


This article presents ten key benefits of electronic data capture (EDC) for clinical research, including:
These advantages arise from the automation and integration of EDC systems, which:
Moreover, EDC systems significantly boost patient engagement and retention rates, underscoring their vital role in modern clinical research.
The landscape of clinical research is undergoing a significant transformation, driven by the rapid adoption of electronic data capture (EDC) solutions. With nearly 80% of clinical trials now leveraging these advanced systems, the shift from traditional methodologies is not merely a trend—it's a necessity for enhancing efficiency and accuracy in data management. This article delves into the ten key benefits of EDC, revealing how these technologies streamline processes, reduce errors, and ultimately improve patient outcomes. What challenges do organizations face in embracing this digital evolution, and how can they ensure they harness the full potential of EDC?
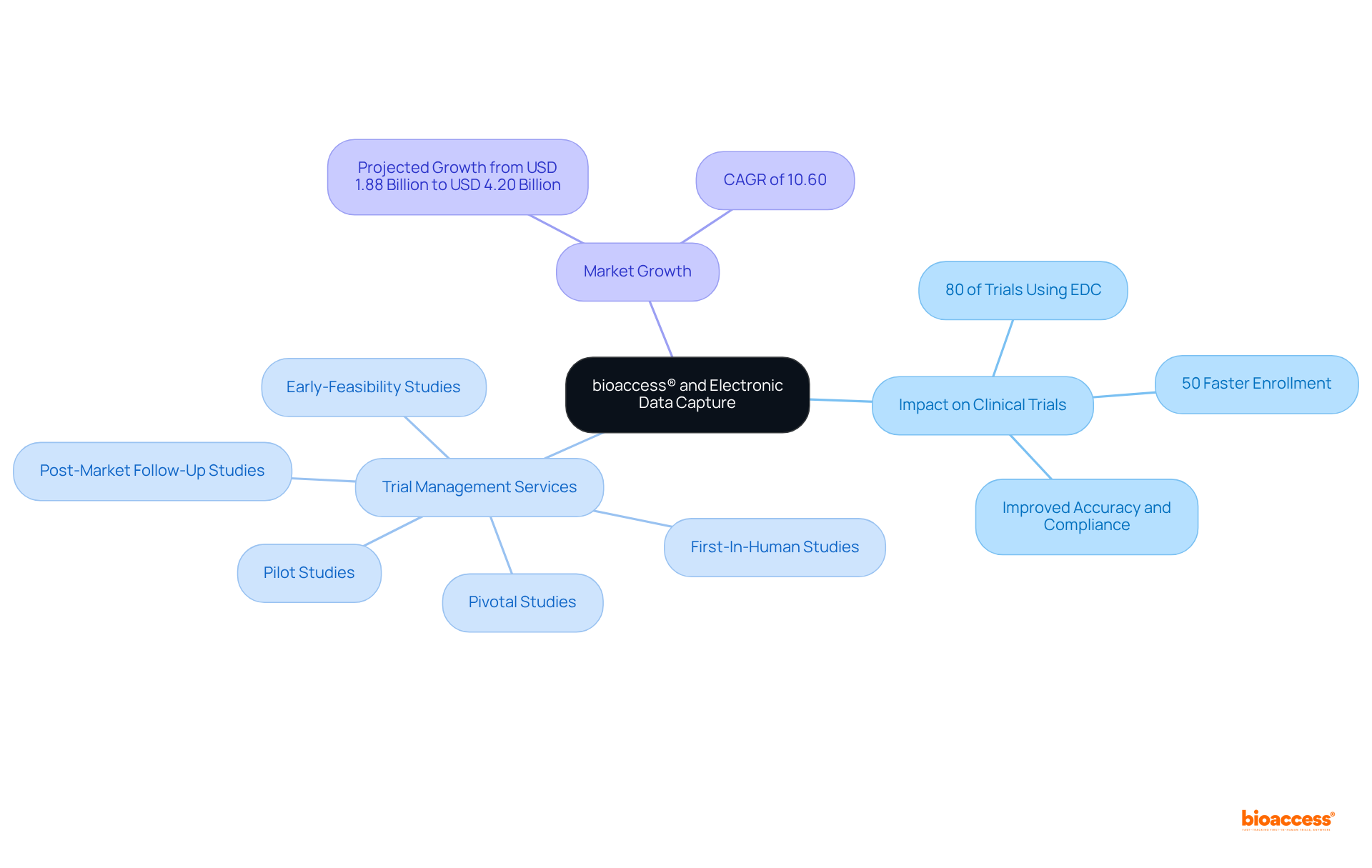
bioaccess® leverages electronic data capture (EDC) solutions to revolutionize research processes across Latin America, ensuring efficient and effective information management. The surge in electronic data capture adoption is notable, with nearly 80% of clinical trials now utilizing these systems, marking a substantial departure from traditional methodologies. By streamlining information collection and enhancing real-time access, bioaccess® not only expedites ethical approvals but also accelerates patient enrollment, achieving rates that are 50% faster than conventional markets. This efficiency not only shortens research timelines but also improves accuracy and regulatory compliance, positioning electronic data capture as an essential tool for innovators in Medtech, Biopharma, and Radiopharma.
