

The advantages of employing an electronic laboratory notebook (ELN) in research are significant, encompassing enhanced data integrity, improved collaboration, and notable cost efficiency. This article elucidates how ELNs streamline data management, enabling real-time collaboration among researchers, while simultaneously reducing operational costs through decreased reliance on physical materials and optimized workflows. Such efficiencies ultimately contribute to more effective research outcomes, underscoring the critical role of ELNs in modern scientific inquiry.
The landscape of clinical research is rapidly evolving, driven by the need for efficiency and accuracy in data management. Electronic laboratory notebooks (ELNs) have emerged as a transformative tool, offering a multitude of benefits that streamline research processes and enhance collaboration among teams. However, as organizations strive to adopt these innovative solutions, they face challenges in user acceptance and integration.
What are the key advantages of transitioning to electronic systems, and how can researchers overcome the hurdles to fully leverage these powerful tools?
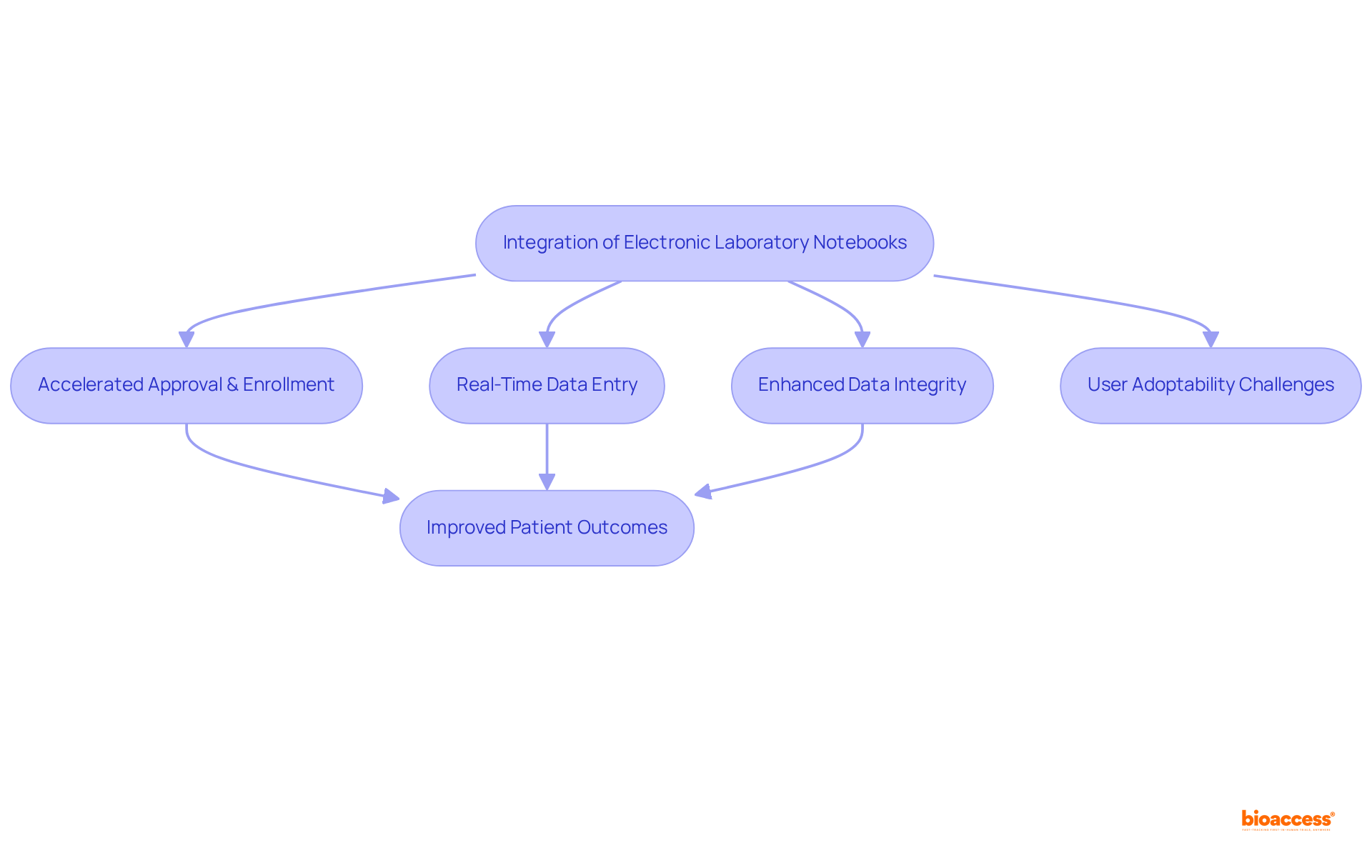
At bioaccess®, the integration of laboratory notebook electronic has fundamentally transformed clinical study methodologies. By employing a laboratory notebook electronic system, bioaccess® accelerates approval and enrollment schedules, enabling studies to be completed more swiftly than traditional methods. The real-time data entry capabilities of these notebooks not only reduce errors but also enhance data integrity, addressing the concerning statistic that 17% of all information is lost in research processes. This advancement fosters quicker decision-making and ultimately leads to improved patient outcomes. Such technological innovation aligns seamlessly with bioaccess®'s commitment to delivering global-first clinical agility, reinforcing its status as a leader in the Medtech, Biopharma, and Radiopharma sectors.
