


The article delineates ten essential abbreviations pertinent to antibodies that every research director must comprehend, underscoring their significance in clinical research and therapeutic applications. It accentuates the necessity of grasping these abbreviations, including:
as they are vital for adeptly navigating the intricacies of antibody research and enhancing treatment strategies in contemporary medicine.
Understanding the language of antibodies is crucial for any research director navigating the complex landscape of immunology. Abbreviations such as mAb, Ig, and Fab frequently appear in scientific literature; grasping their meanings unlocks a wealth of knowledge about antibody applications and their therapeutic potential. As the field evolves, researchers face an ongoing challenge: how can they keep pace with rapid advancements and ensure they are utilizing the most relevant and effective antibody strategies? This article delves into ten essential abbreviations for antibodies, offering insights that can enhance both research and clinical applications.
bioaccess® distinguishes itself by providing expedited medical study services specifically designed for immune response investigations. By leveraging the regulatory efficiency of Latin America alongside the diverse patient populations in the Balkans, bioaccess® secures ethical approvals in a remarkable timeframe of just 4-6 weeks. This swift turnaround is critical for project directors focused on accelerating the development of therapies, specifically the abbreviation for antibodies.
In under 8 weeks, bioaccess® has activated over 50 locations, ensuring that trials can commence swiftly and effectively. Furthermore, bioaccess® delivers FDA/EMA/MDR-ready datasets and centralized monitoring, which are vital components of their comprehensive services.
The collaboration with Caribbean Health Group aims to position Barranquilla as a premier site for trials in Latin America, supported by Colombia's Minister of Health, thereby enhancing the region's appeal for medical studies. For instance, the AMP trials highlighted the importance of timely medical trials, as they assessed the broadly neutralizing agent VRC01, which demonstrated 74% effectiveness against specific HIV strains. This underscores the crucial need for rapid clinical research services in the evolving landscape of therapeutic agents.

Monoclonal proteins (mAbs), which are an abbreviation for antibodies, are laboratory-engineered molecules designed to attach specifically to targeted antigens. Originating from a single clone of B cells, they ensure both uniformity and specificity in their interactions. mAbs, the abbreviation for antibodies, play a pivotal role in diagnostics and therapeutics, particularly within oncology and autoimmune diseases, where they have transformed treatment paradigms. Notably, approximately 80 mAbs have received marketing approval, with 12 approved by the FDA in 2018, underscoring the ongoing development in this field.
