


This article underscores the critical importance of Class III medical devices, which are essential for sustaining human life. Due to their high-risk nature, these devices require stringent regulatory approval. Notable examples include:
Each of these devices plays a vital role in enhancing patient outcomes and necessitates extensive clinical trials to ensure their safety and efficacy. The detailed examination of their functions and regulatory processes highlights the challenges and responsibilities that come with their development.
The landscape of healthcare is increasingly shaped by Class III medical devices, which are crucial for sustaining life and enhancing patient outcomes. These high-risk instruments, such as pacemakers and implantable defibrillators, necessitate rigorous premarket approval processes due to their significant impact on health. As innovations in technology and regulatory practices evolve, understanding the complexities and opportunities within this sector becomes essential.
What challenges do manufacturers face in navigating the intricate approval landscape?
How can they leverage emerging trends to bring life-saving devices to market more efficiently?
bioaccess® is dedicated to accelerating the development of class iii medical devices examples, leveraging Colombia's competitive advantages. These include substantial cost reductions of over 30% compared to North America and Western Europe, alongside remarkable regulatory efficiency, with ethical approvals secured in just 4-6 weeks. The total review process involving IRB/EC and MoH (INVIMA) in Colombia spans approximately 90-120 days, significantly enhancing the efficiency of clinical trials.
Colombia boasts a robust healthcare system, ranked #22 by the World Health Organization and recognized globally for its excellence. This positions bioaccess® as a key enabler for Medtech, Biopharma, and Radiopharma innovators, allowing them to conduct clinical trials more efficiently than in traditional markets. The diverse patient population, with over 95% covered by universal healthcare, ensures effective patient recruitment.
Moreover, Colombia offers attractive R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, making it an ideal location for clinical studies. This strategic advantage not only accelerates the timeline for introducing life-saving products to market but also ensures that innovators can capitalize on the expanding healthcare market in Latin America.

Class III medical instruments represent high-risk products that are essential for sustaining or supporting human life. These instruments require premarket approval (PMA) from regulatory authorities due to their inherent risks. Some Class III medical devices examples are:
Their significance in healthcare cannot be overstated, as they play a critical role in preventing severe health impairments and enhancing patient outcomes. For instance, pacemakers not only regulate heart rhythms but also substantially lower the risk of sudden cardiac arrest, thereby improving survival rates. Likewise, implantable defibrillators are capable of detecting life-threatening arrhythmias and delivering shocks to restore normal heart function, underscoring their vital function in emergency cardiac care. The ongoing advancements in Class III instruments continue to transform healthcare, highlighting their crucial role in modern medicine.