As the market for laboratory notebook electronic systems is projected to reach USD 718.7 million by 2030, with an estimated market value of USD 576 million in 2024, the adoption of these tools is becoming increasingly vital for organizations striving to optimize their clinical trial processes and enhance operational efficiency. However, it is crucial to acknowledge that user adoptability remains a significant challenge in the effective implementation of electronic learning networks, a hurdle that organizations must overcome to fully capitalize on their benefits. As Scott Gottlieb pointed out, the pharmaceutical industry must strive to make drug development less expensive and more efficient to effectively leverage advancements in science.
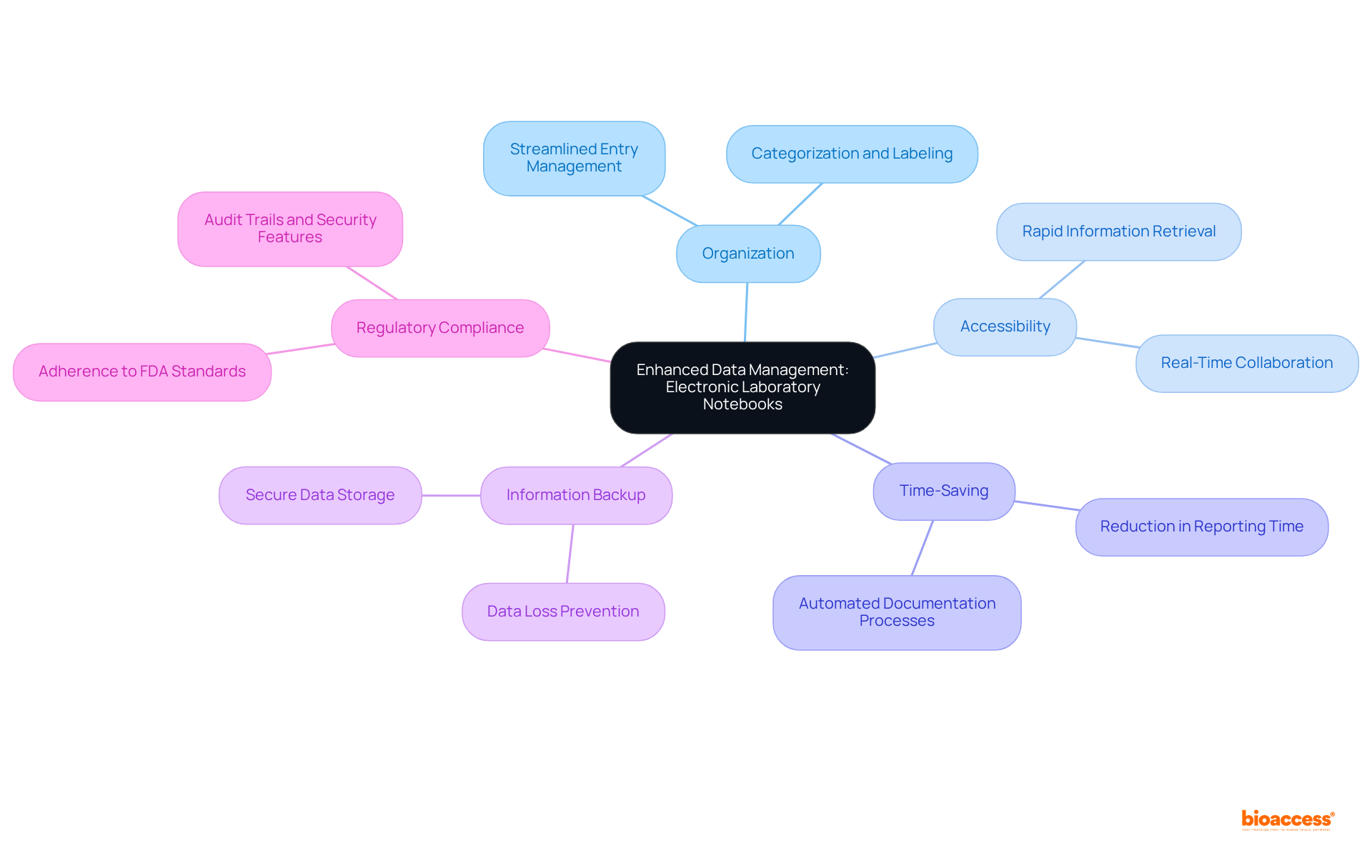
Laboratory notebook electronic significantly enhances the organization and accessibility of research information, making it indispensable in the realm of clinical research. Unlike conventional paper notebooks, a laboratory notebook electronic offers streamlined organization, labeling, and retrieval of entries. This functionality not only saves valuable time but also ensures that researchers can swiftly access critical information when required. Furthermore, a laboratory notebook electronic supports information backup and restoration, safeguarding against data loss while ensuring compliance with regulatory standards.