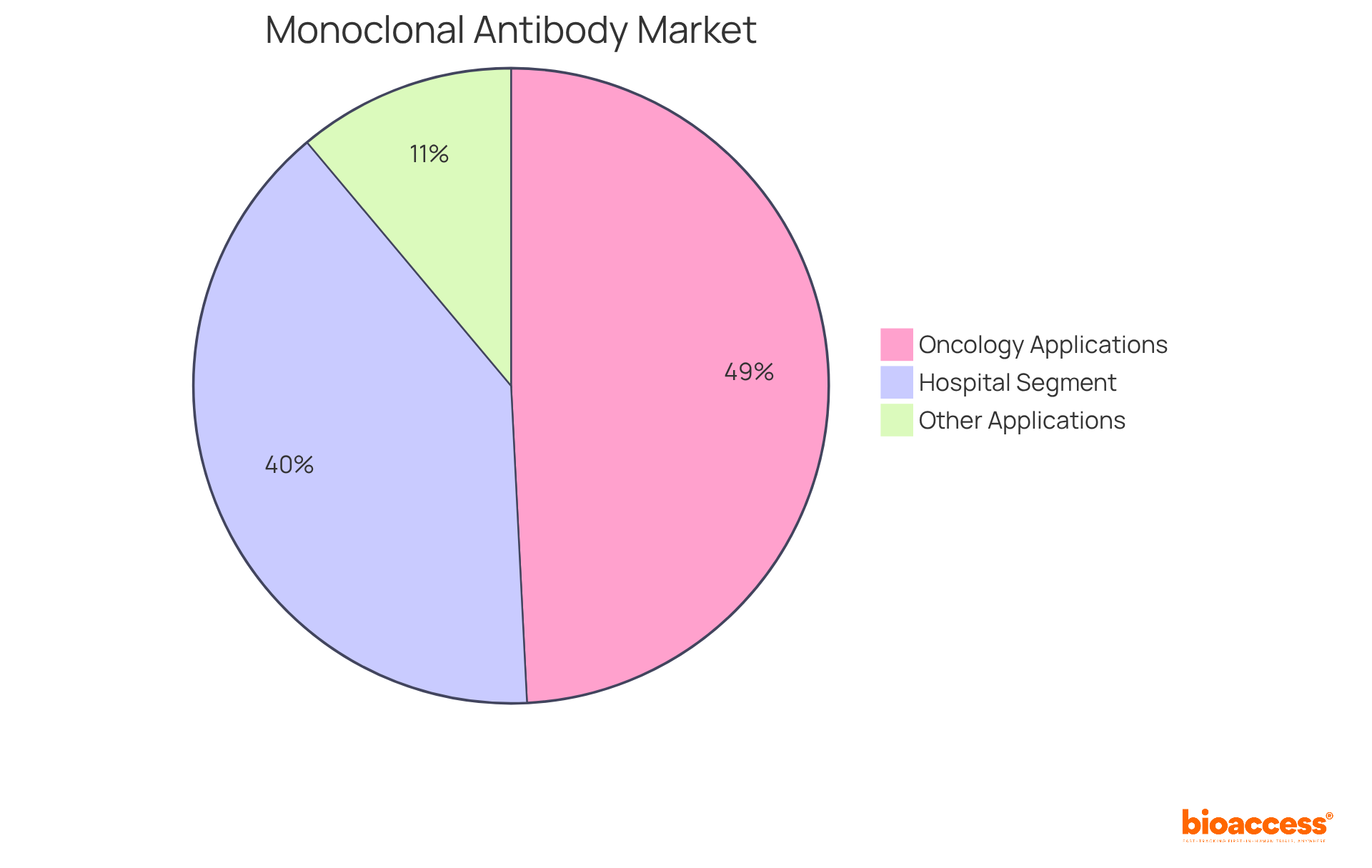
The oncology segment alone accounted for 49.2% of the applications market for mAbs in 2022, while the hospitals segment held the largest market share of 39.7% due to the increasing adoption of mAbs for cancer treatment. Researchers emphasize that mAbs, the abbreviation for antibodies, not only enhance diagnostic capabilities but also provide targeted therapies that minimize off-target effects, thereby improving patient outcomes.
As Mark Wingertzahn, Senior Vice President of Clinical Development at Invivyd, observes, "Monoclonal therapies represent one of the most transformative breakthroughs in modern medicine, providing rapid, targeted protection by utilizing the immune system’s natural defenses."
Furthermore, the development of humanized mAbs, the abbreviation for antibodies, has addressed previous immunogenicity concerns, solidifying their importance in clinical research and patient care. The mAbs market is projected to reach USD 494.53 billion by 2030, growing at a CAGR of 11.04% from 2023 to 2030, underscoring their increasing relevance and investment.
Immunoglobulins (Ig), known as the abbreviation for antibodies, are categorized into five primary types: IgG, IgA, IgM, IgD, and IgE, each serving unique roles in the immune response. Understanding these immunoglobulin types, the abbreviation for antibodies, is crucial for selecting appropriate proteins for research and therapeutic applications, particularly in the evolving landscape of clinical research.
IgG is the most prevalent immunoglobulin, comprising approximately 70-85% of the total immunoglobulin pool in human serum. The abbreviation for antibodies is essential for long-term immunity, effectively neutralizing pathogens and facilitating opsonization. Notably, the abbreviation for antibodies, IgG, can be transferred across the placenta, providing passive immunity to neonates, thereby highlighting its critical role in early life protection.
IgA accounts for about 5-15% of the immune protein pool and is predominantly found in mucosal regions such as saliva, tears, and breast milk. This immunoglobulin, often referred to by the abbreviation for antibodies, is vital for mucosal immunity, defending against pathogens at these surfaces. Research indicates that the daily production of IgA, the abbreviation for antibodies, surpasses that of any other immunoglobulin class, underscoring its significance in maintaining mucosal health.
IgM is the first antibody produced during an immune response, representing 5-10% of the immunoglobulin pool. Its unique pentameric structure serves as the abbreviation for antibodies, enabling effective binding to antigens and positioning it as a key player in the initial defense against infections, thereby establishing its importance in early immune responses.