Class III medical instruments are indispensable in modern healthcare, often fulfilling life-saving roles. Notable examples include:
These examples underscore the life-saving potential of Class III instruments, highlighting their critical role in elevating patient care and enhancing quality of life, all supported by the expertise of bioaccess in managing clinical trials.

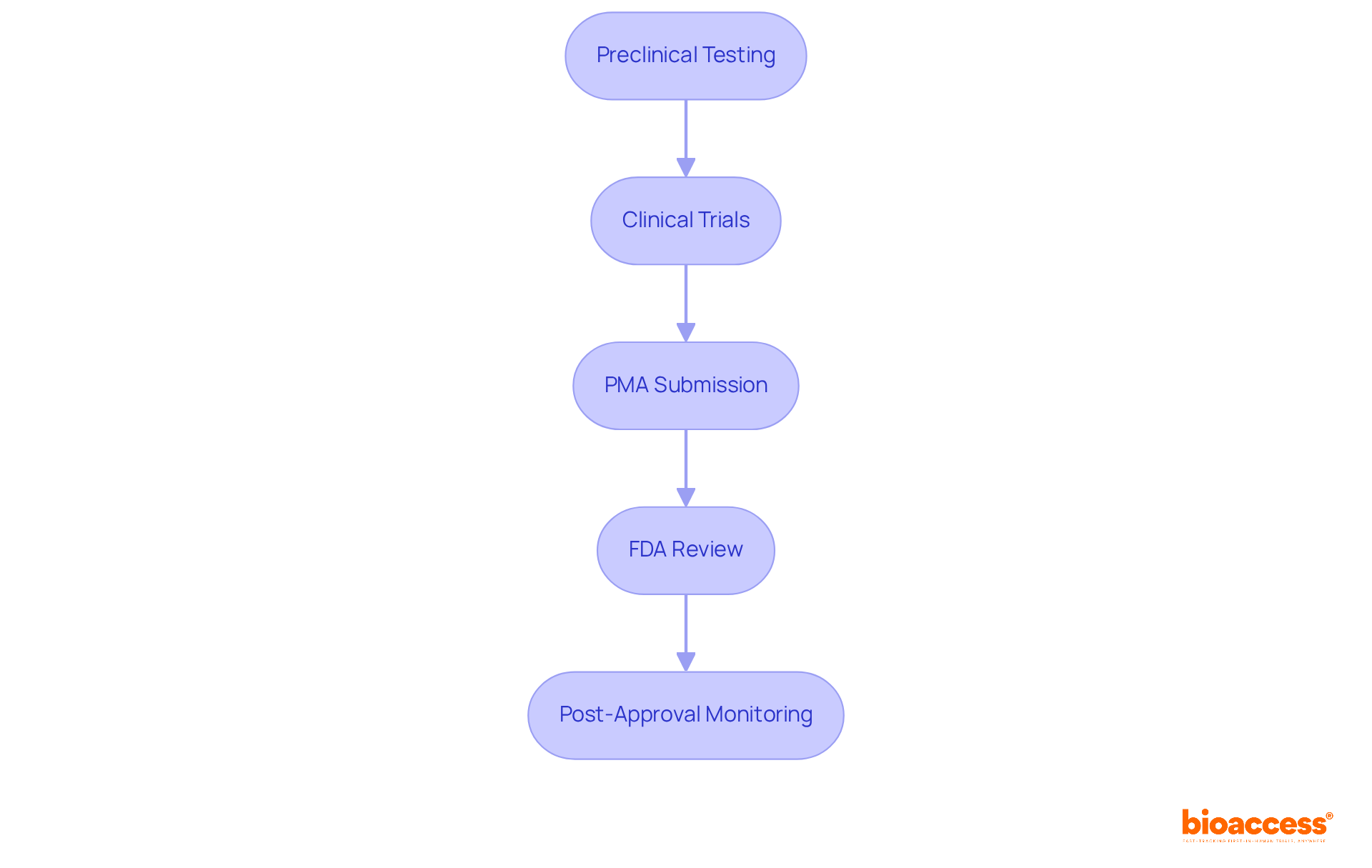
The Premarket Approval (PMA) process for Class III devices encompasses several critical steps that are essential for ensuring safety and efficacy in clinical research.
Preclinical Testing: Initial security data is collected through laboratory and animal studies, establishing a foundation for further investigation.
Clinical Trials: Human studies are carried out to demonstrate the apparatus's reliability and effectiveness, providing vital proof for regulatory assessment.
PMA Submission: A comprehensive application is compiled and submitted to the FDA, including clinical data, manufacturing details, and labeling information.
FDA Review: The FDA thoroughly evaluates the application, which may involve consultations with advisory committees to ensure all aspects are considered.
Post-Approval Monitoring: After receiving approval, manufacturers must carry out post-market surveillance to observe the product's performance in real-world environments, ensuring continued safety and effectiveness.
The intricacy of this procedure is underscored by the fact that class III medical devices examples, including essential products such as implantable pacemakers and insulin pumps, endure the most stringent regulatory oversight due to their potential hazards to patients. As of 2025, updates to the PMA process reflect ongoing efforts to streamline submissions and enhance efficiency, addressing challenges such as participant recruitment and regulatory compliance. Engaging proactively with regulatory bodies can significantly reduce delays, improving the likelihood of successful outcomes in the PMA journey.
Post-market compliance for class III medical devices examples encompasses several essential considerations.
Adverse Event Reporting: Manufacturers are mandated to report any adverse events or device failures to the FDA within specified timelines. This includes severe injuries or fatalities associated with the apparatus, which must be reported within 30 calendar days or within 5 workdays if urgent corrective measures are required. The FDA's dependence on prompt reporting is essential for protecting public health, as delays can obstruct the recognition of health issues.
Post-Market Surveillance Studies: Continuous research is vital for assessing the long-term security and efficacy of class III medical devices examples. These studies assist in collecting real-world data, crucial for evaluating performance beyond initial clinical trials. The FDA urges producers to establish robust post-market monitoring systems to enhance patient well-being.