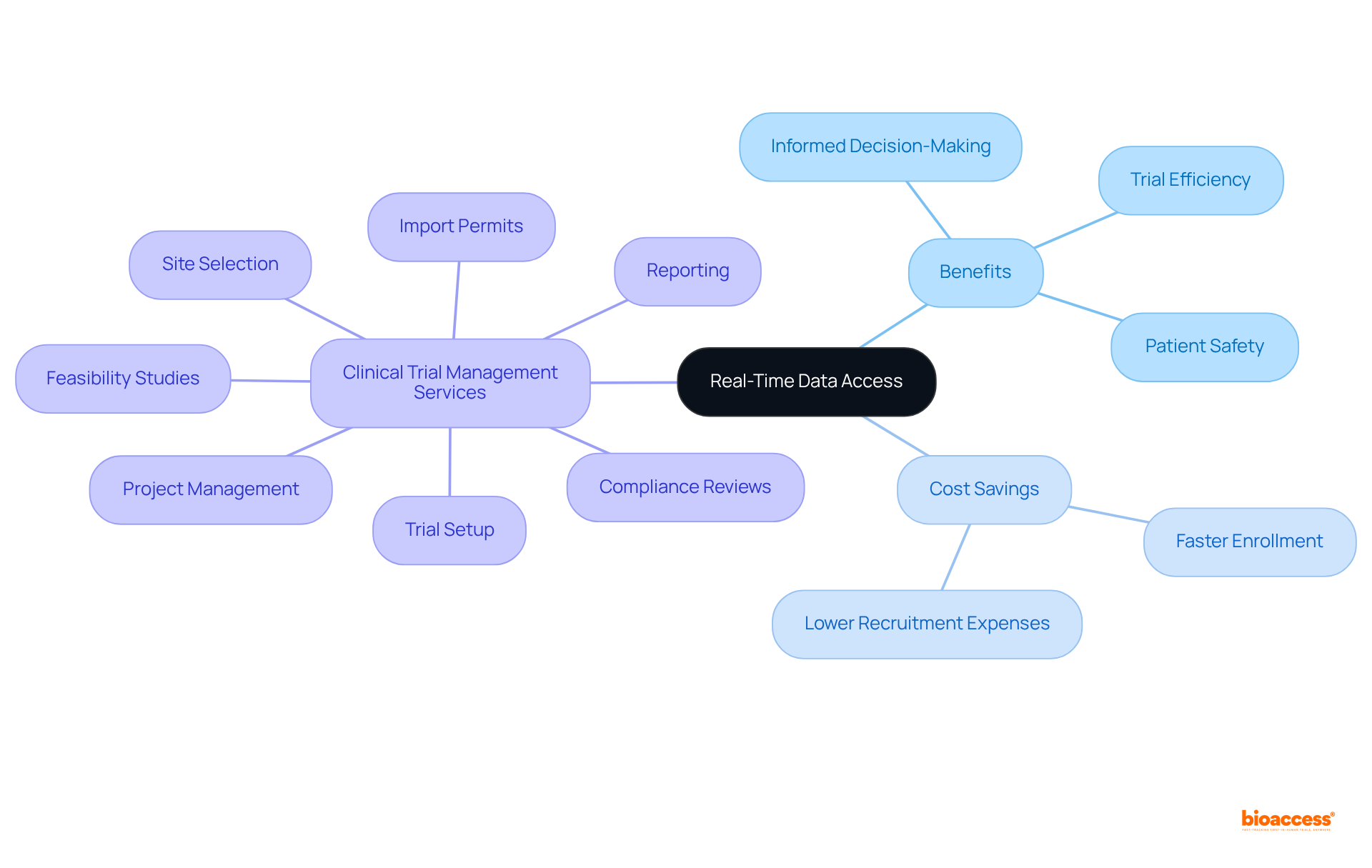
Furthermore, bioaccess® offers comprehensive trial management services, including:
This empowers clients to navigate the complexities of trials with unparalleled expertise. The global market for electronic data capture (EDC) is projected to grow from USD 1.88 billion in 2024 to USD 4.20 billion by 2032, driven by increasing demand for real-time information collaboration and the imperative for effective regulatory compliance. As industry leaders recognize the critical role of electronic data capture in streamlining trials, the integration of advanced technologies continues to enhance operational efficiency and patient engagement, solidifying the status of electronic data capture as a cornerstone of contemporary research.
Electronic data capture (EDC) frameworks revolutionize information gathering in clinical research by automating processes and significantly reducing reliance on manual input. This automation not only minimizes the time researchers dedicate to data collection but also allows them to concentrate on critical analysis and interpretation. For instance, electronic data capture systems facilitate real-time data entry, providing immediate access to information and eliminating delays typically associated with traditional paper-based methods.
The impact of manual data entry errors is considerable, with studies indicating that as much as 30% of records may be compromised due to human error. Peter Sondergaard, Senior Vice President and Global Head of Research at Gartner, aptly states, "information is often regarded as more valuable than oil, but like oil, it requires proper mining and analysis to generate meaningful insights." By automating electronic data capture, EDC systems significantly reduce these errors, thereby enhancing the overall integrity of clinical trials.