IgD is present in minimal amounts, accounting for less than 1% of total plasma immunoglobulin. Primarily located on the surface of B lymphocytes, the abbreviation for antibodies plays a role in their activation and maturation, although its precise functions remain under investigation, indicating an area ripe for further research.
IgE, despite being the least abundant, plays a crucial role in mediating allergic reactions and defending against parasitic infections. It binds to allergens and triggers histamine release from mast cells, contributing to the symptoms of allergies, which underscores the need for targeted therapeutic strategies involving the abbreviation for antibodies.
Recent advancements in immune protein engineering are broadening the therapeutic roles of these immunoglobulins, particularly IgG and IgA, known as the abbreviation for antibodies, in addressing previously undruggable diseases. This underscores their importance in modern medicine and the necessity for continued collaboration in clinical research to harness their full potential.

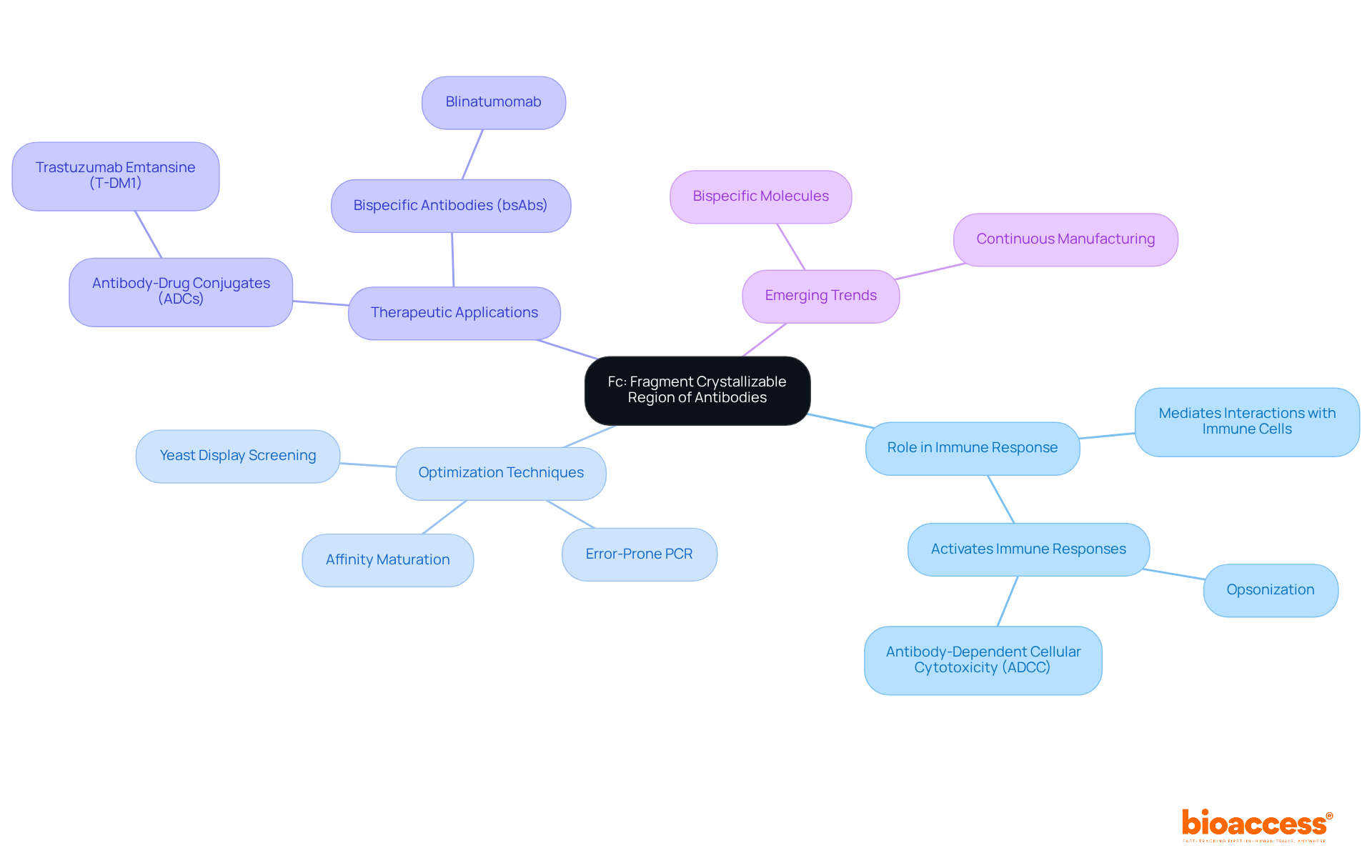
The fragment crystallizable (Fc) region of immunoglobulins is crucial in mediating interactions with immune cells and complement proteins, essential for activating immune responses such as opsonization and antibody-dependent cellular cytotoxicity (ADCC). Recent research underscores that optimizing the Fc region can markedly enhance the therapeutic effectiveness of immunoglobulins in medical applications.
