


The article delineates ten significant benefits of decentralized clinical trials (DCTs) that are instrumental in achieving expedited research outcomes. These benefits include:
By leveraging technology, DCTs streamline processes, enhance participant access, and foster greater diversity in recruitment. This ultimately culminates in more efficient and effective clinical research, underscoring the transformative potential of DCTs in the Medtech landscape.
Decentralized clinical trials (DCTs) are revolutionizing the field of medical research, presenting innovative solutions to longstanding challenges in participant recruitment and retention. By harnessing technology and digital tools, DCTs not only accelerate the research process but also improve accessibility for a broader and more diverse participant pool.
However, as the industry embraces this transformative shift, critical questions emerge:
This article delves into the extensive benefits of DCTs, illustrating how they can facilitate quicker research outcomes while adeptly navigating the complexities of regulatory compliance and participant engagement.
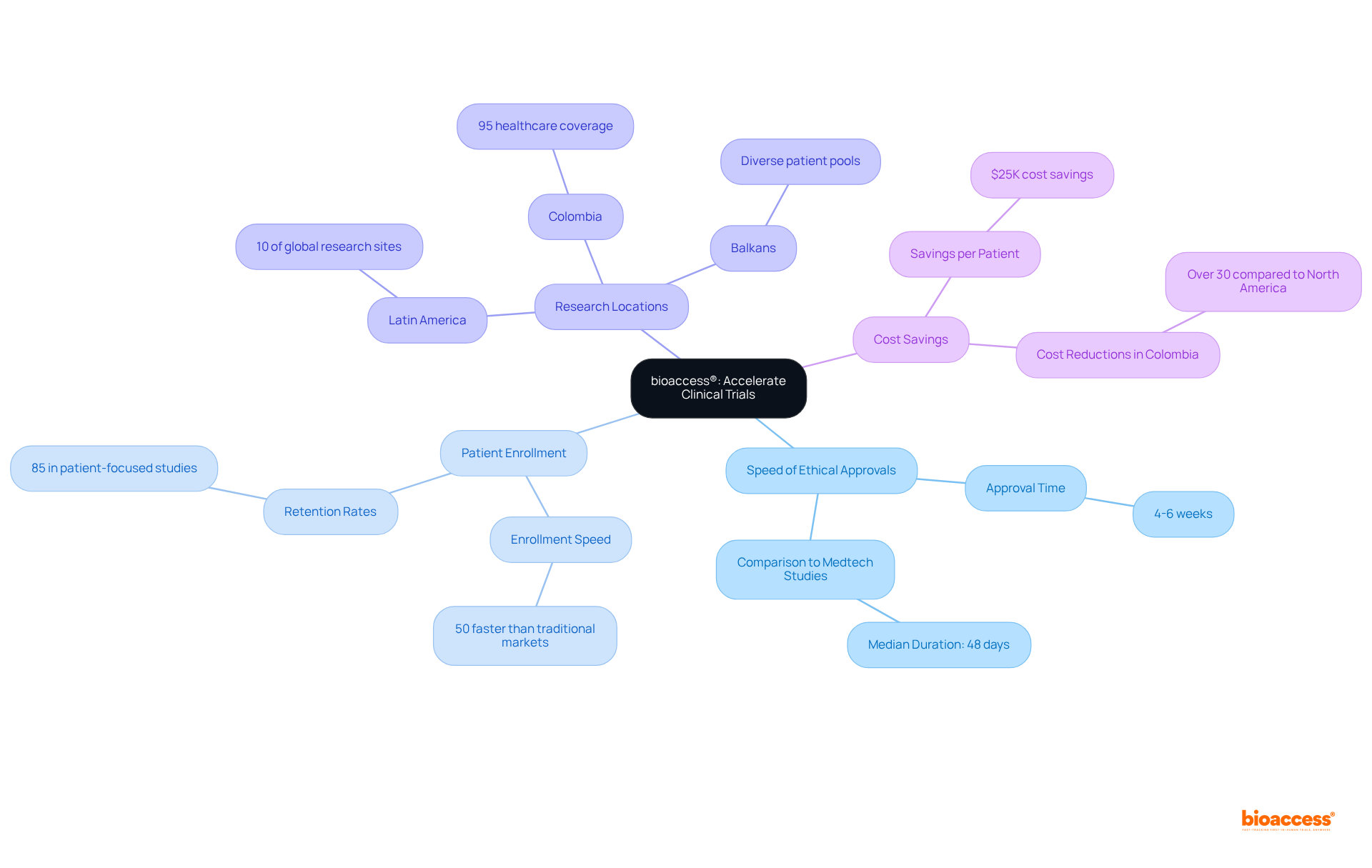
bioaccess® leverages its extensive expertise in the regulatory landscapes of Latin America, the Balkans, and Australia to facilitate rapid ethical approvals and expedited patient enrollment. This global-first agility empowers Medtech, Biopharma, and Radiopharma innovators to conduct research studies with exceptional speed, ensuring that new therapies reach the market efficiently.
This innovative approach demonstrates how decentralized health studies can optimize research schedules and outcomes, ultimately accelerating the development of medical devices and treatments. As industry leaders have noted, the potential cost savings of $25K per patient through expedited enrollment further highlight the efficiency of bioaccess®'s processes.
Decentralized medical studies empower individuals to participate in research from the comfort of their homes or local healthcare facilities, significantly alleviating travel challenges and time constraints. This convenience not only attracts a broader spectrum of participants but also cultivates a more inclusive environment, allowing those who previously faced barriers to engage in clinical research.
By harnessing digital tools and telehealth solutions, dct clinical trials improve accessibility and streamline the participant experience. Notably, studies incorporating decentralized trial elements have reported enrollment rates that are 50% faster than traditional methods, a statistic that aligns with bioaccess®'s achievement of enhancing patient enrollment rates by the same margin.