Clinical researchers have noted that automating data collection processes leads to improved accuracy and productivity. One researcher remarked that 'the transition to EDC has transformed our workflow, enabling us to focus on what truly matters—patient outcomes and data analysis.'
The advantages of using electronic data capture in clinical research are clear:
As the healthcare landscape evolves, with 70% of healthcare organizations actively considering the use of generative AI, the adoption of electronic data capture solutions will be essential for organizations aiming to enhance their research capabilities and deliver timely, high-quality outcomes.
A substantial benefit of electronic data capture systems is their ability for real-time information access. This feature empowers medical researchers to consistently oversee trial progress and make well-informed choices based on the latest data. With real-time insights, research teams can swiftly identify issues, adjust protocols, and enhance patient safety, ultimately leading to improved trial outcomes.
Notably, bioaccess® enables treatment-naive cardiology or neurology cohorts to be enrolled 50% faster than Western sites, translating to significant savings of $25K per patient with FDA-ready data—no rework, no delays. Research indicates that access to real-time information can lower recruitment expenses and facilitate flexible trial designs, which are essential for enhancing operational procedures.
Furthermore, the use of electronic data capture systems simplifies information gathering and validation processes, ensuring that the details used for decision-making are precise and trustworthy. As industry specialists emphasize, possessing timely information at their fingertips is crucial for effective decision-making in healthcare, enabling teams to react proactively to challenges and seize opportunities as they emerge.
This capability not only boosts the efficiency of medical trials but also greatly aids the overall success of research initiatives. Additionally, bioaccess offers comprehensive clinical trial management services, including:
This further supports the success of clinical research.
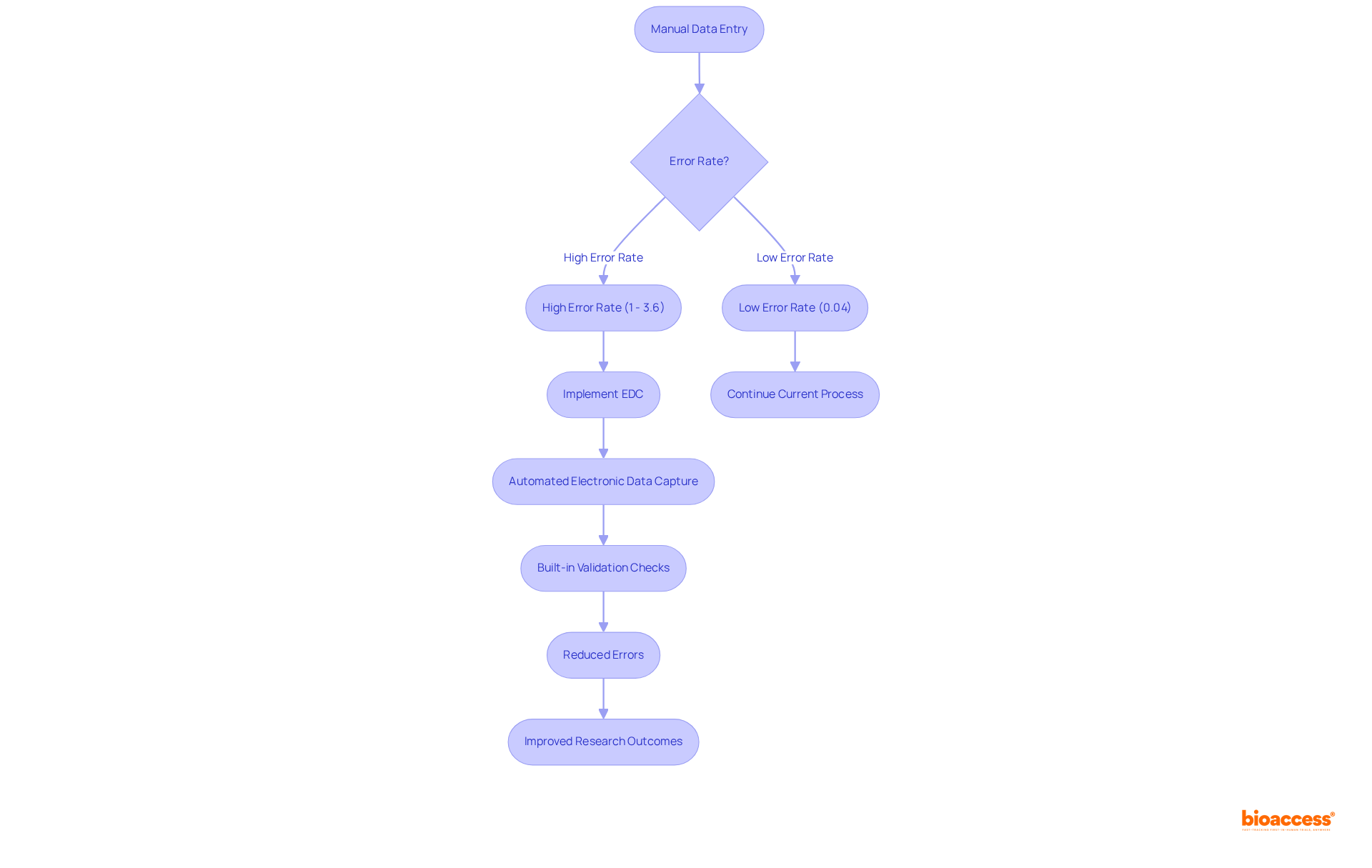
Automated electronic data capture (EDC) technologies are essential in minimizing entry errors by eliminating the need for manual procedures. These systems incorporate built-in validation checks and logic rules that promptly alert users to any discrepancies during data entry. This proactive approach not only enhances the accuracy of information but also significantly reduces the time required for data cleaning and correction, ultimately leading to more reliable research outcomes.