Quality System Regulations (QSR): Adherence to QSR is critical for ensuring consistent manufacturing practices. Following these regulations helps preserve the quality and security of class III medical devices examples throughout their lifecycle, from production to post-market oversight.
Periodic Safety Update Reports (PSURs): Producers must provide routine updates to the FDA concerning their product's performance and any new information on risks. These reports play an important role in keeping the FDA informed about potential risks and the overall risk profile of the apparatus, thereby facilitating timely regulatory actions when necessary.
The significance of these compliance measures cannot be overstated, as they are essential to safeguarding patient safety and ensuring the effectiveness of medical products in the market.
Implementing a Quality Management System (QMS) for Class III medical devices encompasses several essential elements:
Clinical trials for Class III medical devices are structured into several key phases, each serving a distinct purpose in the development process.
Pilot Studies: These small-scale studies are crucial for assessing the feasibility of the device and refining trial protocols. They typically involve a small group of healthy participants to evaluate security and initial effectiveness.
Pivotal Trials: Larger and more comprehensive, pivotal trials are designed to provide definitive evidence of a device's reliability and effectiveness. These trials are essential for acquiring Premarket Approval (PMA) from regulatory agencies, as they gather comprehensive data needed to prove adherence to quality standards. A notable example is ReGelTec's Early Feasibility Study in Barranquilla, Colombia, where eleven patients with degenerative disc disease were successfully treated with the HYDRAFIL™ hydrogel. This research underscores the significance of pivotal trials in advancing medical technologies, as it not only evaluates safety and effectiveness but also contributes to the body of evidence required for regulatory approval. The overall probability of success (POS) for drug development programs, particularly for class III medical devices examples, is approximately 13.8%, highlighting the challenges faced during pivotal trials. Furthermore, Flow-FX's first-in-human clinical trial of the Flow-Screw apparatus for intraosseous antibiotic delivery in Colombia further exemplifies the critical role of pivotal trials in the medical technology landscape.
