialogue that clarifies regulatory requirements and effectively addresses moral considerations. This collaboration is crucial for achieving quicker consents; studies indicate that expedited assessments can be completed an average of 44 days faster than full-board evaluations. For instance, our partnerships have enabled approvals in as little as 4-6 weeks, significantly enhancing the pace of trial initiation.
Industry leaders assert that such collaboration not only streamlines processes but also builds trust among stakeholders, ensuring that both regulatory compliance and ethical integrity are maintained. As the landscape of medical research evolves, the significance of combining regulatory and ethics approval in Australia remains paramount, driving innovation and improving patient outcomes. By working together, we can navigate the complexities of clinical research more effectively, ultimately benefiting all parties involved.

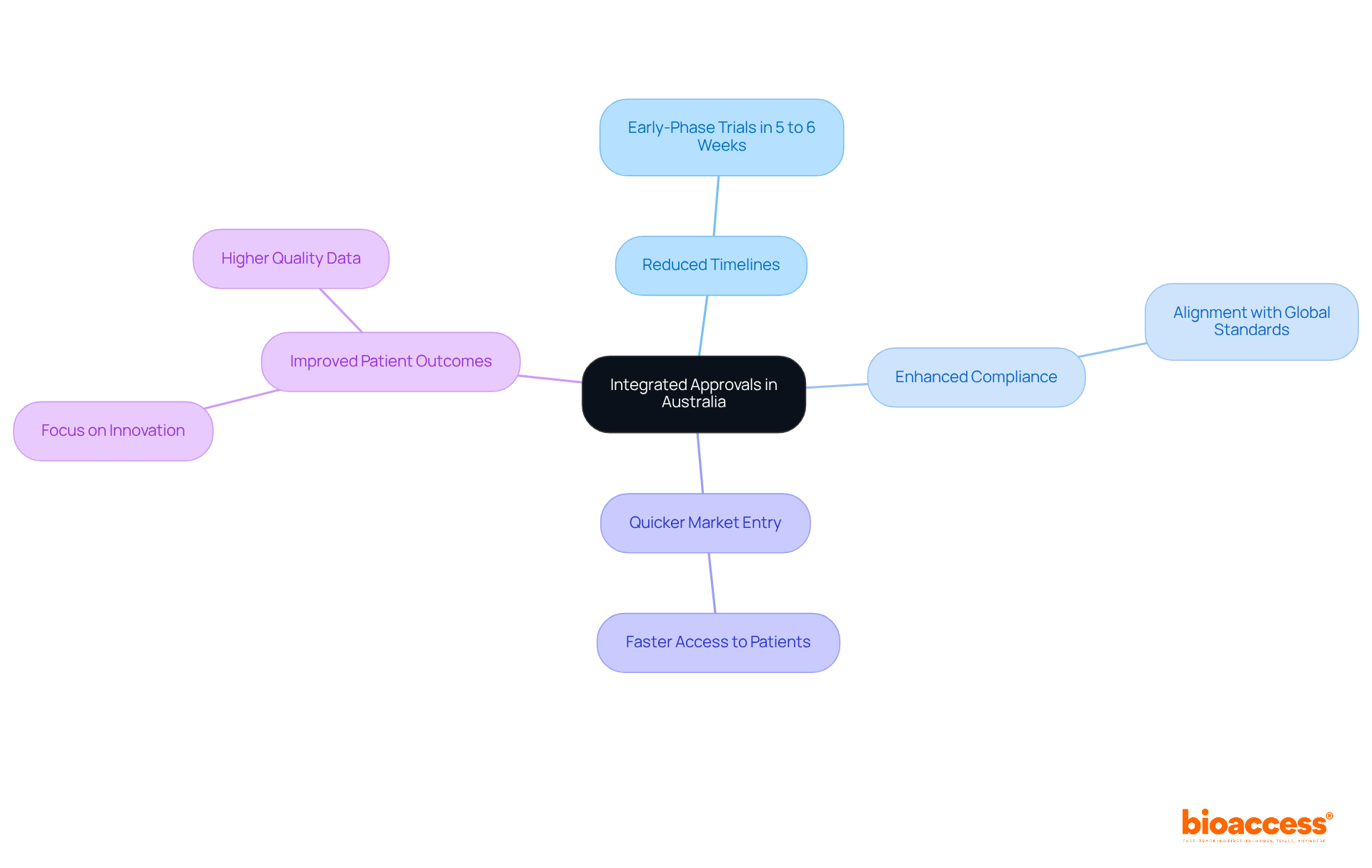
Combining regulatory and ethics approval in Australia offers substantial advantages for clinical study sponsors. This streamlined approach significantly reduces timelines, enabling companies to initiate early-phase trials within just 5 to 6 weeks - much quicker than in other regions. Notably, statistics reveal that approximately 85% to 90% of New Drug Applications (NDAs) submitted to the FDA receive authorization, underscoring the effectiveness of a well-structured regulatory framework.