Techniques such as affinity maturation, which incorporates error-prone PCR and yeast display screening, are employed to engineer Fc variants that not only boost ADCC but also extend the serum half-life of immunoglobulins through improved binding to Fc receptors. A notable example is trastuzumab emtansine (T-DM1), an antibody-drug conjugate that illustrates the advantages of Fc optimization in cancer treatment.
Clinical trial data reveal that modifications to the Fc region can lead to improved patient outcomes, highlighting the importance of this region in developing next-generation therapeutic agents. As Emilia O'Connor articulates, "Antigen molecules are made up of two identical heavy chains and two identical light chains, which consequently provide the antigen two binding sites." This understanding is crucial for researchers focused on innovating and refining therapies, which often utilize the abbreviation for antibodies.
Furthermore, the emergence of bispecific molecules (bsAbs) in optimizing therapeutic proteins signifies a pivotal trend in the field, further accentuating the necessity for a comprehensive understanding of the Fc region's role.
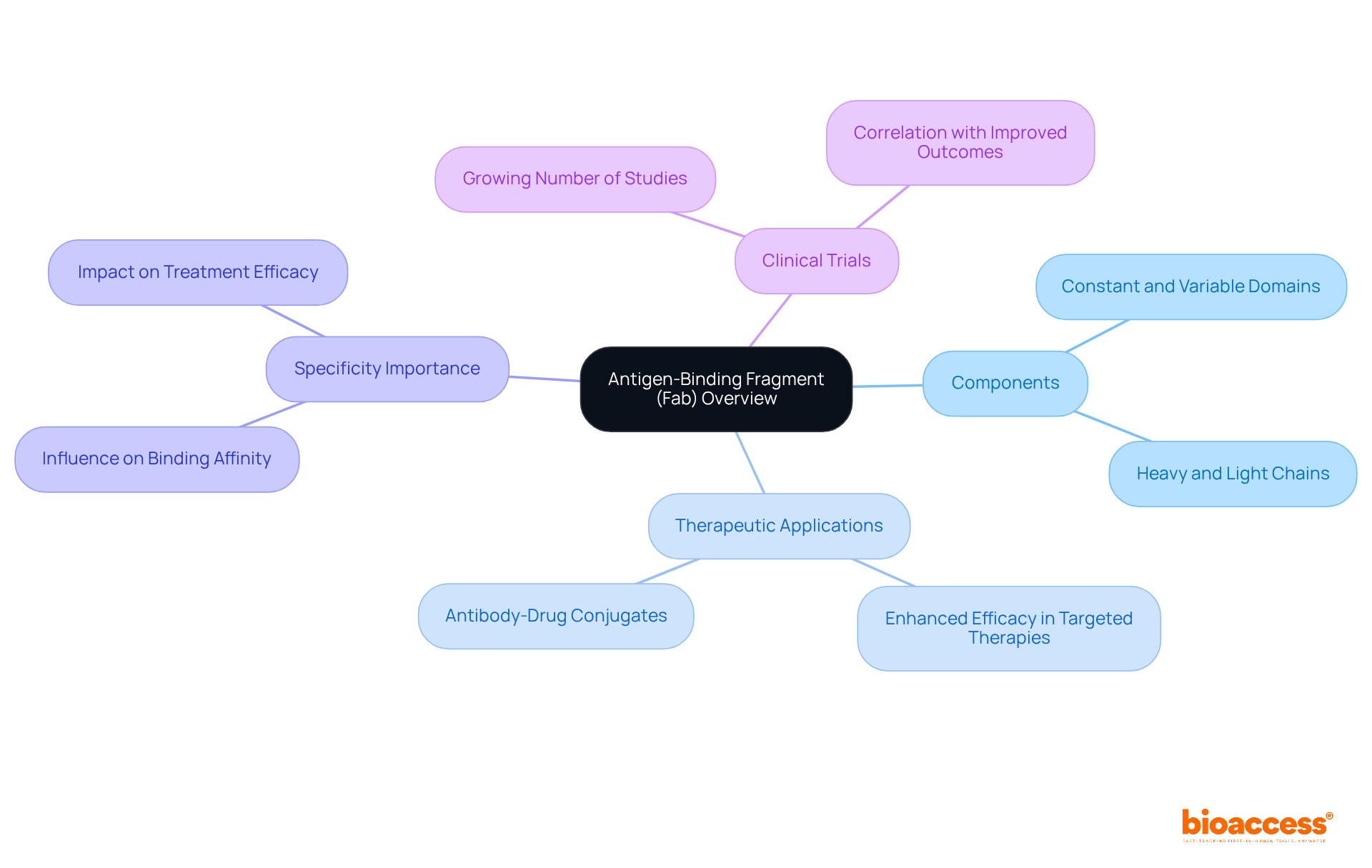
The antigen-binding fragment (Fab) is an essential component of immunoglobulins, crucial for binding to specific antigens. Each Fab fragment comprises one constant and one variable domain from both the heavy and light chains, which is vital for its specificity. The increasing utilization of Fab fragments in therapeutic applications, especially in targeted therapies, highlights their significance in antibody research, where the abbreviation for antibodies is commonly used. Recent studies have demonstrated that Fab fragments can significantly enhance the efficacy of antibody-drug conjugates, which serve as an abbreviation for antibodies, facilitating more precise targeting of cancer cells.