Post-Market Studies: Once a product is approved, ongoing post-market studies are conducted to monitor long-term performance and gather additional data on its impact on patient outcomes. These studies are vital for ensuring that equipment continues to meet safety standards and for identifying any potential long-term effects that may not have been apparent during initial trials.
Clinical researchers emphasize the significance of pivotal trials, noting that they frequently serve as the determining factor in the market introduction of class III medical devices examples. For instance, advancements in technology and methodologies, including the integration of AI and machine learning, have led to increased success rates in pivotal trials, reflecting a commitment to innovation and patient-centered solutions. As of 2025, updates in pivotal trials for medical devices indicate a growing trend towards utilizing these advanced technologies to enhance trial efficiency and outcomes.
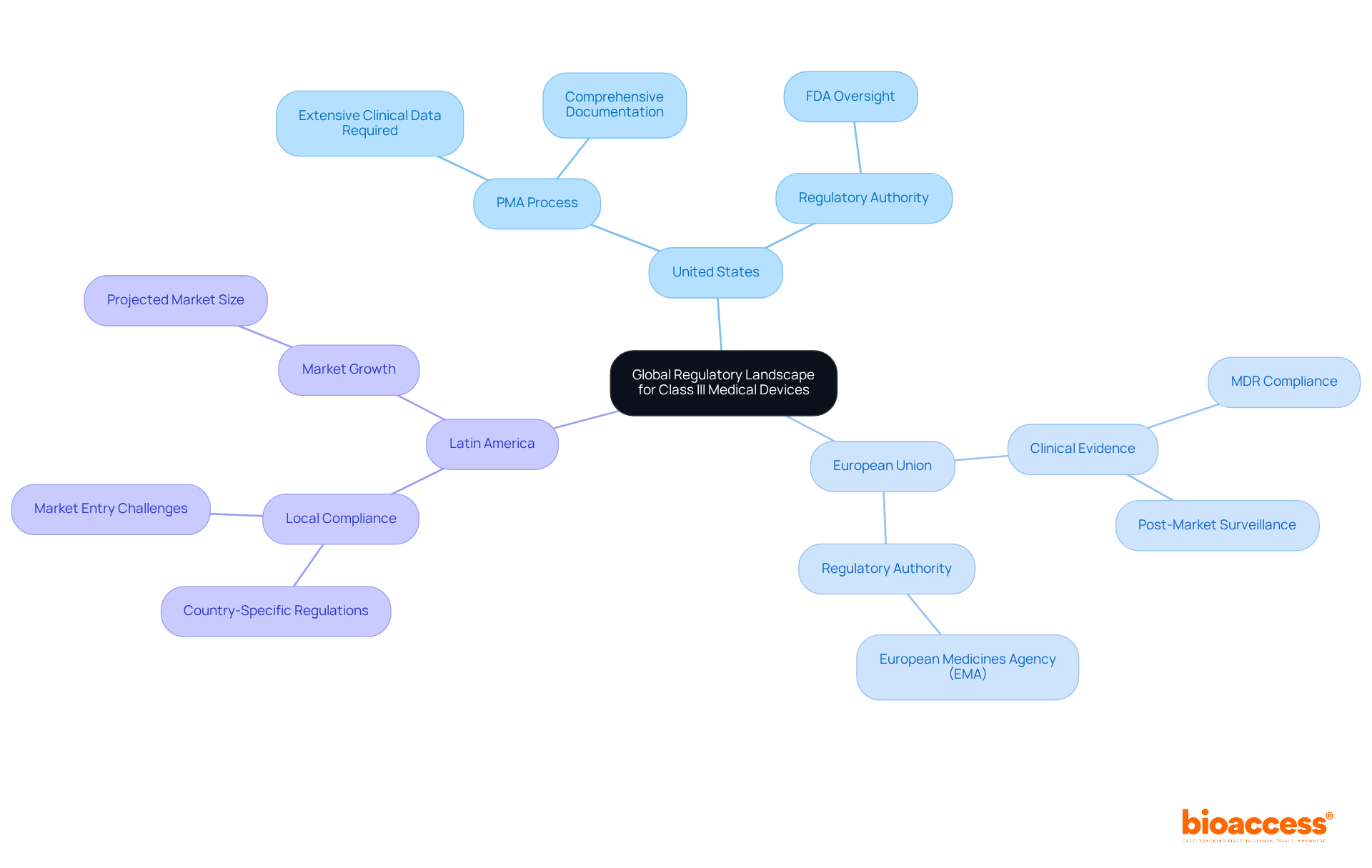
The global regulatory landscape for Class III medical devices presents significant variations across regions, a factor crucial for manufacturers aiming to navigate these complexities effectively.
United States: The FDA governs the approval process, mandating a Premarket Approval (PMA) for high-risk devices. This thorough process necessitates extensive clinical data to demonstrate reliability and efficacy, along with comprehensive documentation.
European Union: Under the Medical Device Regulation (MDR), the EU emphasizes the necessity of clinical evidence and robust post-market surveillance. The MDR, effective since 2021, mandates that manufacturers conduct thorough assessments to ensure compliance with safety and performance standards.
Latin America: Countries such as Brazil and Mexico have developed their own regulatory frameworks, often drawing from FDA and EU standards. Navigating these regulations is crucial for successful market entry, as local compliance requirements can vary significantly. The healthcare market in Latin America is anticipated to reach approximately $37.23 billion by 2025, highlighting the opportunity for groundbreaking medical tools in this area. However, manufacturers must remain vigilant regarding the unique regulatory challenges and opportunities that exist. Bioaccess® leverages over 20 years of expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, offering a customized approach to guide manufacturers through these complexities.
Grasping these regulatory variations is imperative for manufacturers seeking to launch Class III medical devices into diverse markets, as each region presents distinct challenges and opportunities.