Statistics indicate that manual data entry can have an error rate averaging around 1%, which tends to escalate in complex scenarios. In research and medical environments, error rates may vary from 0.04% to 3.6%. For example, processing 1,000 orders could result in approximately 10 errors, highlighting the critical need for automation in clinical trials. By implementing electronic data capture solutions, organizations can achieve error rates as low as 0.04% in research settings, demonstrating the efficacy of these tools in enhancing data integrity.
Real-world examples illustrate the success of electronic data capture in reducing information discrepancies. Organizations that have adopted these systems report improved accuracy and efficiency, allowing researchers to focus on critical analyses rather than data correction. As Pierce Smith notes, 'Despite the best intentions, mistakes are simply unavoidable' in manual data entry processes. Experts in information management emphasize that electronic data capture and automation are vital for acquiring high-quality data in clinical research, as it not only streamlines processes but also fosters a culture of accuracy and reliability in data handling. Furthermore, ARDEM's automated information capture solutions achieve an impressive 99.97% accuracy rate, showcasing the effectiveness of automated methods in bolstering data integrity. Additionally, the case study of Rexel Canada illustrates how automation has successfully minimized errors and saved time, providing a tangible example of the discussed benefits.
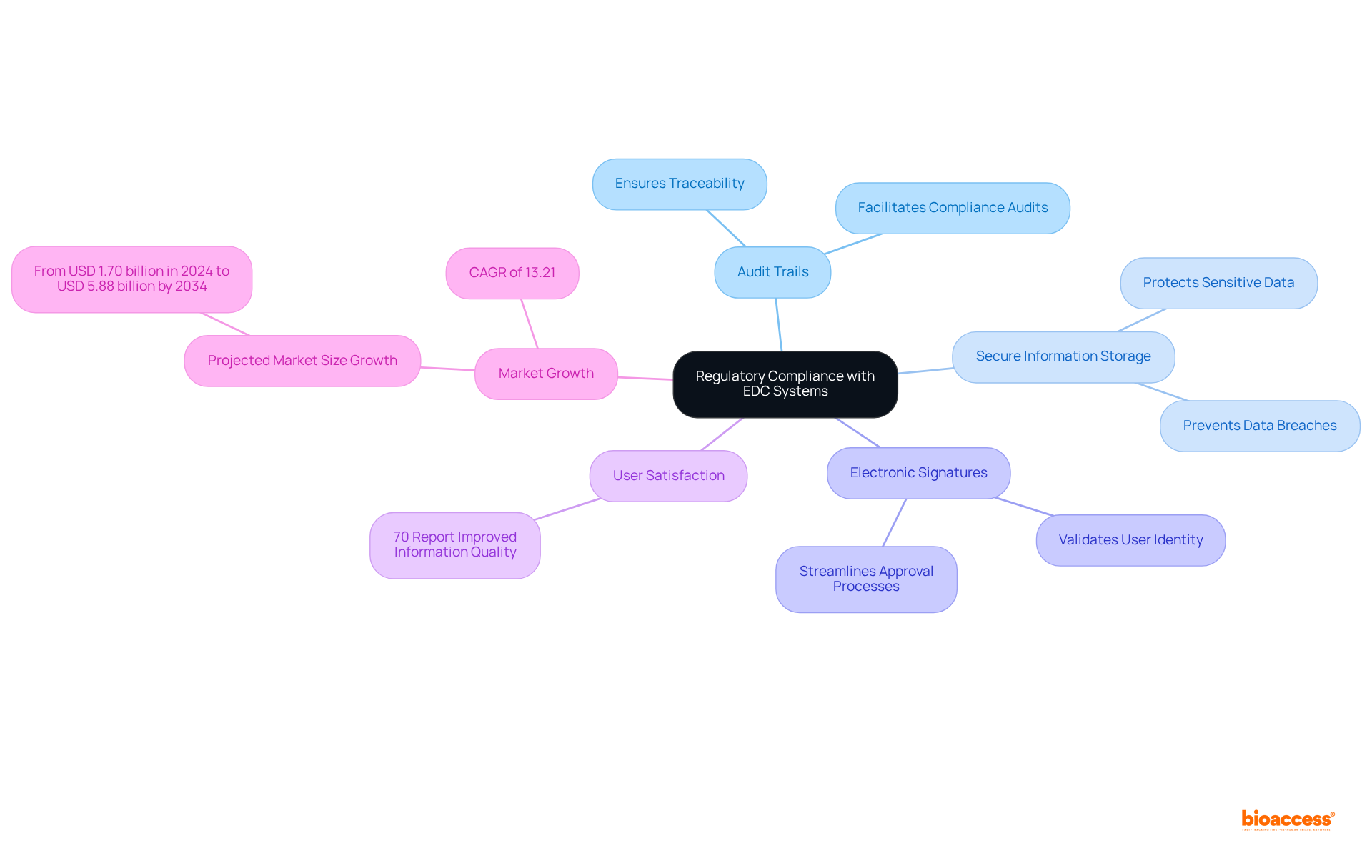
Electronic data capture platforms are essential for ensuring regulatory compliance, strictly adhering to industry standards such as FDA 21 CFR Part 11. These platforms boast robust features, including:
These features are critical for meeting regulatory requirements. By utilizing electronic data capture, researchers can maintain compliance throughout the trial process, thereby significantly enhancing the credibility of their findings. Notably, 70% of EDC users report improved information quality compared to traditional methods, underscoring the efficiency of EDC in patient-related research.