Furthermore, research trials indicate that employing Fab fragments correlates with improved therapeutic outcomes, showcasing their potential across various applications. Notably, researchers emphasize that the specificity provided by Fab fragments, the abbreviation for antibodies, is critical for developing effective therapeutic agents, as it directly influences binding affinity and overall treatment efficacy.
Statistics reveal that Fab fragments are a focal point in clinical trials, with a growing number of studies exploring their applications in targeted therapies, further emphasizing their relevance in the evolving landscape of immunological research.
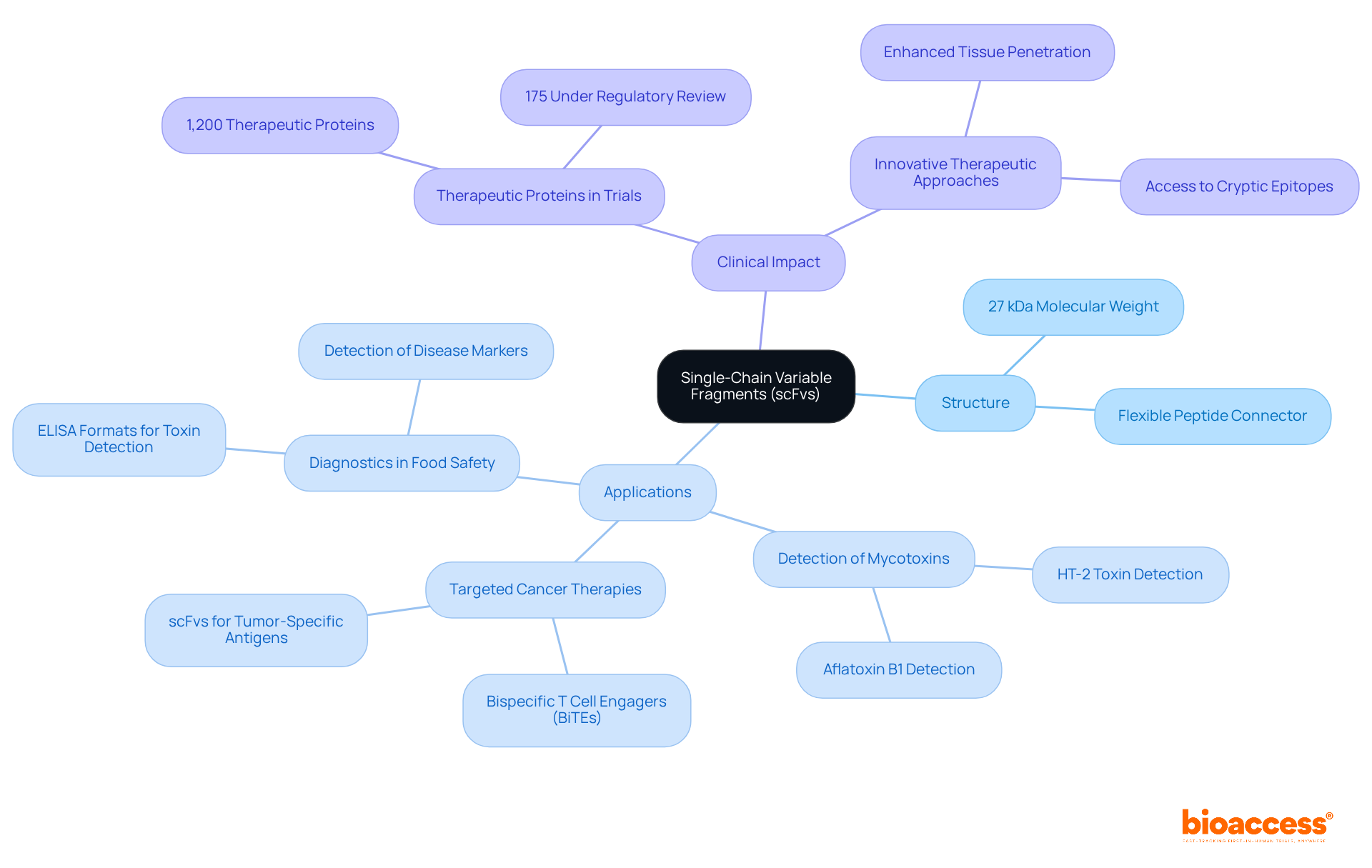
Single-chain variable fragments (scFvs) represent a groundbreaking advancement in engineered proteins, derived from the variable regions of heavy and light chains and interconnected by a flexible peptide connector. With a molecular weight of approximately 27 kDa, scFvs are significantly smaller than traditional immunoglobulins, facilitating enhanced tissue penetration and expedited clearance from the body. This distinctive structure empowers scFvs to bind antigens with exceptional specificity and affinity, thereby increasing their value in therapeutic and diagnostic applications.
By 2025, an estimated 1,200 therapeutic proteins are undergoing clinical trials, with around 175 currently under regulatory review, illustrating the dynamic landscape of medical development. Within this context, scFvs are pivotal, showcasing versatility across various applications, such as:
Notably, scFvs have been engineered to target tumor-specific antigens, resulting in the innovative creation of bispecific T cell engagers (BiTEs) that redirect T cells towards cancer cells, thus enhancing treatment outcomes.