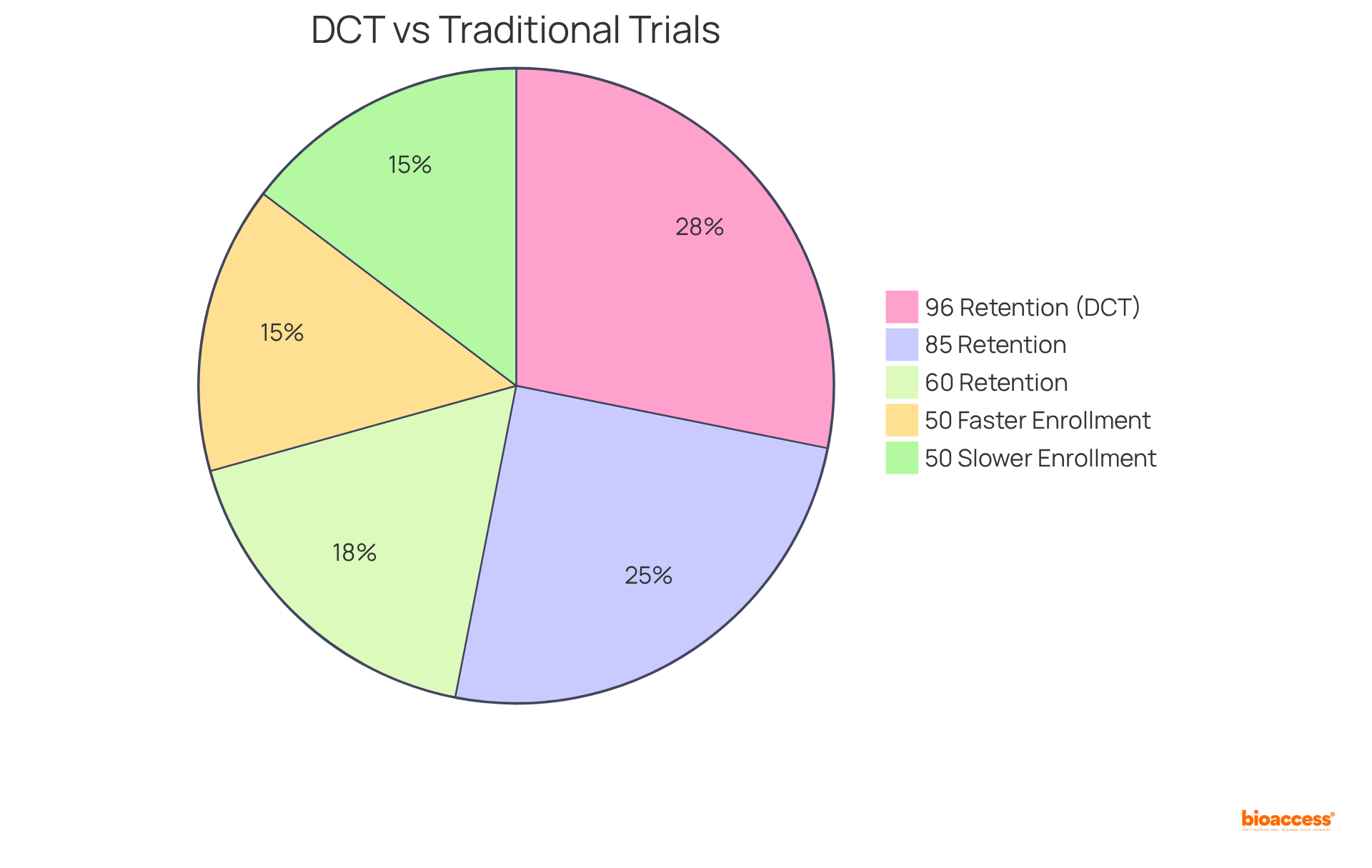
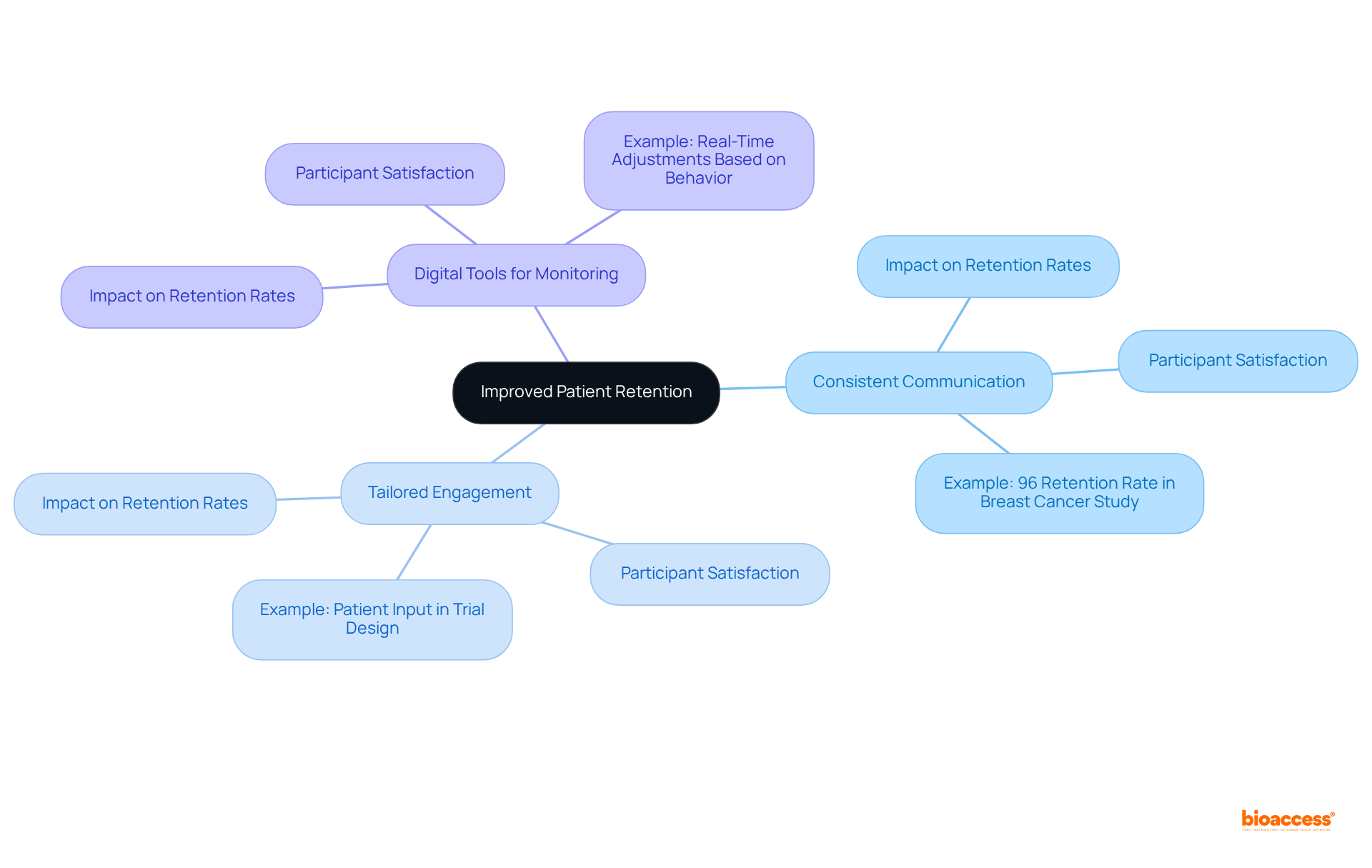
Furthermore, retention rates in decentralized clinical studies can soar to as high as 85%, compared to 60% in conventional settings, with some fully decentralized studies, such as a Phase 4 breast cancer investigation, achieving an impressive 96% retention rate. This transformation not only leads to increased enrollment but also enhances data quality, positioning dct clinical trials as a crucial advancement in research.