As the global electronic data collection market is projected to grow at a CAGR of 13.21%, the demand for these solutions will continue to rise, driven by the need for effective data management and regulatory compliance. This trend highlights the increasing reliance on electronic data capture solutions to navigate the complexities of compliance in clinical trials, ensuring that innovative therapies meet the stringent safety and efficacy standards set by regulatory authorities.
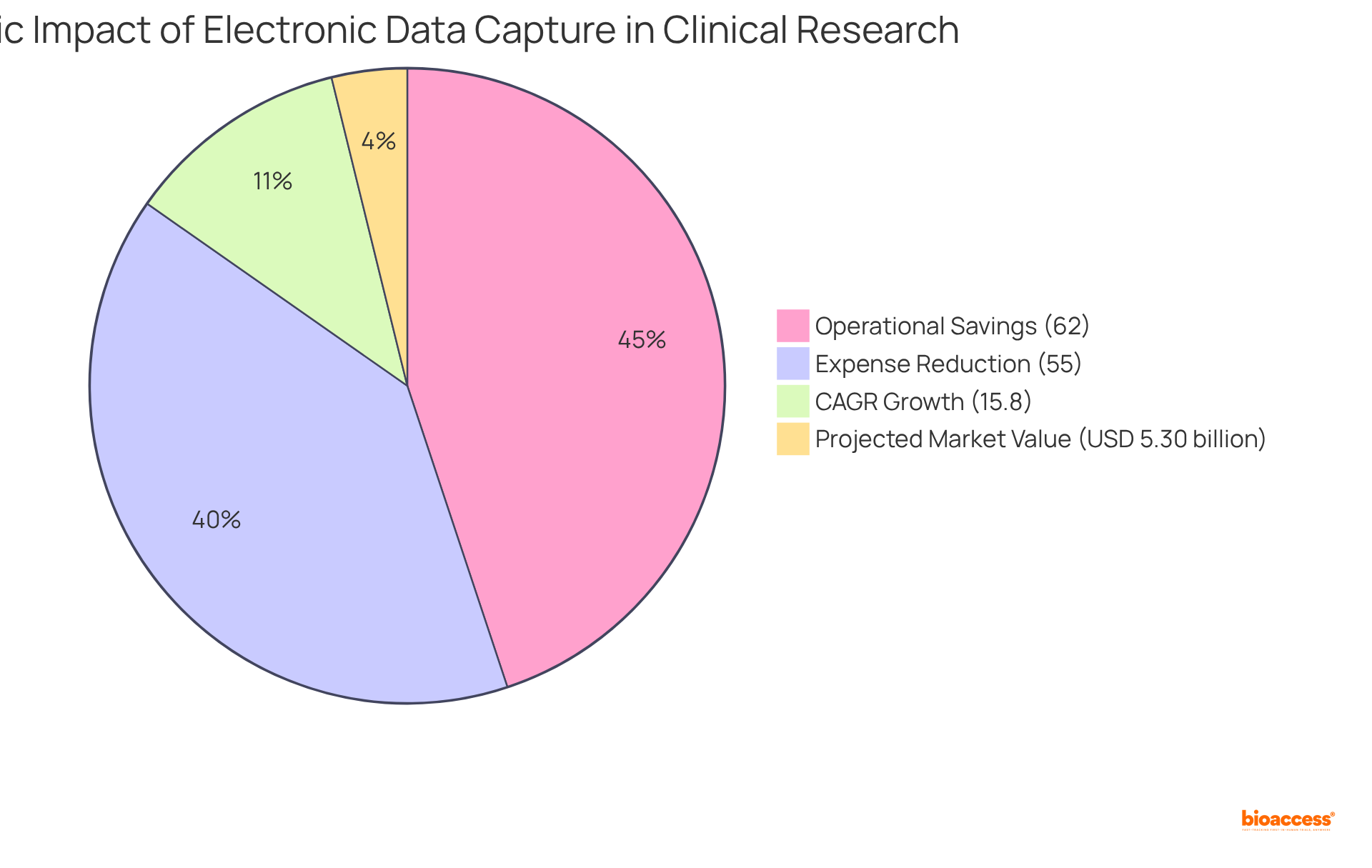
Implementing electronic data capture solutions can lead to substantial reductions in research costs. By automating information gathering and reducing dependence on physical storage and manual work, organizations can attain operational savings of up to 62% relative to conventional methods. Electronic data capture solutions not only streamline processes but also significantly reduce the time needed for data cleaning and analysis, thereby enhancing cost efficiency.