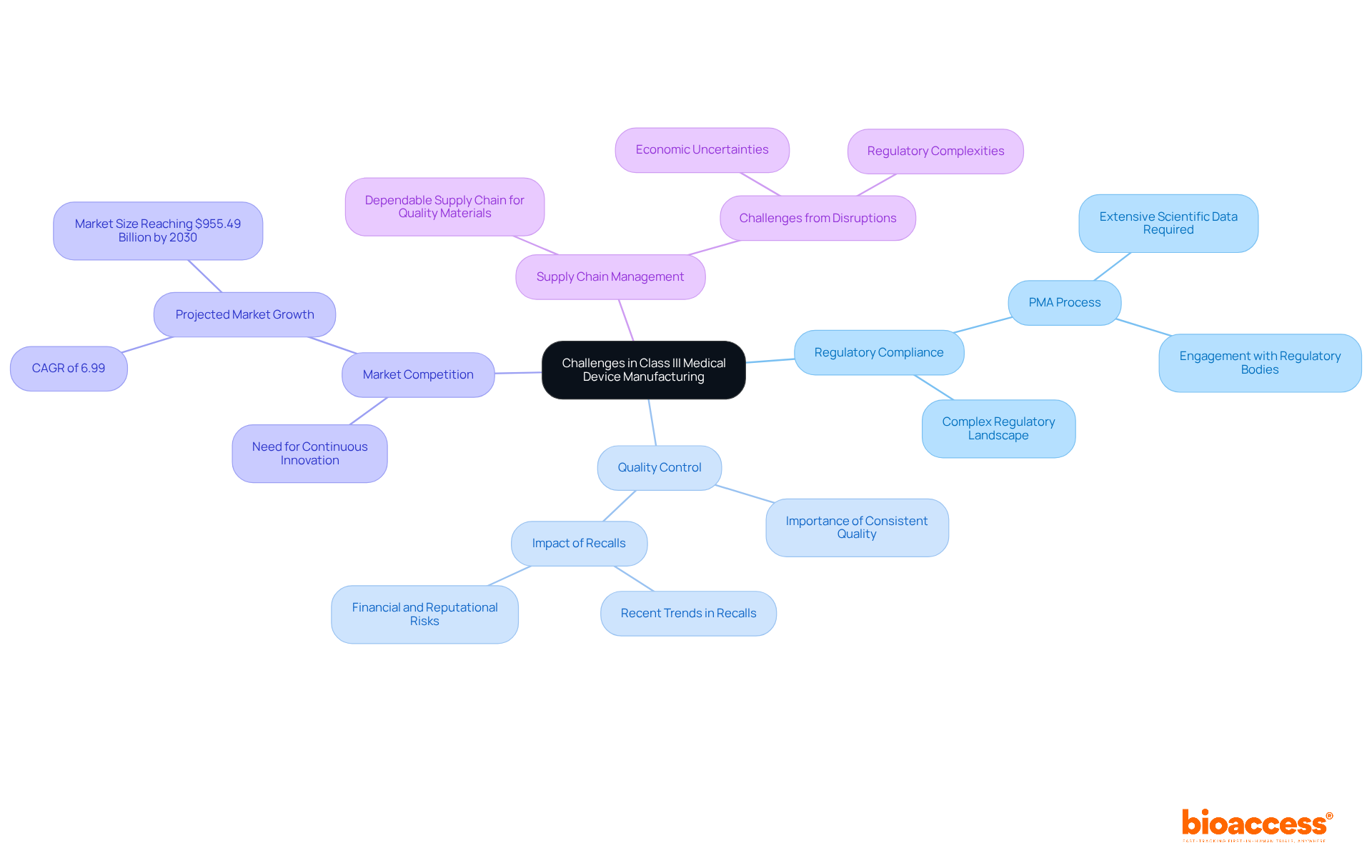
Manufacturers of class iii medical devices examples face a multitude of significant challenges that can influence their market success.
Regulatory Compliance: The regulatory landscape for Class III devices is both intricate and demanding. Representing approximately 9% of the industry, these devices must adhere to the most stringent standards, necessitating the Premarket Approval (PMA) process. This process requires comprehensive scientific data to demonstrate reliability and efficacy, making early engagement with regulatory bodies essential to clarify expectations and streamline submissions.
Quality Control: Ensuring consistent quality across production batches is vital for consumer protection. Failures in quality control can lead to recalls, jeopardizing patient safety and severely affecting a company's reputation and financial health. Recent trends indicate a rise in recalls among Category II products, underscoring the importance of rigorous quality management systems.
Market Competition: The high stakes associated with class iii medical devices examples foster fierce competition. Manufacturers must innovate continuously while optimizing their processes to remain competitive. The U.S. medical equipment market is projected to grow at a CAGR of 6.99%, reaching $955.49 billion by 2030, amplifying the need for companies to effectively differentiate their products.
Supply Chain Management: A dependable supply chain for high-quality materials is essential for timely production. Disruptions in the supply chain can lead to delays in product market entry, complicating compliance with regulatory timelines. As the industry evolves, manufacturers must navigate new challenges, including economic uncertainties and regulatory complexities.
Industry leaders stress the necessity of comprehending these regulatory frameworks to mitigate risks associated with misclassification and potential recalls. By proactively addressing these challenges, manufacturers can enhance their ability to deliver innovative medical solutions to patients in a timely manner.