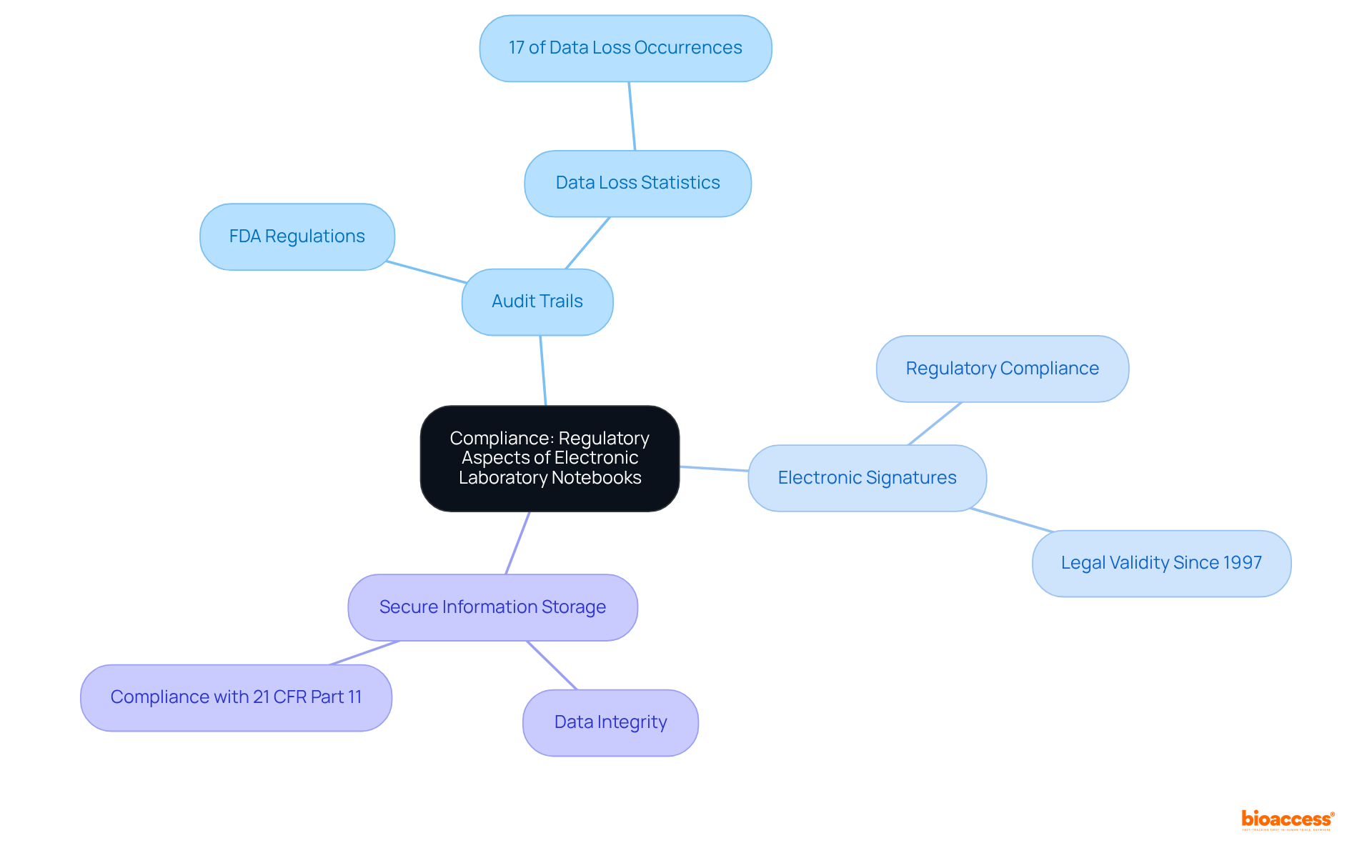
Adhering to regulatory standards is crucial in clinical research. Electronic laboratory records are specifically created to fulfill these strict requirements. Laboratory notebook electronic systems encompass critical components such as:
These components are essential for complying with FDA regulations like 21 CFR Part 11. Research indicates that the adoption of electronic lab notebooks significantly reduces the likelihood of data loss; notably, the absence of digital records accounts for 17% of data loss occurrences. By employing a laboratory notebook electronic, researchers can ensure that their documentation is not only precise but also compliant, thereby enhancing the integrity of their studies and facilitating smoother regulatory reviews. Furthermore, the FDA has recognized electronic records as legally valid since 1997, underscoring the importance of electronic laboratory notebook electronic in contemporary scientific settings. As the market for laboratory notebook electronic continues to expand, their role in ensuring compliance and enhancing study efficiency becomes increasingly vital.