The anticipated compound annual growth rate (CAGR) for electronic data capture (EDC) technologies is projected at 15.8% from 2024 to 2033, reflecting the growing recognition of the economic benefits of electronic data capture in clinical research. Moreover, the worldwide electronic data capture market is projected to achieve around USD 5.30 billion by 2025, highlighting the growing acceptance of these solutions. As organizations continue to adopt electronic data capture, they can anticipate enhanced information accuracy and operational efficiency, ultimately resulting in better patient outcomes and lowered research-related costs.
Additionally, the worldwide eClinical solutions market is expected to attain US$17.61 billion by 2025, highlighting the significance of electronic data capture technologies in the broader context of research. Electronic data capture networks can also lower information gathering expenses by 55%, thereby strengthening their role in improving cost effectiveness. Adherence to industry standards remains essential, ensuring that EDC platforms not only enhance operational metrics but also maintain the integrity of research in healthcare.
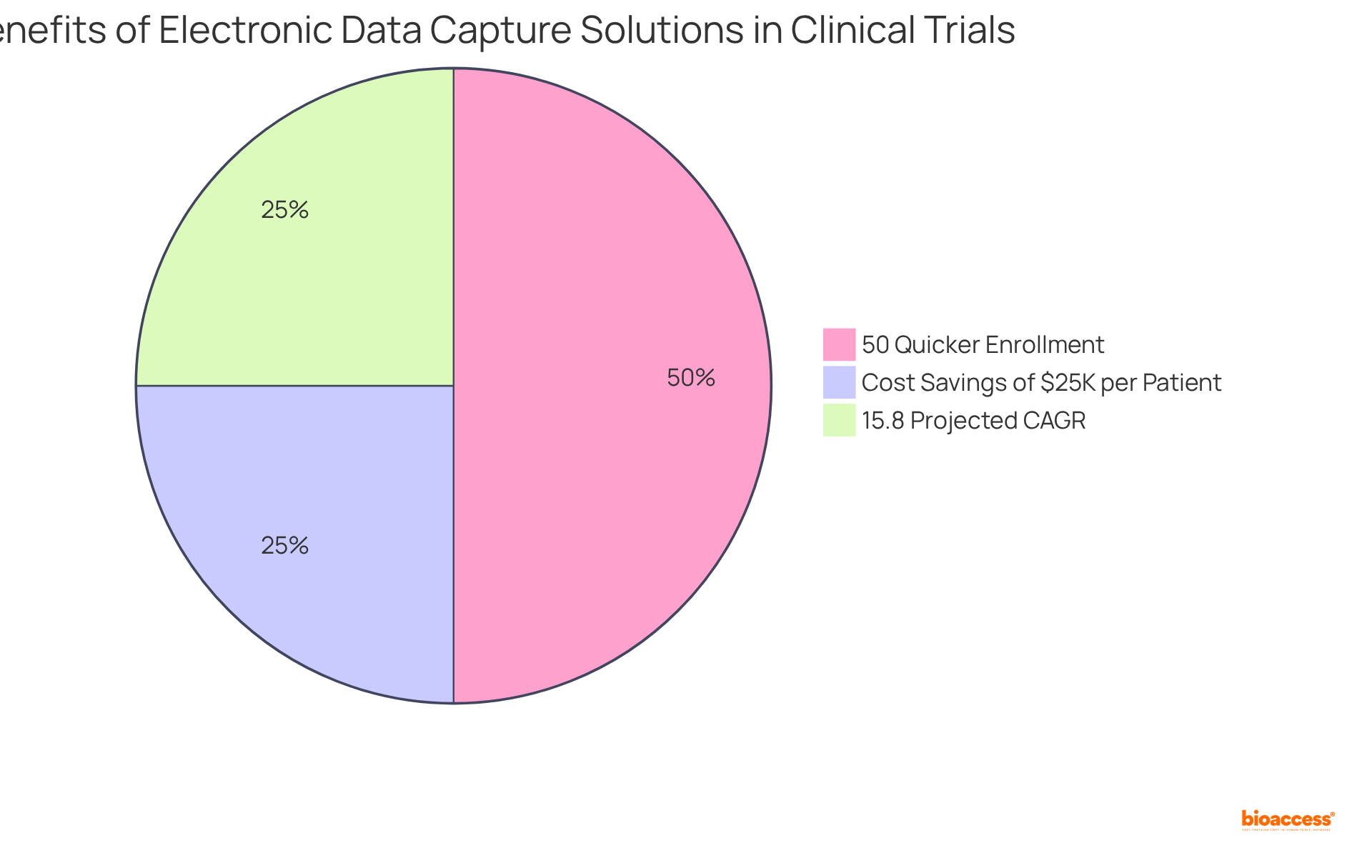
Flexible electronic data capture (EDC) solutions enable clinical trials to scale effectively, accommodating diverse participant numbers, data types, and study designs. This versatility makes electronic data capture solutions ideal for both small pilot studies and expansive large-scale trials. Recent trends indicate that trials utilizing electronic data capture platforms can achieve a 50% quicker patient enrollment rate compared to conventional methods, significantly enhancing operational efficiency.