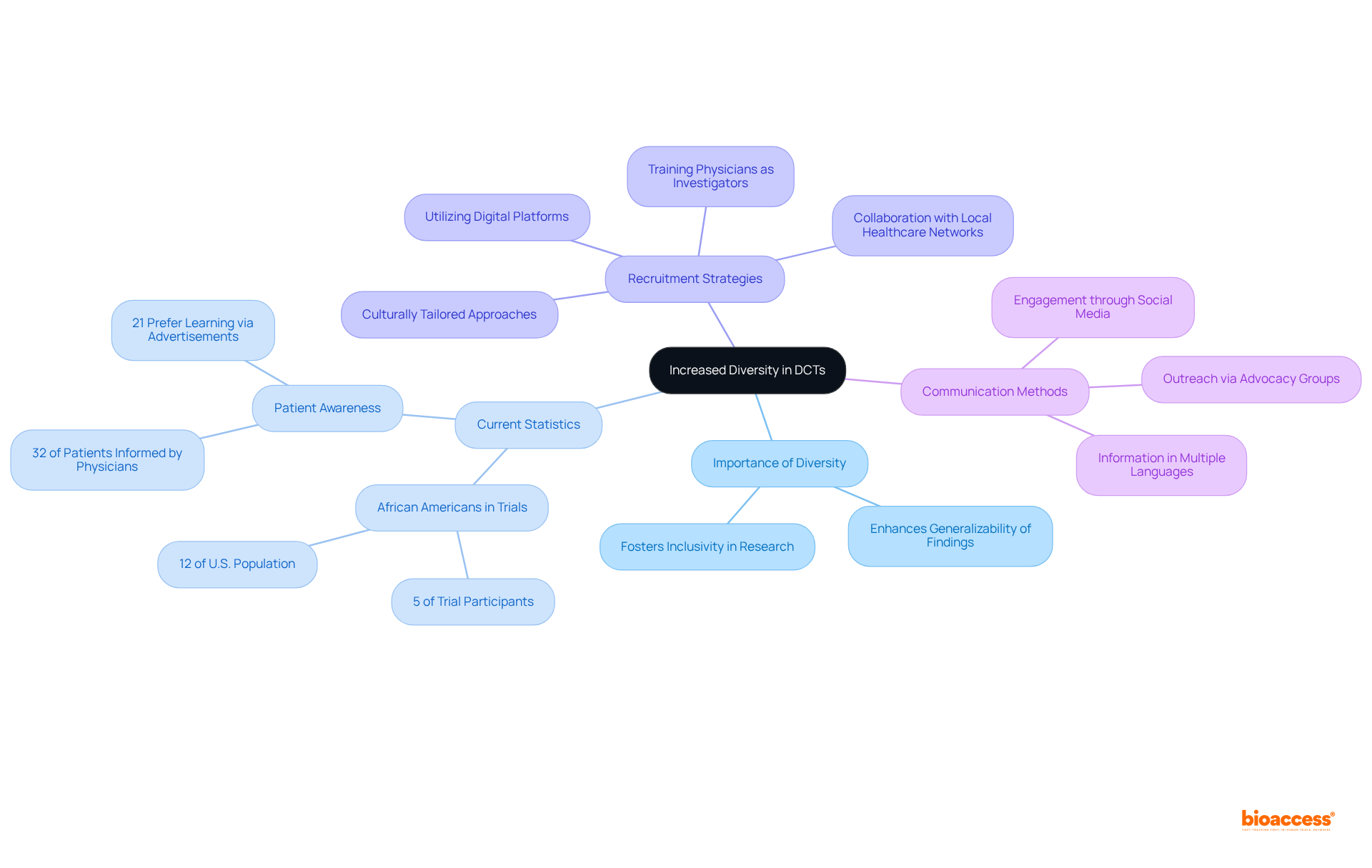
DCT clinical trials are pivotal in enhancing the recruitment of diverse demographics by eliminating geographical barriers and leveraging technology to engage underrepresented communities. This innovative approach not only fosters inclusivity in research but also enhances the generalizability of findings. For instance, research indicates that African Americans, who constitute 12% of the U.S. population, represent merely 5% of trial participants, underscoring the urgent need for improved representation.
To effectively attract a broader spectrum of individuals, DCTs implement culturally tailored recruitment strategies and collaborate with local healthcare networks. This approach ensures that medical research mirrors the demographics of the wider population, which is essential for developing treatments that are effective across various demographic groups. Furthermore, predictive analytical models can pinpoint sites that fulfill both enrollment and diversity objectives, facilitating a more nuanced recruitment strategy.