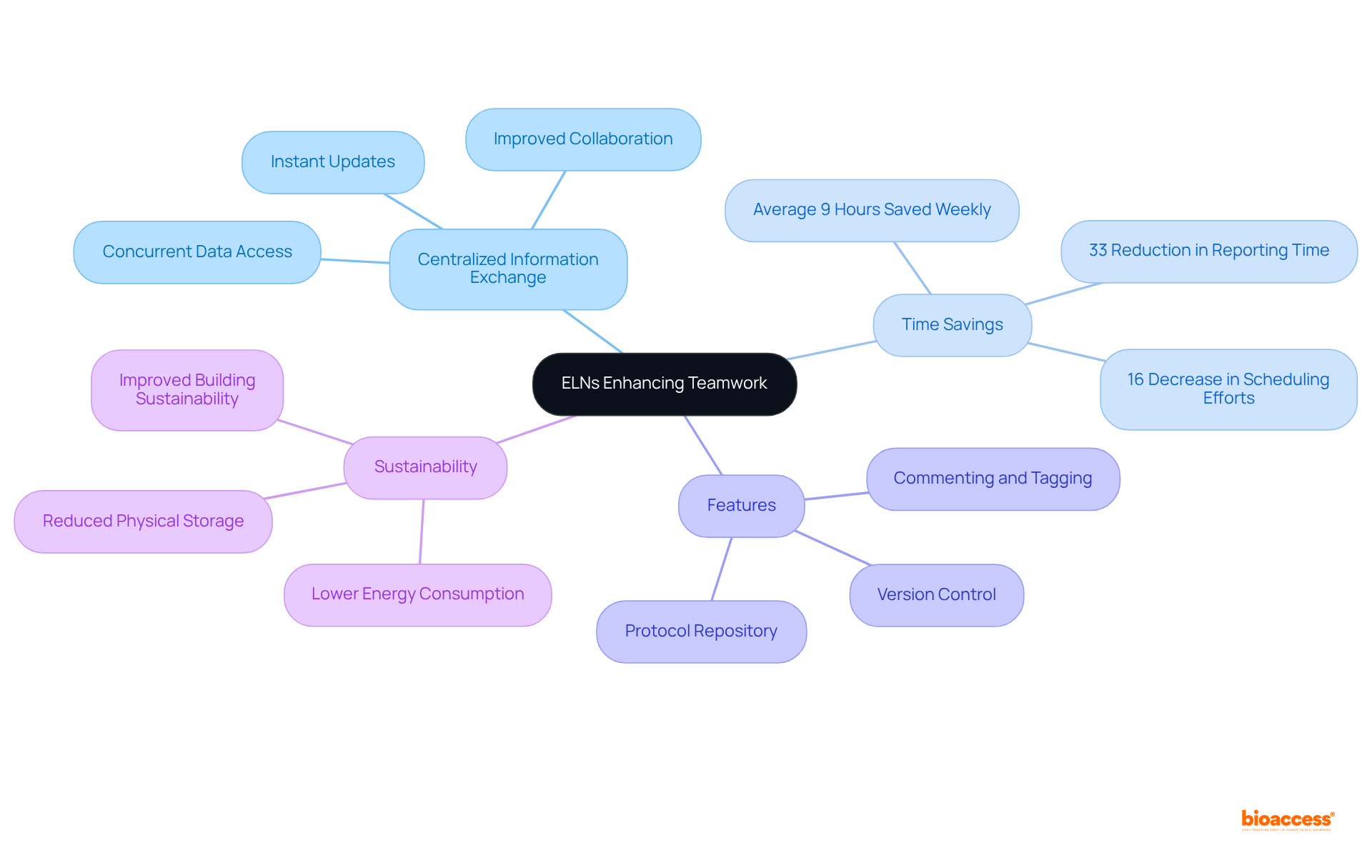
Laboratory notebook electronic significantly enhances teamwork by providing a centralized platform for group members to exchange information, insights, and updates instantly. This capability is especially advantageous in multi-disciplinary environments, where diverse expertise is crucial. By facilitating concurrent access to data, laboratory notebook electronic tools enable researchers to collaborate more effectively, regardless of their physical locations. With features such as commenting, tagging, and version control, these tools ensure that all team members remain aligned and informed throughout the research process.