With bioaccess®, you can enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, resulting in $25K savings per patient with FDA-ready data—no rework, no delays. Furthermore, electronic data capture (EDC) platforms are designed to assist with intricate data management requirements, ensuring researchers can uphold data integrity and optimize resource distribution, regardless of trial scale.
As the demand for decentralized research trials grows, the adaptability of electronic data capture (EDC) platforms to meet diverse needs becomes increasingly crucial, solidifying their role as indispensable tools in contemporary medical research. Additionally, the growth of electronic data capture technologies is projected to reach a compound annual growth rate (CAGR) of 15.8% from 2024 to 2033, underscoring their rising significance.
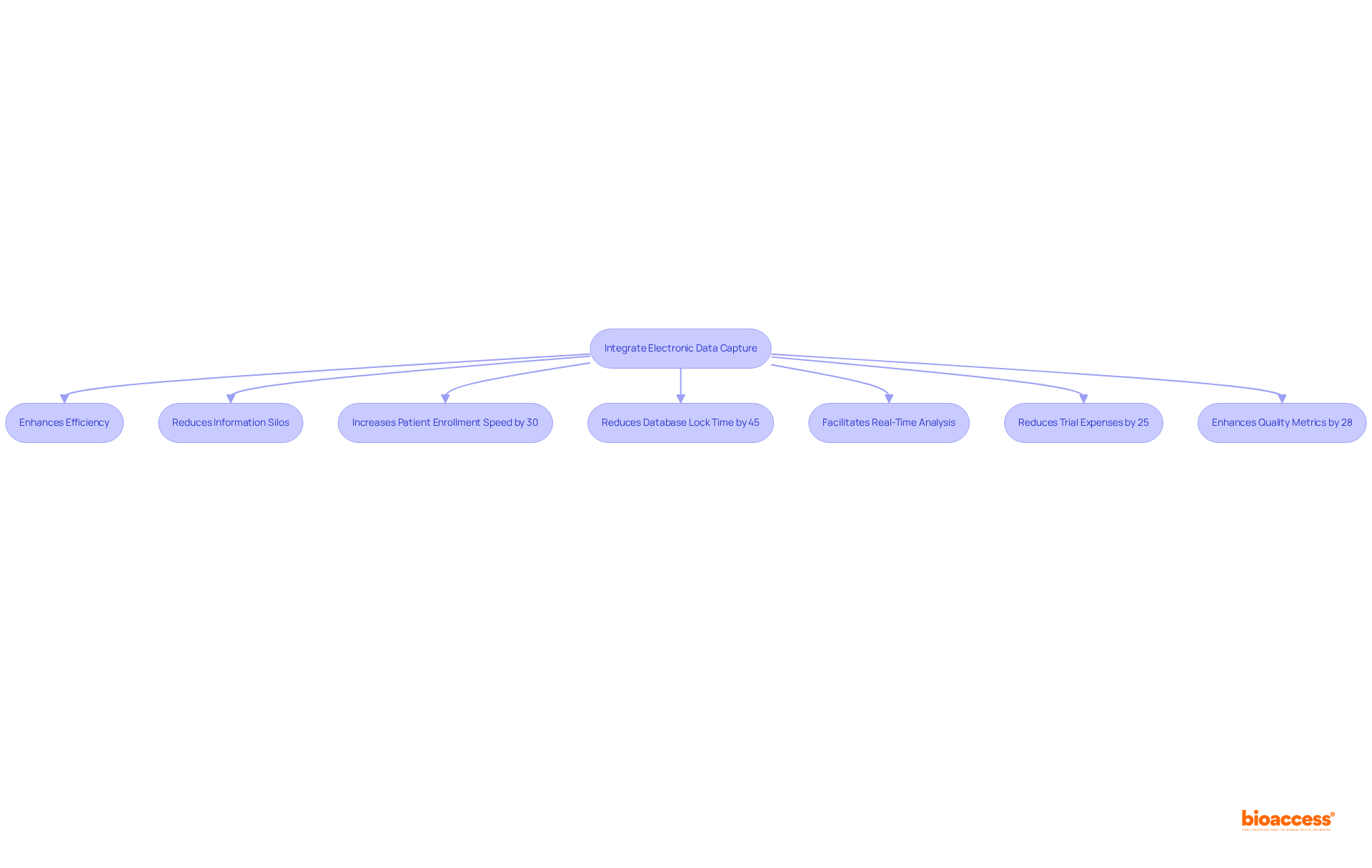
Incorporating electronic data capture technologies with current clinical research tools significantly enhances the efficiency of the research process, particularly within the realm of medical device clinical trials overseen by bioaccess. The integration of electronic data capture frameworks with information management platforms, electronic health records (EHR), and other software solutions creates a unified information ecosystem. This integration not only streamlines workflows but also reduces information silos and enhances the precision of analysis.
For instance, research trials that utilize cohesive, automated information management workflows have shown a remarkable 30% increase in the speed of patient enrollment and a 45% reduction in the time required to achieve database lock compared to those relying on disjointed frameworks. Moreover, the adoption of electronic data capture frameworks facilitates real-time information analysis, which is crucial in urgent medical scenarios, such as assessing potential heart attack instances. This capability not only mitigates delays in medical intervention but also significantly elevates patient care and outcomes.