As the sector evolves, it is imperative to adopt strategies that prioritize diversity in research studies. This includes:
Notably, only 32% of patients reported that their physicians had ever provided information about research studies, highlighting a significant communication gap that necessitates attention. Moreover, 21% of patients prefer to learn about trials through advertisements, emphasizing the need for varied communication methods. By implementing these strategies, DCT clinical trials can substantially improve the representation of diverse populations in clinical research, ultimately leading to more equitable healthcare solutions.
Digital care teams employ a range of strategies to enhance patient retention, including:
By providing individuals with easy access to study information and resources, along with offering flexible involvement options, dct clinical trials significantly improve the experience for participants. This proactive approach not only keeps individuals engaged throughout the trial but also reduces dropout rates, thereby ensuring that the study maintains its statistical power and integrity.
Trials designed with genuine patient input achieve higher recruitment rates, better retention, and more meaningful results. As Ingrid Oakley-Girvan, PhD, MPH, notes, effective engagement requires a nuanced understanding of the needs of participants. This can be accomplished through the thoughtful application of digital health technologies (DHTs), which streamline communication and facilitate real-time adjustments based on participant behavior.
A notable example is a decentralized Phase 4 breast cancer study that achieved a remarkable 96% patient retention rate, underscoring the effectiveness of dct clinical trials in enhancing retention rates. Additionally, dct clinical trials utilizing decentralized technologies present significant cost-saving opportunities across various phases of research, further amplifying their appeal in medical studies.
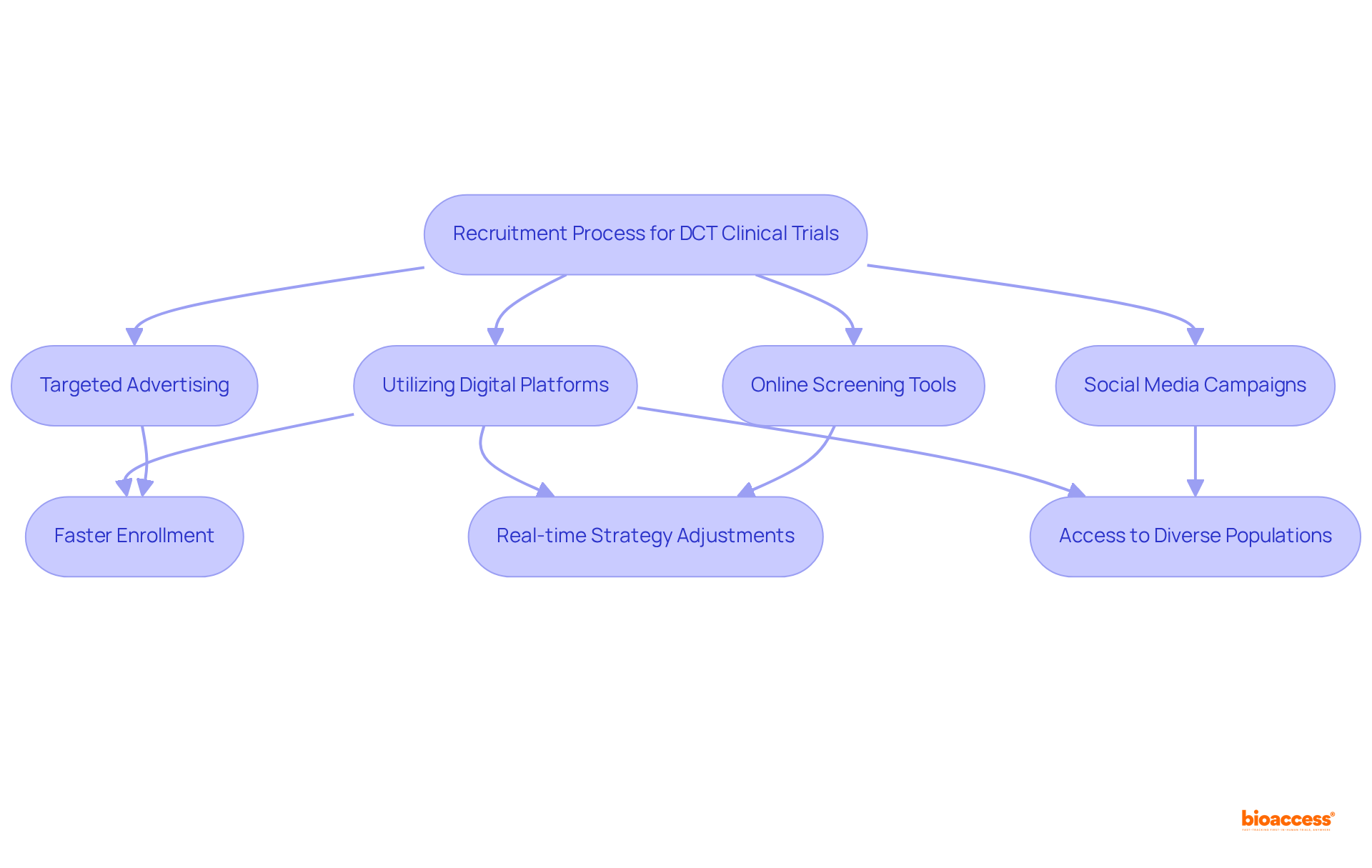
DCT clinical trials represent a transformative approach in clinical research, leveraging technology to enhance recruitment efforts. By utilizing digital platforms for effective outreach and engagement, researchers can swiftly identify and enroll qualified individuals through targeted advertising, social media campaigns, and online screening tools. This integration of technology not only accelerates the recruitment process but also allows for real-time adjustments to strategies based on participant feedback and engagement metrics. Consequently, DCT clinical trials achieve enrollment objectives more effectively than conventional site-based studies, significantly shortening the overall timeline for research.
With over 80% of clinical trials encountering delays due to recruitment challenges—costing approximately $1.89 billion, or 40% of the total budget—adopting digital platforms has become essential. For instance, platforms like Accelerated Enrollment Solutions offer access to a database of 100 million patients, including 20 million prescreened individuals, facilitating quicker access to potential participants. The COVID-19 pandemic has further underscored the significance of DCT clinical trials, showcasing their feasibility and advantages in reaching diverse populations. As a result, decentralized technologies are revolutionizing recruitment methods and enhancing the overall effectiveness of research studies.