Research indicates that teams utilizing a laboratory notebook electronic can save an average of 9 hours each week, allowing them to redirect this time towards priority activities, thus boosting productivity. For example, a study revealed that researchers experienced a 33% reduction in time spent on reporting and a 16% decrease in scheduling efforts after adopting an ELN. Such enhancements not only streamline workflows but also cultivate a culture of teamwork, as team members can easily access project information in their laboratory notebook electronic without the need for extensive verbal explanations.
Moreover, electronic lab notebooks contribute to sustainability by reducing the physical storage space required for traditional lab notebooks, leading to lower energy consumption and improved building sustainability. The integration of electronic lab notebooks into scholarly practices exemplifies how technology can elevate collaboration, making it more efficient and effective.
Integrating a laboratory notebook electronic with essential research tools like analysis software and laboratory information management systems can significantly enhance workflow efficiency. This integration not only facilitates automated data transfer but also minimizes manual entry errors, leading to a marked increase in overall productivity. Researchers have reported saving an average of 9 hours per week after adopting ELNs, with some scientists noting time savings of up to 17 hours weekly. This shift allows them to concentrate on their primary investigative activities rather than administrative tasks.
The seamless transfer of information between systems creates a unified environment for inquiry, where knowledge is readily accessible, thereby enhancing processes and fostering collaboration. For instance, users of SciNote—a widely adopted ELN trusted by over 90,000 researchers across more than 100 countries—have observed that the platform's integration capabilities empower them to manage projects and data more effectively. This ultimately leads to faster research outcomes and improved compliance with regulatory standards.
Such changes not only boost operational efficiency but also address the growing demand for digital solutions in research facilities. This is underscored by the anticipated growth of the ELN market, projected to reach USD 922.4 million by 2032. To effectively implement a laboratory notebook electronic, consider initiating a pilot project that integrates your current tools, allowing your team to experience these advantages firsthand.