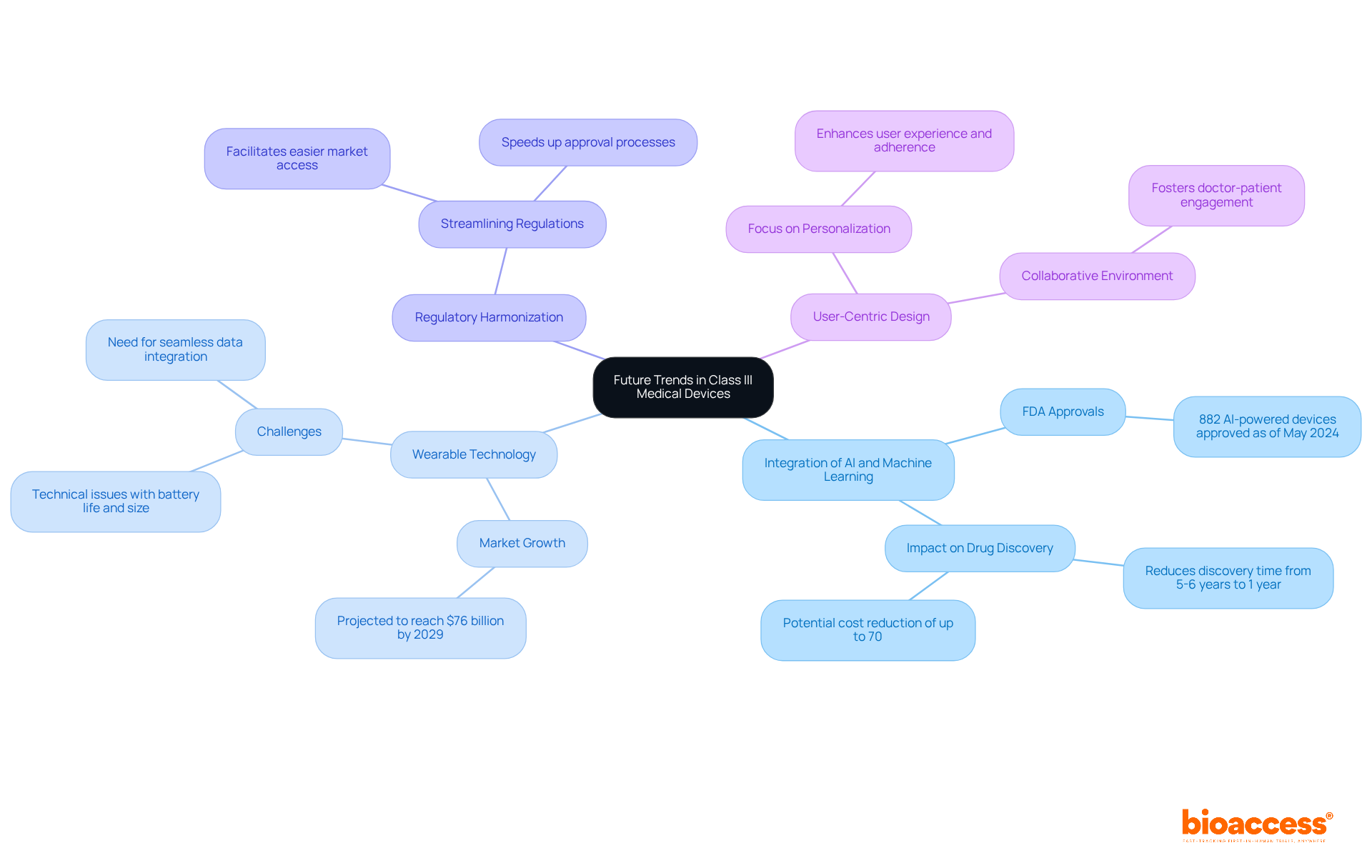
Future trends in Class III medical devices are characterized by several key innovations:
Integration of AI and Machine Learning: The incorporation of AI and machine learning is revolutionizing device functionality and patient monitoring. These technologies enable equipment to analyze vast amounts of data, enhancing diagnostic accuracy and personalizing treatment plans. As of May 2024, the FDA has authorized 882 products powered by AI and machine learning, reflecting the increasing dependence on these technologies in healthcare. This integration not only improves healthcare but also significantly decreases drug discovery time, demonstrating the transformative potential of AI in the medical field.