As bioaccess focuses on Early-Feasibility Studies and First-In-Human Studies, the strategic advantage of incorporating electronic data capture frameworks emerges, enabling pharmaceutical firms to reduce trial expenses by an average of 25% and enhance quality metrics by 28%. The future of clinical research will increasingly depend on such integrations to drive innovation and optimize research investments.
User-friendly electronic data capture (EDC) platforms are pivotal in enhancing patient involvement by providing intuitive interfaces that streamline information entry. When patients engage with straightforward and accessible platforms, their likelihood of remaining engaged throughout the study increases significantly. Moreover, electronic data capture solutions that incorporate mobile access empower patients to submit data effortlessly from their devices, thereby enriching their experience and boosting retention rates.
Current trends reveal that trials utilizing electronic data capture systems are experiencing notable improvements in patient retention. In Colombia, the collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a premier hub for medical trials, with the endorsement of the Minister of Health. This initiative, alongside GlobalCare Clinical Trials' partnership with bioaccess™, has resulted in over a 50% reduction in recruitment time and remarkable 95% retention rates.
As the demand for personalized and efficient communication escalates, the integration of user-friendly electronic data capture solutions becomes essential for cultivating deeper connections between patients and research.
Utilizing advanced analytics within electronic data capture systems significantly enhances research outcomes. By employing sophisticated analytical tools, researchers can extract valuable insights into trial performance, patient demographics, and treatment efficacy. For instance, bioaccess enables treatment-naive cardiology or neurology cohorts to be enrolled 50% faster than Western sites, yielding substantial savings of $25K per patient with FDA-ready data—no rework, no delays. This clearly demonstrates the potential of analytics in optimizing research processes.
Moreover, bioaccess offers extensive trial management services, including:
All of which are crucial for successful trial execution. Analytics-driven patient engagement initiatives have shown a 22% enhancement in treatment adherence, underscoring the importance of information in improving patient outcomes. These insights empower researchers to make informed decisions, facilitating timely adjustments to study protocols that ultimately lead to more successful trials.
Current trends reveal a growing reliance on cloud-based analytics solutions, which accounted for 60% of the market share in 2023, due to their scalability and flexibility. As the clinical data analytics market is projected to reach USD 188,305 million by 2033, integrating electronic data capture into clinical trials is not merely advantageous but essential for driving innovation and efficiency within the pharmaceutical industry.
The transformative potential of electronic data capture (EDC) in clinical research is profound. By modernizing data collection methods, EDC solutions enhance operational efficiency, accuracy, and regulatory compliance, establishing a new benchmark for clinical trials. As organizations increasingly adopt these advanced technologies, the benefits become evident: improved data integrity, accelerated patient enrollment, and reduced research costs are just a few advantages that position EDC as an essential tool in the contemporary research landscape.
Throughout this discussion, various key points illustrate the multifaceted benefits of EDC:
All contribute to a more streamlined and effective research process. The capability to integrate EDC with existing clinical tools further amplifies these advantages, fostering a cohesive and efficient workflow that empowers researchers to focus on critical analyses and patient outcomes. Moreover, the anticipated growth of the EDC market underscores the increasing recognition of its role in driving innovation within the healthcare sector.
Ultimately, the shift towards electronic data capture signifies a crucial evolution in clinical research methodologies. As the industry embraces these advancements, stakeholders are urged to explore the integration of EDC solutions to enhance their research capabilities. By harnessing the power of technology, organizations can not only improve trial outcomes but also contribute to a more efficient and effective healthcare system overall.
What is bioaccess® and how does it impact clinical research?
bioaccess® leverages electronic data capture (EDC) solutions to streamline research processes in Latin America, enhancing information management and expediting ethical approvals and patient enrollment, achieving rates 50% faster than traditional methods.
What are the key benefits of using electronic data capture in clinical trials?
The key benefits include increased efficiency, reduced errors in data collection, real-time access to information, improved accuracy, and enhanced regulatory compliance, all of which contribute to shorter research timelines.
What types of trial management services does bioaccess® offer?
bioaccess® offers a range of trial management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies.
How is the market for electronic data capture expected to grow in the coming years?
The global market for electronic data capture (EDC) is projected to grow from USD 1.88 billion in 2024 to USD 4.20 billion by 2032, driven by the demand for real-time information collaboration and effective regulatory compliance.
What role does automation play in electronic data capture?
Automation in electronic data capture significantly reduces reliance on manual input, minimizing data entry errors, enhancing data integrity, and allowing researchers to focus on data analysis and patient outcomes.
How does real-time data access benefit medical researchers?
Real-time data access allows researchers to monitor trial progress, make informed decisions based on the latest data, identify issues quickly, and enhance patient safety, ultimately improving trial outcomes.
What cost savings can be achieved through the use of bioaccess® for clinical trials?
Bioaccess® enables faster enrollment of treatment-naive cardiology or neurology cohorts, resulting in significant savings of $25K per patient with FDA-ready data, with no rework or delays.
What additional services does bioaccess® provide to support clinical research?
In addition to trial management services, bioaccess® offers feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting to support the success of clinical research initiatives.