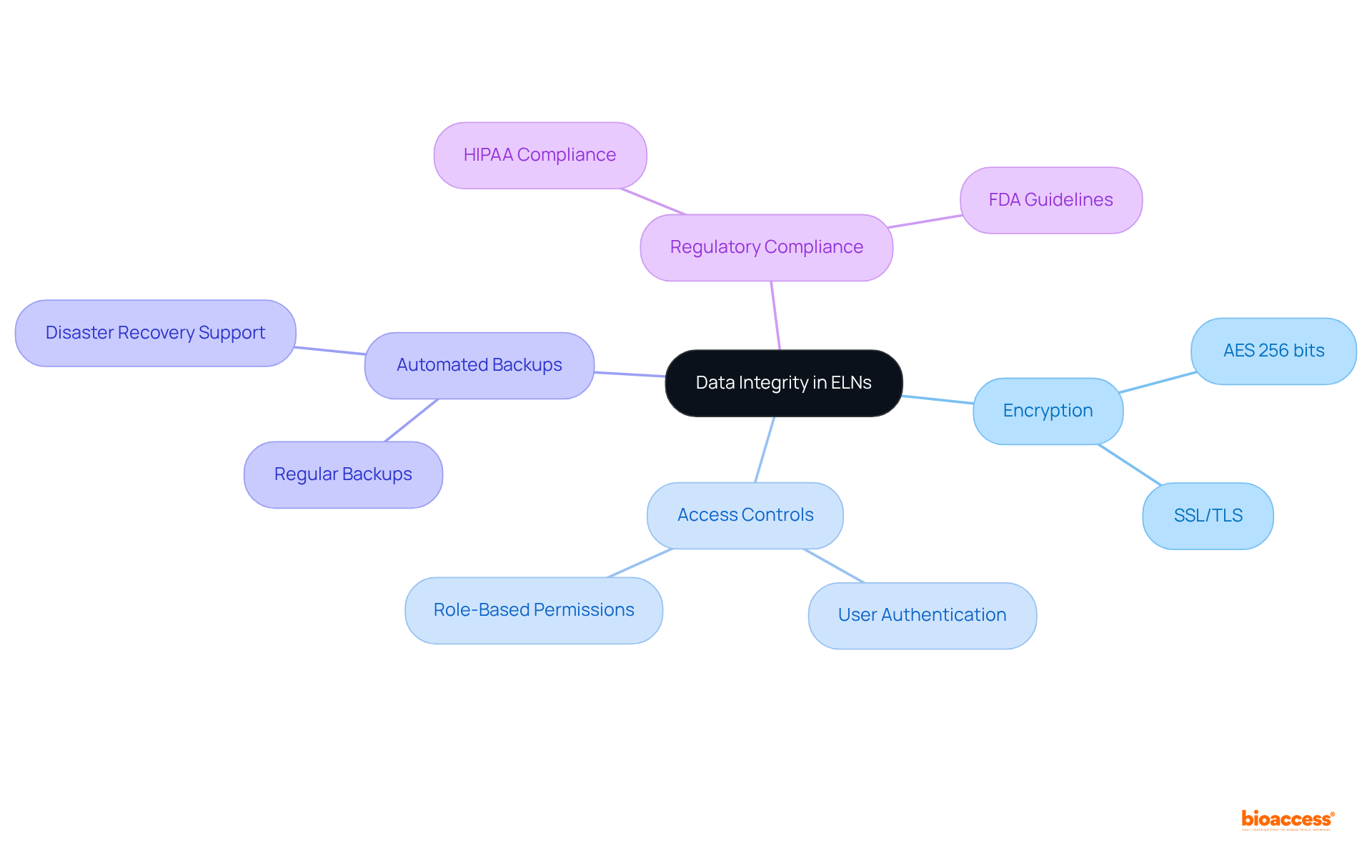
Data integrity is paramount in clinical research, and laboratory notebook electronic (LNE) systems are equipped with advanced security features designed to safeguard sensitive information. These notebooks typically employ robust encryption techniques, such as Advanced Encryption Standard (AES) with key lengths of 256 bits for data at rest, alongside Secure Sockets Layer (SSL) or Transport Layer Security (TLS) for data in transit. This ensures that details remain secure and unaltered. Access controls, including user authentication and role-based permissions, empower organizations to regulate who can view or modify specific entries, thereby enhancing information protection.
Moreover, consistent automated backups are essential to electronic lab notebooks, significantly reducing the risk of information loss and ensuring continuity in scientific endeavors. Cybersecurity specialists emphasize that these security measures not only protect information but also bolster adherence to regulatory standards, such as HIPAA and FDA guidelines. For instance, numerous electronic lab notebooks automatically generate audit trails and maintain version control, which are crucial for meeting regulatory obligations and ensuring information integrity.
The effectiveness of these security features is evident in the rising adoption of laboratory notebook electronic systems across laboratories, as they ensure a secure environment for managing sensitive information while fostering collaboration among teams. By integrating these sophisticated security protocols, electronic lab notebooks play a vital role in preserving the integrity of clinical information, ultimately facilitating the advancement of medical innovations. To further enhance data security, organizations should prioritize user training and awareness, given that 95% of security breaches stem from human errors.
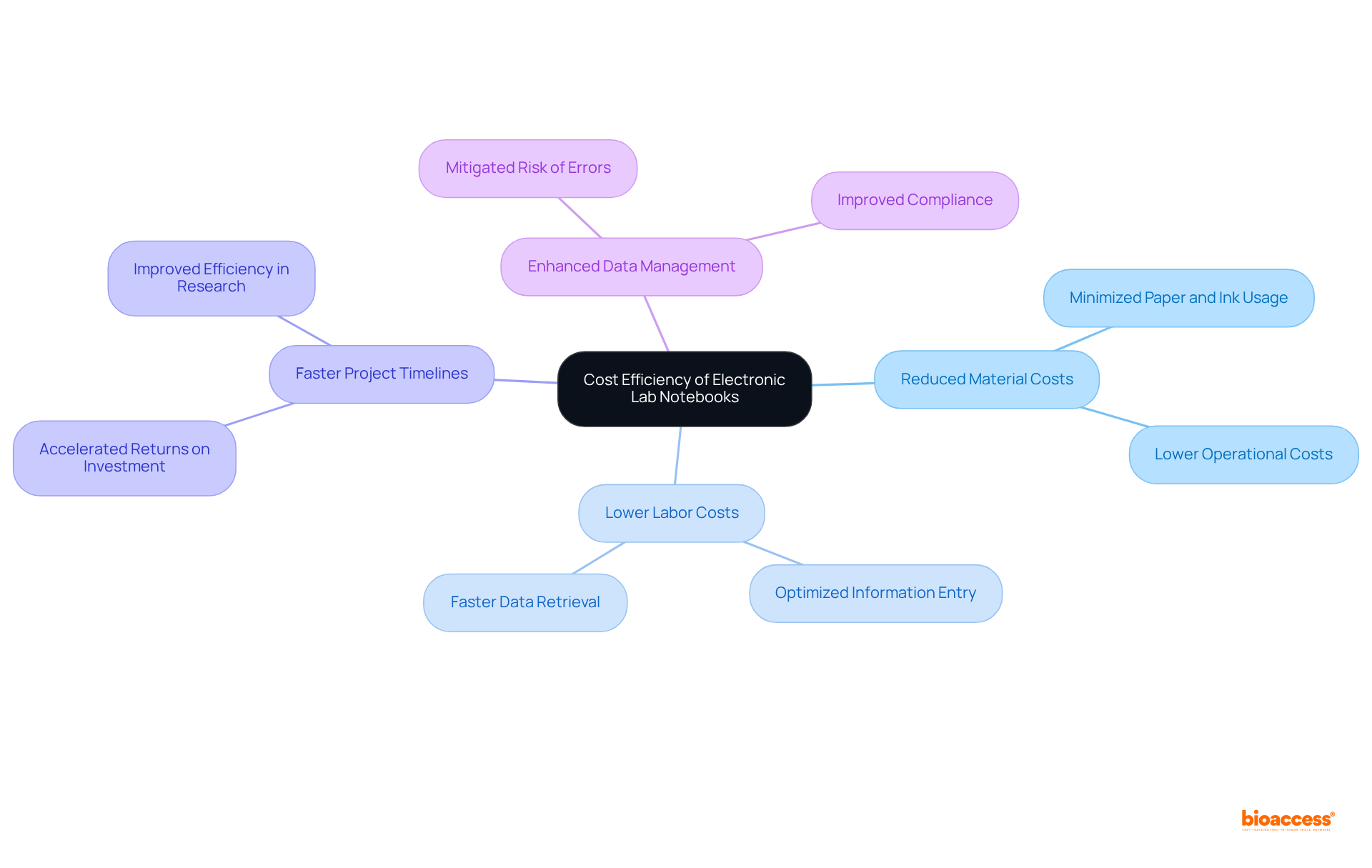
Adopting a laboratory notebook electronic system presents a compelling opportunity for research institutions to achieve substantial cost reductions. By significantly minimizing the reliance on physical materials like paper and ink, electronic lab notebooks effectively lower operational costs. Moreover, the efficiency gained from optimized information entry and retrieval processes leads to marked decreases in labor costs.