Notably, bioaccess® enables enrollment 50% faster than traditional markets, highlighting the benefits of DCT clinical trials. This evolution in clinical research not only addresses existing challenges but also paves the way for more efficient and inclusive studies moving forward.
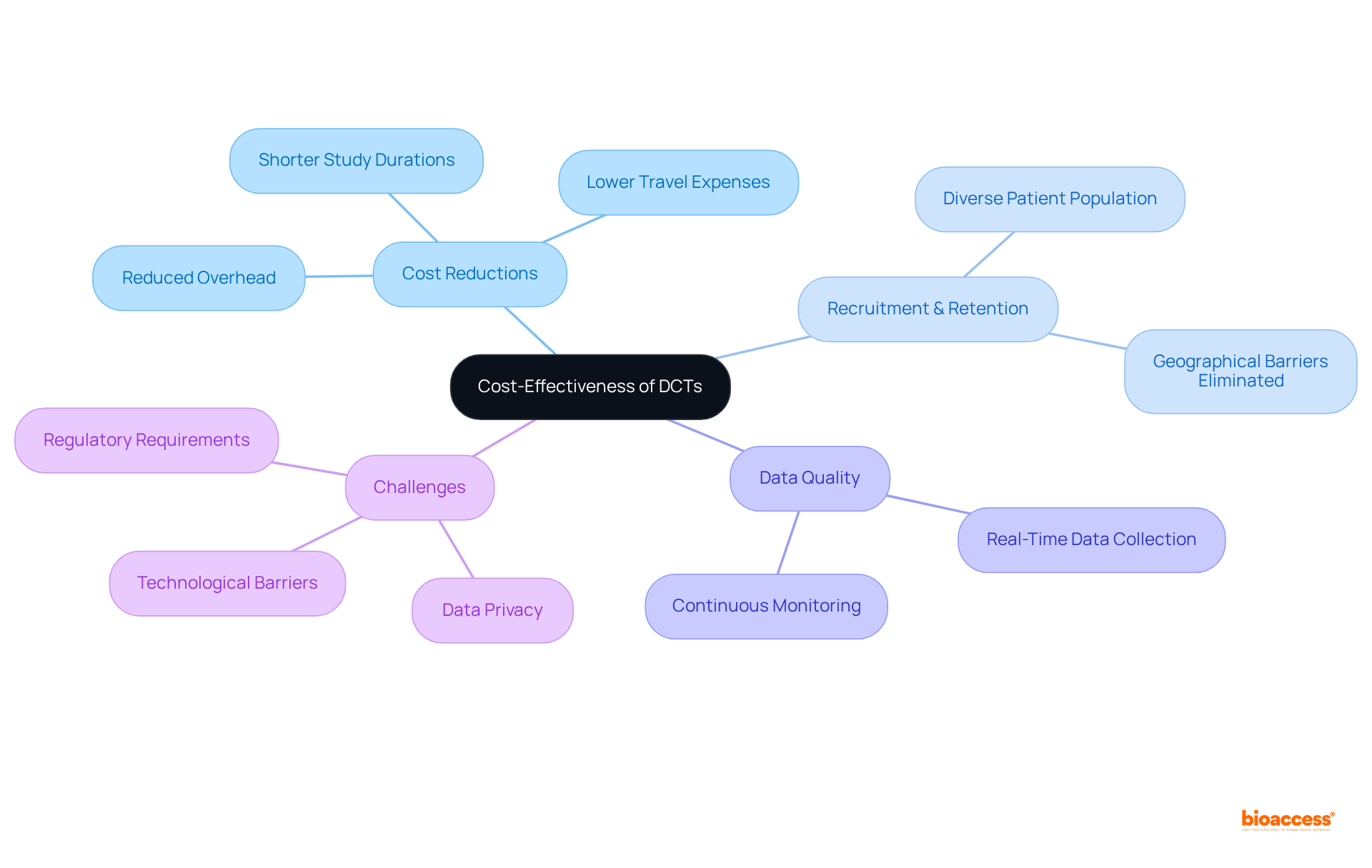
DCT clinical trials present a significant opportunity for sponsors to achieve considerable cost reductions by minimizing the need for physical locations and their associated overhead costs. By conducting experimental activities in participants' residences or nearby centers, these trials substantially decrease travel expenses and logistical challenges. Moreover, DCT clinical trials improve recruitment and retention rates, facilitating a more diverse patient population by eliminating geographical barriers. The expedited recruitment and retention rates that characterize decentralized clinical studies lead to shorter study durations, further contributing to overall cost reduction.
Additionally, these trials enhance data quality through continuous, real-time data collection, enabling more precise tracking of patient progress. This financial efficiency empowers sponsors to allocate resources more strategically, optimizing the effectiveness of their research budgets. As the pharmaceutical sector grapples with escalating research and development expenses, DCT clinical trials emerge as a viable solution, potentially conserving 5% to 30% or more in study expenditures while fostering a more inclusive and patient-centric research environment.
However, it is crucial to address the challenges posed by digital communication technologies, such as data privacy and regulatory requirements, to ensure successful implementation.