Wearable Technology: There is a growing demand for wearable gadgets that offer real-time health information, improving patient involvement and self-management. The market for consumer and medical-grade wearables is projected to grow significantly, with estimates suggesting it could reach $76 billion by 2029. However, challenges such as technical issues related to battery life and size must be addressed to fully realize the potential of these gadgets. These wearables facilitate continuous health monitoring and contribute to proactive healthcare management by minimizing disease risks and improving early diagnosis.
Regulatory Harmonization: Efforts are underway to streamline regulations across various regions, promoting easier market access for innovative medical products. This harmonization is essential for speeding up the approval process and ensuring that innovative technologies reach individuals more quickly.
User-Centric Design: There is an increasing focus on creating user-friendly tools that enhance the experience and adherence of individuals. This trend aligns with the shift towards personalized healthcare, where devices are customized to meet individual needs, ultimately improving health outcomes. Furthermore, wearable technology fosters a collaborative environment between doctors and patients, emphasizing the importance of user engagement in the design process.
These trends indicate a significant shift towards more innovative, efficient, and patient-focused medical technologies, including examples of Class III medical devices, paving the way for advancements that will transform healthcare delivery.
The exploration of Class III medical devices underscores their pivotal role in modern healthcare, particularly in life-saving applications. These devices—ranging from pacemakers to implantable defibrillators—demand rigorous regulatory scrutiny to guarantee their safety and efficacy. It is essential for stakeholders in the medical technology sector to grasp the complexities of their development, which includes the critical roles of clinical trials and post-market compliance.
Key insights presented throughout the article detail the significance of Class III devices and the challenges encountered in their manufacturing and approval processes. The advantages of conducting clinical trials in regions like Colombia, bolstered by organizations such as bioaccess®, illustrate the potential for accelerated innovation in Latin America. Furthermore, ongoing advancements in technology, such as AI integration and user-centric design, are shaping the future landscape of medical devices, promising improved patient outcomes and streamlined regulatory processes.
As the healthcare industry evolves, the emphasis on innovation and compliance remains paramount. Stakeholders are urged to stay informed about regulatory changes and emerging trends in Class III medical devices. By embracing these developments, manufacturers can enhance their capacity to deliver effective medical solutions, ultimately contributing to better health outcomes for patients worldwide.
What is bioaccess® and its role in medical device development?
bioaccess® is dedicated to accelerating the development of Class III medical devices in Latin America, particularly leveraging Colombia's competitive advantages such as cost reductions, regulatory efficiency, and a robust healthcare system.
What are the cost advantages of developing medical devices in Colombia?
Developing medical devices in Colombia can lead to cost reductions of over 30% compared to North America and Western Europe.
How efficient is the regulatory process for clinical trials in Colombia?
Ethical approvals in Colombia can be secured in just 4-6 weeks, and the total review process involving IRB/EC and the Ministry of Health (INVIMA) takes approximately 90-120 days.
What is the significance of Class III medical devices in healthcare?
Class III medical devices are high-risk products essential for sustaining or supporting human life and require premarket approval due to their inherent risks. They play a critical role in preventing severe health impairments and enhancing patient outcomes.
Can you provide examples of Class III medical devices?
Examples of Class III medical devices include pacemakers, implantable defibrillators, prosthetic heart valves, and cochlear implants.
How do pacemakers contribute to patient care?
Pacemakers regulate heart rhythms and significantly lower the risk of sudden cardiac arrest, thereby improving survival rates and overall quality of life for patients with arrhythmias.
What is the function of implantable defibrillators?
Implantable defibrillators monitor and correct abnormal heart rhythms, providing immediate assistance for individuals at risk of sudden cardiac arrest.
What benefits do cochlear implants provide?
Cochlear implants restore auditory perception for individuals with severe hearing loss, greatly improving their communication skills and overall quality of life.
How do breast implants relate to Class III medical devices?
Breast implants are used for both reconstructive and cosmetic purposes, significantly affecting individuals' self-esteem and body image, thereby enhancing emotional well-being.
What role do deep brain stimulators play in healthcare?
Deep brain stimulators are surgically implanted in the brain to address neurological disorders such as Parkinson's disease, alleviating severe symptoms and improving daily functioning.
How does bioaccess® support clinical trials for Class III medical devices?
bioaccess® enhances the efficiency of clinical trials by providing expertise in managing the regulatory process and facilitating patient recruitment in Colombia’s diverse healthcare landscape.