Organizations that have implemented electronic lab notebooks report expedited project timelines, which can accelerate returns on investment. The financial implications of electronic learning networks extend to clinical trial budgets as well; enhanced data management and compliance can mitigate the risk of costly delays and errors.
Collectively, the financial benefits associated with electronic lab notebooks render them a strategic investment for any research group aiming to enhance productivity and optimize resource utilization.
Modern electronic laboratory notebooks are distinguished by their user-friendly features, which are designed to cater to researchers of all skill levels. Intuitive interfaces, customizable templates, and streamlined navigation tools simplify the entry process significantly. For instance, drag-and-drop functionality and mobile access empower researchers to document their work effortlessly, whether in the lab or remotely. These usability enhancements not only elevate the overall user experience but also play a crucial role in the widespread acceptance of electronic lab notebooks within research teams.
As the demand for effective information management systems continues to rise, the emphasis on user-friendly features remains instrumental in shaping the development of laboratory notebook electronic, ensuring they meet the needs of contemporary researchers. Market projections indicate that the electronic lab notebook market is anticipated to reach USD 1,211.06 million by 2034, underscoring the significance of these advancements.
Vega Shah, PhD, asserts that 'successful adoption is intertwined with the people and culture of science,' emphasizing the essential role of usability in facilitating this transition. Furthermore, the increasing focus on compliance with regulatory standards accelerates the adoption of laboratory notebook electronic systems, as user-friendly features aid researchers in efficiently meeting these requirements.
Laboratory notebook electronic provides robust archival capabilities that are essential for long-term information retention and retrieval. By automatically storing all entries, laboratory notebook electronic systems guarantee that researchers maintain a comprehensive record of their work over time. This functionality is particularly vital for compliance with regulatory requirements, notably the NARA/OMB Federal-Wide Transition to Electronic Records mandate, which necessitates that all Federal Records, including the laboratory notebook electronic, transition to electronic format by June 30, 2024.