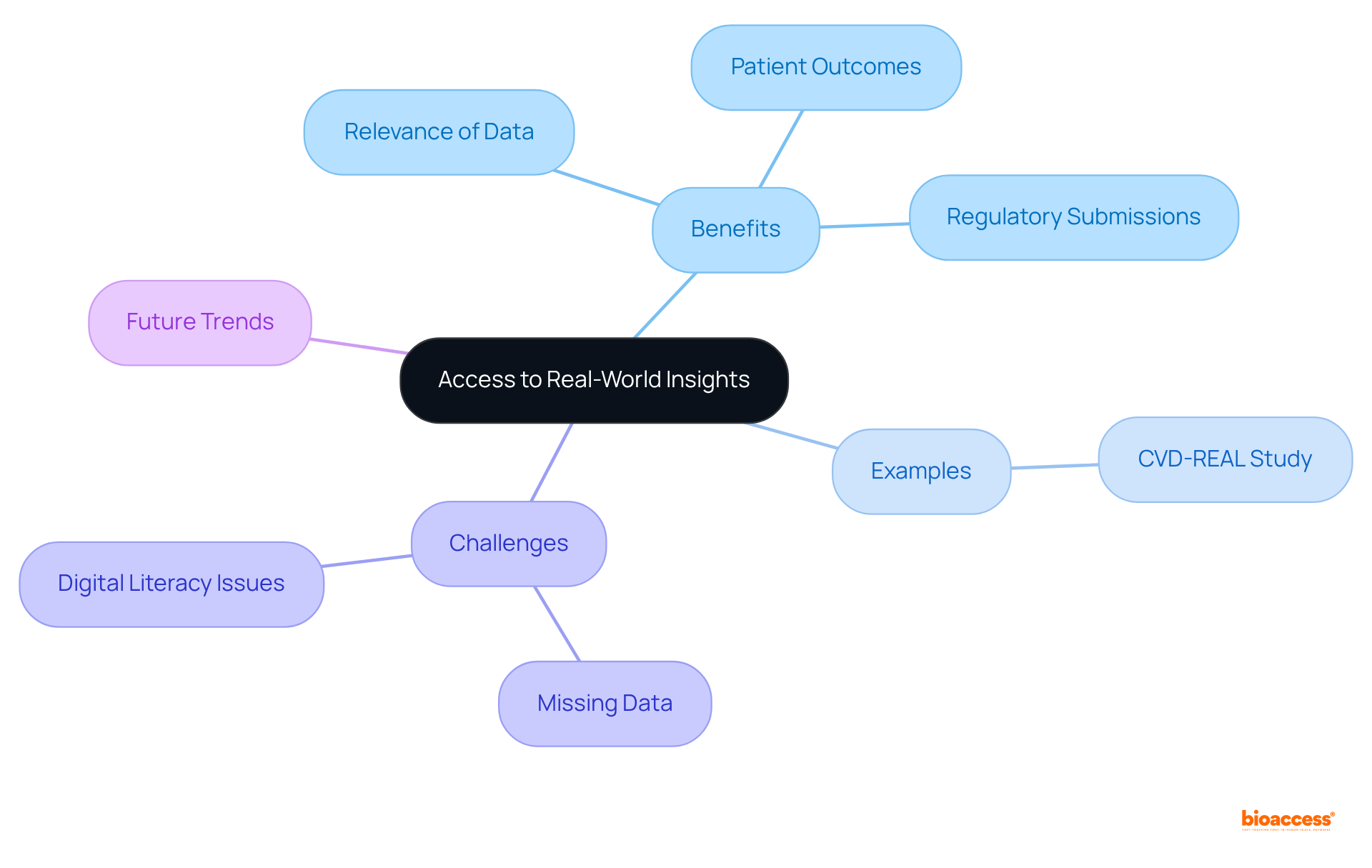
Decentralized research trials revolutionize the collection of real-world information by enabling individuals to participate in studies within their everyday environments. This approach significantly enhances the relevance of the data gathered, as it reflects the true experiences and behaviors of participants. By integrating real-world insights into clinical research, dct clinical trials offer a more comprehensive understanding of treatment effects and patient outcomes. Such enriched data is vital for regulatory submissions and can inform future research initiatives, ultimately leading to more effective therapies.
For example, studies like the CVD-REAL, which involved over 300,000 patients, illustrate how real-world evidence can uncover substantial treatment benefits that traditional randomized controlled trials (RCTs) may overlook.
Nevertheless, challenges persist, such as the statistic indicating that 50% of diary entries may be missing, complicating data interpretation. Additionally, the COVID-19 pandemic has accelerated the adoption of dct clinical trials, highlighting their ability to gather diverse and representative data while alleviating the burden on participants.
As organizations increasingly recognize the importance of these methodologies, the integration of digital clinical tools into research is expected to grow, enhancing the overall quality and relevance of health data. Furthermore, the EU Clinical Trials Regulation may lead to a more standardized assessment process for dct clinical trials, ensuring that the benefits of these studies are maximized while addressing potential challenges, such as the exclusion of individuals lacking digital literacy.
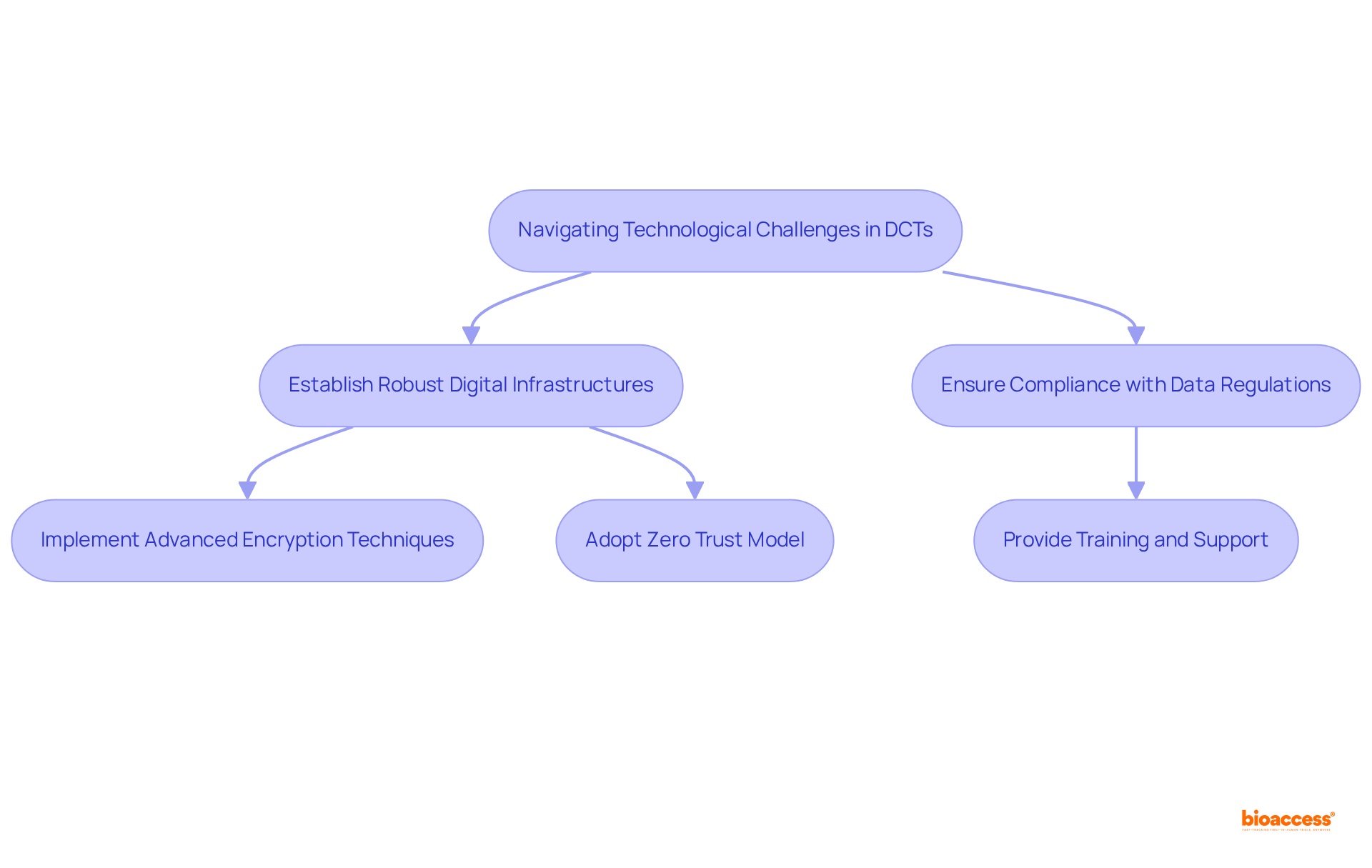
DCT clinical trials present significant advantages, yet they also introduce technological challenges that must be effectively managed to ensure successful implementation. Key issues such as data security, user privacy, and the digital divide can impede the effectiveness of DCT clinical trials.
To navigate these complexities, researchers must prioritize the establishment of robust digital infrastructures while ensuring compliance with evolving data protection regulations. This includes:
Moreover, providing comprehensive training and support for participants is essential to enhance their understanding of data privacy measures. As emphasized by industry specialists, organizations must remain vigilant against cyber threats, particularly as the volume of data produced in research studies continues to rise.