Medtech leaders have observed that these streamlined processes not only enhance compliance with global standards but also cultivate a more agile research environment. Companies utilizing unified endorsements have reported quicker market entry and improved patient outcomes, especially when combining regulatory and ethics approval in Australia, allowing them to focus on innovation rather than navigating complex regulatory landscapes.
Real-world examples further illustrate the success of this approach. Avance Clinical, a prominent player in the CRO sector, has guided various biotech companies through the intricacies of trials, demonstrating how combined endorsements can boost operational efficiency. By combining regulatory and ethics approval in Australia, bioaccess® empowers innovators to deliver safe and effective medical products to market, ultimately benefiting patients and healthcare systems alike.
As Australia continues to refine its regulatory landscape, the benefits of integrated approvals will only become more pronounced, positioning the country as a leader in clinical research and development by 2025.
The integration of regulatory and ethics approval in Australia is a pivotal strategy for enhancing the efficiency of clinical research. This streamlined approach not only accelerates the authorization process but also fosters a collaborative environment that prioritizes compliance and ethical integrity. By leveraging the expertise of organizations like bioaccess®, innovators can navigate the complexities of regulatory frameworks with greater agility, ultimately reducing the time to market for groundbreaking medical products.
Key advantages of combining these approval processes include:
Moreover, the financial incentives available through Australia’s R&D tax rebate empower organizations to innovate without the burden of excessive costs. The emphasis on ethical considerations ensures that patient safety remains at the forefront of research initiatives, reinforcing trust and credibility in the outcomes.
As the landscape of clinical research continues to evolve, embracing integrated regulatory and ethics approvals will be essential for driving innovation and improving patient outcomes. Stakeholders should consider the benefits of this approach, not only for expediting their research endeavors but also for contributing to a more effective and ethical healthcare system. By collaborating effectively, the potential for advancing medical research in Australia is vast, paving the way for a future where innovation and patient safety coexist harmoniously.
What is bioaccess® and how does it streamline the regulatory and ethics approval process in Australia?
bioaccess® is a service that combines regulatory and ethics approval processes in Australia, leveraging local knowledge and international standards to facilitate swift study initiation, typically within 4 to 6 weeks.
Why is the efficiency of the approval process important for Medtech, Biopharma, and Radiopharma innovators?
The efficiency is crucial for these innovators as it allows them to accelerate their research and development timelines, minimizing bureaucratic delays and enabling rapid responses to market demands and regulatory requirements.
What services does bioaccess® provide to help organizations expedite their time to market?
bioaccess® offers comprehensive research management services, including feasibility studies, site selection, compliance assessments, setup, import permits, project management, and reporting, allowing organizations to secure authorizations in just 4-6 weeks.
How does bioaccess®'s approval timeline compare to traditional markets like the US and EU?
The approval timeline of 4-6 weeks with bioaccess® is significantly shorter than the typical 6-9 months seen in traditional markets like the US and EU.
What advantages do organizations gain by using bioaccess® for their approval processes?
Organizations benefit from improved operational agility, reduced costs, and the ability to seize emerging market opportunities more effectively, which provides a competitive edge in the healthcare landscape.
How does bioaccess® ensure compliance with global standards and regulations?
bioaccess® emphasizes adherence to international regulations and moral standards, ensuring that all trials meet high scientific and ethical benchmarks, which streamlines the approval process.
What is the typical timeframe for achieving regulatory approval through bioaccess®?
bioaccess® typically achieves regulatory approval in just 6-8 weeks.
Why is adherence to ethical standards important in medical research?
Upholding ethical standards fosters trust and credibility in research results, protects participant well-being, enhances marketability, and ultimately leads to more successful studies and quicker access to innovative treatments.
How does the evolving regulatory framework in Australia impact market access for innovators?
As Australia enhances its regulatory framework, including tighter requirements for electronic Common Technical Documents (eCTD), the potential for quicker market access is expected to grow, making Australia an attractive destination for Medtech and Biopharma innovators.