Researchers can readily access historical information, simplifying the referencing of past experiments and findings—an aspect crucial for ongoing studies and publications. Furthermore, research indicates that scientists save an average of nine hours each week by utilizing laboratory notebook electronic systems, significantly reducing the time spent on administrative tasks and allowing for greater focus on research activities.
The adoption of electronic laboratory notebooks fosters transparency and traceability, as these laboratory notebook electronic systems provide a permanent record of laboratory activities, mitigating risks associated with information loss and transcription errors. Additionally, it is essential to delineate archiving responsibilities in multi-site trials to ensure compliance and effective information management. This organized approach not only facilitates adherence to data retention policies but also enhances the overall efficiency of documentation.
The flexibility of laboratory notebook electronic systems (ELNs) is crucial for ensuring their relevance in the face of evolving scientific demands and technological advancements. Many laboratory notebook electronic solutions now offer customizable options, allowing organizations to tailor the software to their unique workflows and requirements.
As LaToya Lee Jones, Partner and Quality + Compliance Lead, aptly notes, "A laboratory notebook electronic (ELN) is an experiment and project-centric application that facilitates the storage and retrieval of both structured and unstructured information in fundamental and innovative exploration and discovery." This capability not only enhances operational efficiency but also empowers organizations to maintain a competitive edge in a rapidly changing environment.
For example, the introduction of no-code configurability in platforms like Sapio ELN enables scientists to swiftly adapt their data management systems to meet shifting experimental needs, significantly boosting productivity and quality. Furthermore, the trend towards community-driven extensions, such as LabIMotion, illustrates how laboratory notebook electronic systems can evolve to support interdisciplinary studies, ensuring they meet the diverse requirements of modern laboratories.
With LabWare LIMS boasting a 98% customer satisfaction rate, it is clear that the integration of customizable features and community-driven innovations is vital for the future-proofing of research labs.
The adoption of electronic laboratory notebooks (ELNs) signifies a pivotal advancement in clinical research, providing researchers with enhanced efficiency, improved data management, and adherence to regulatory standards. Transitioning from traditional paper notebooks to electronic systems allows organizations to streamline workflows, safeguard data integrity, and promote collaboration among team members, ultimately resulting in more effective and timely research outcomes.
Key insights from the discussion underscore the myriad benefits of utilizing ELNs, including:
All of these foster a more productive research environment. The financial benefits, such as cost savings and an increased return on investment, further highlight the strategic importance of integrating electronic laboratory notebooks into research practices. Additionally, the adaptability of these systems ensures they evolve in tandem with the shifting demands of scientific inquiry.
Given these compelling advantages, organizations are urged to adopt electronic laboratory notebooks as essential tools for future-proofing their research capabilities. By prioritizing user training and proactively addressing adoption challenges, research teams can unlock the full potential of ELNs, paving the way for innovation and improved patient outcomes in clinical research. The transition to digital solutions not only boosts operational efficiency but also positions organizations to excel in an increasingly competitive landscape.
What is bioaccess® and how does it utilize electronic laboratory notebooks?
bioaccess® integrates electronic laboratory notebooks to transform clinical study methodologies, accelerating approval and enrollment schedules, and enabling quicker completion of studies compared to traditional methods.
What are the benefits of using electronic laboratory notebooks in clinical research?
Electronic laboratory notebooks enhance data integrity through real-time data entry, reduce errors, improve decision-making, and ultimately lead to better patient outcomes.
What is the projected market growth for electronic laboratory notebooks?
The market for electronic laboratory notebooks is projected to reach USD 718.7 million by 2030, with an estimated value of USD 576 million in 2024.
What challenges exist in the adoption of electronic laboratory notebooks?
User adoptability is a significant challenge in effectively implementing electronic laboratory notebooks, which organizations must overcome to fully benefit from these tools.
How do electronic laboratory notebooks enhance data management?
They provide streamlined organization, labeling, and retrieval of research information, saving time and allowing researchers to quickly access critical data. They also support information backup and restoration.
What regulatory aspects are associated with electronic laboratory notebooks?
Electronic laboratory notebooks help ensure compliance with regulatory standards by including features such as audit trails, electronic signatures, and secure information storage, which are essential for meeting FDA regulations like 21 CFR Part 11.
How do electronic laboratory notebooks reduce data loss?
The adoption of electronic laboratory notebooks significantly reduces data loss, addressing the statistic that 17% of data loss occurs due to the absence of digital records.
Are electronic records recognized by regulatory bodies?
Yes, the FDA has recognized electronic records as legally valid since 1997, highlighting the importance of electronic laboratory notebooks in modern scientific research.