By proactively addressing these obstacles, DCT clinical trials can unlock their full potential, which leads to faster enrollment and improved research outcomes, ultimately benefiting the entire research landscape.
Regulatory compliance stands as a cornerstone in decentralized clinical studies, essential for preserving the integrity and ethical conduct of research. These studies must adhere to the same rigorous regulatory requirements as traditional studies, encompassing:
By establishing robust compliance frameworks and proactively engaging with regulatory authorities during the trial design phase, researchers can adeptly navigate the complexities of DCT clinical trials while upholding the highest ethical standards. This unwavering commitment to adherence not only safeguards the individuals involved but also bolsters the credibility of research outcomes.
Specialists in regulatory affairs emphasize that maintaining ethical standards is vital for fostering trust among participants and the broader public, ultimately enhancing the overall integrity of research studies. Furthermore, with approximately $1.89 billion earmarked for patient recruitment within the pharmaceutical research budget in the United States, the importance of adhering to regulatory standards becomes even more pronounced.
Katherine Ruiz aptly states, "It’s the balance that we need to strike between technology and human interaction," underscoring the necessity for a comprehensive approach to compliance that incorporates robust pharmacovigilance systems to monitor safety throughout the studies.
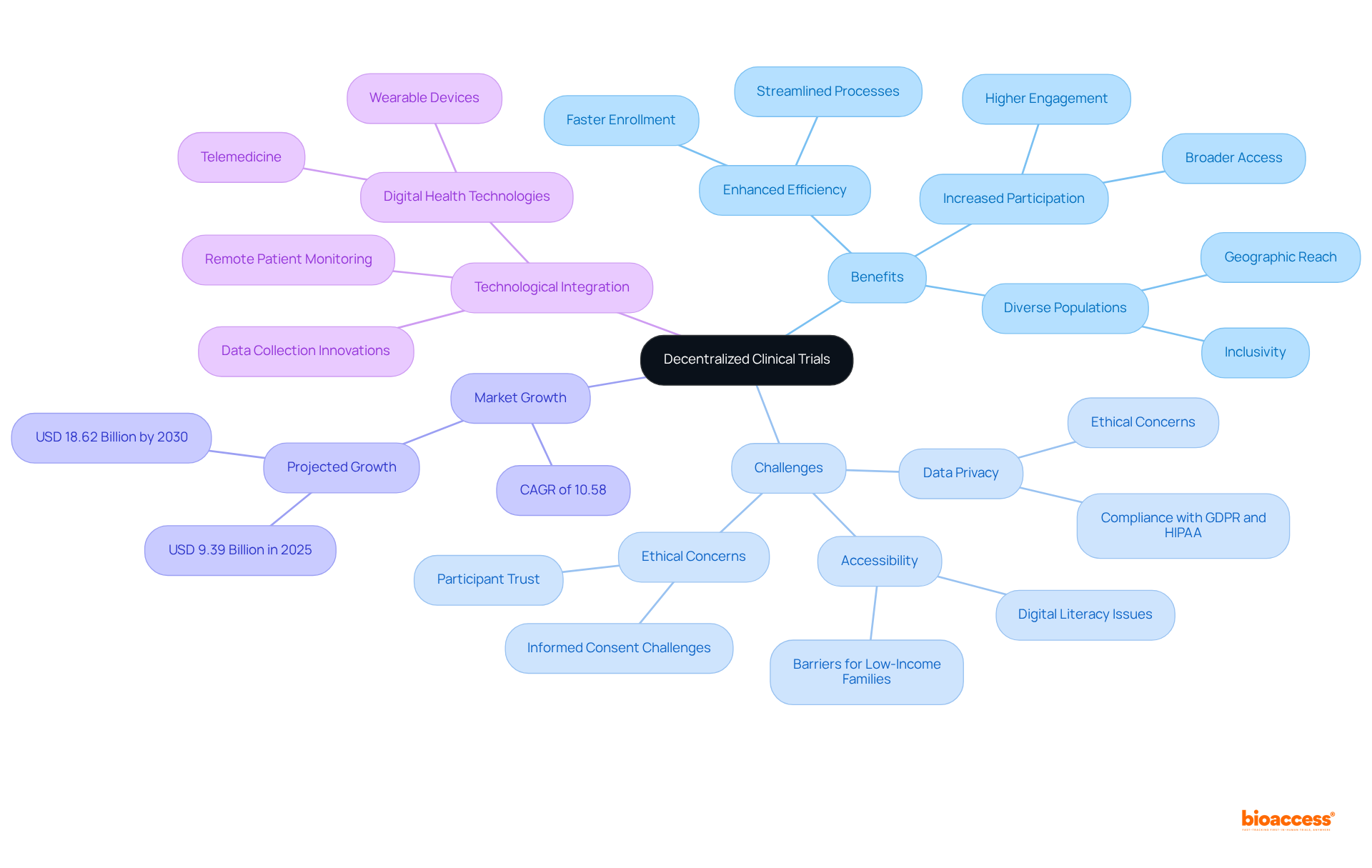
The future of medical studies is progressively directed towards decentralization, driven by technological advancements and an increased emphasis on patient-focused research. As DCT clinical trials evolve, they are positioned to significantly enhance study efficiency, boost participant involvement, and broaden access to diverse populations. Notably, the decentralized research market is projected to grow from USD 9.39 billion in 2025 to USD 18.62 billion by 2030, reflecting a compound annual growth rate of 10.58%. This expansion is fueled by the integration of digital health technologies, such as telemedicine and wearable devices, which facilitate remote patient monitoring and data collection.
Moreover, DCT clinical trials empower researchers to streamline processes, which reduces logistical barriers and accelerates enrollment by up to 50% compared to traditional methods. This transformation not only fosters inclusivity but also ensures that research studies are more representative of the populations they aim to serve. However, it is crucial to address ethical concerns surrounding patient data privacy and security, as well as potential obstacles to participation for low-income families and individuals with limited computer literacy. Adherence to data protection regulations like GDPR and HIPAA is essential for maintaining participant trust and upholding the integrity of the research process.
As Dr. Shaalan Beg, a prominent figure in medical research, notes, "Patients and individuals with cancer are highly inclined to participate in a research study if they are merely approached." This statement underscores the significance of outreach and accessibility in enhancing trial participation. The ongoing shift towards decentralization is set to redefine the clinical research landscape, paving the way for more effective and timely DCT clinical trials. By leveraging technology, researchers can amass extensive data, improve patient engagement, and ultimately drive better health outcomes.
Decentralized clinical trials (DCTs) signify a transformative shift in the landscape of medical research, presenting substantial advantages that enhance the efficiency and inclusivity of studies. By leveraging technology, DCTs expedite ethical approvals, foster improved patient engagement, and broaden participant access, ultimately resulting in quicker and more diverse research outcomes. This innovative approach not only addresses traditional barriers in clinical trials but also optimizes the overall research process, ensuring that new therapies can reach the market promptly.
The article underscores several key benefits of DCTs, such as:
With the capability to conduct studies remotely, DCTs dismantle geographical barriers, enabling researchers to gather real-world insights that accurately reflect the experiences of a diverse patient population. Furthermore, the cost-effectiveness of DCTs allows sponsors to allocate resources more efficiently, thereby enhancing the viability of these trials.
As the clinical research landscape continues to evolve, the adoption of decentralized methodologies is crucial for fostering inclusivity and improving health outcomes. Embracing DCTs not only streamlines the research process but also ensures that studies are representative of the populations they aim to serve. By prioritizing accessibility, diversity, and patient engagement, the future of clinical trials can be shaped into a more efficient, equitable, and effective system that ultimately benefits all stakeholders involved.
What is bioaccess® and what does it offer for clinical trials?
bioaccess® is a platform that accelerates clinical trials by leveraging expertise in the regulatory landscapes of Latin America, the Balkans, and Australia. It facilitates rapid ethical approvals and expedited patient enrollment, allowing Medtech, Biopharma, and Radiopharma innovators to conduct research studies quickly and efficiently.
How fast can ethical approvals be secured through bioaccess®?
Ethical approvals can be secured in just 4-6 weeks, which is significantly faster than the median duration of 48 days for Medtech studies.
How does patient enrollment speed compare to traditional markets?
Patient enrollment through bioaccess® occurs at a pace that is 50% quicker than that of traditional markets.
What are the advantages of conducting medical research in Latin America?
Conducting medical research in Latin America, which represents 10% of the world's research sites, offers strategic advantages, including faster ethical approvals and patient enrollment.
What are the potential cost savings associated with expedited enrollment?
The potential cost savings can amount to $25,000 per patient through expedited enrollment processes.
What are decentralized clinical trials (DCTs) and how do they benefit participants?
Decentralized clinical trials allow individuals to participate in research from home or local healthcare facilities, alleviating travel challenges and time constraints. This convenience attracts a broader range of participants and creates a more inclusive environment for clinical research.
How does the enrollment rate in decentralized clinical trials compare to traditional methods?
Studies that incorporate decentralized trial elements have reported enrollment rates that are 50% faster than traditional methods.
What are the retention rates in decentralized clinical studies?
Retention rates in decentralized clinical studies can reach as high as 85%, compared to 60% in conventional settings, with some fully decentralized studies achieving retention rates of 96%.
How do DCTs enhance diversity in clinical trials?
DCTs enhance diversity by eliminating geographical barriers and using technology to engage underrepresented communities, ensuring that medical research reflects the demographics of the wider population.
What strategies are being implemented to improve participant diversity in clinical trials?
Strategies include training physicians without research experience to serve as investigators, providing information in multiple languages, and utilizing digital platforms for outreach, particularly among populations disproportionately affected by health conditions.
What communication gaps exist regarding research study information?
Only 32% of patients reported that their physicians had ever provided information about research studies, indicating a significant communication gap. Additionally, 21% of patients prefer to learn about trials through advertisements, highlighting the need for varied communication methods.