Clinical researchers emphasize that the reduced size of scFvs provides superior access to cryptic epitopes, particularly advantageous in tumor microenvironments. Getachew Gezehagn Kussia highlights this potential, stating, "the smaller size of scFv proteins allows for easy tissue penetration of tumors and access to cryptic epitopes," which underscores their promise in pioneering therapies.
The practical applications of scFvs are evident in diagnostics, where they have been effectively utilized to detect harmful substances in food products and identify disease markers across various conditions. For instance, the development of an HT-2 toxin-specific ELISA format illustrates the tangible implications of scFvs in safeguarding food safety. The ongoing research and development surrounding scFvs underscore their potential to revolutionize both therapeutic and diagnostic fields, establishing them as essential tools in contemporary immune research.
Antibody-dependent cellular cytotoxicity (ADCC), the abbreviation for antibodies, plays a pivotal role in the immune response by directing immunoglobulins to target and eliminate harmful tissues. This sophisticated process occurs when an immune protein binds to a foreign substance on a target structure, subsequently enlisting effector units, such as natural killer (NK) cells. These NK cells are essential as they trigger programmed cell death in the affected target structure.
The significance of this mechanism is particularly pronounced in cancer treatment, where the abbreviation for antibodies, monoclonal antibodies (mAbs), is engineered to enhance ADCC against tumor cells. Understanding and leveraging ADCC can lead to more effective therapeutic strategies, highlighting the importance of continued research and collaboration in the Medtech landscape.

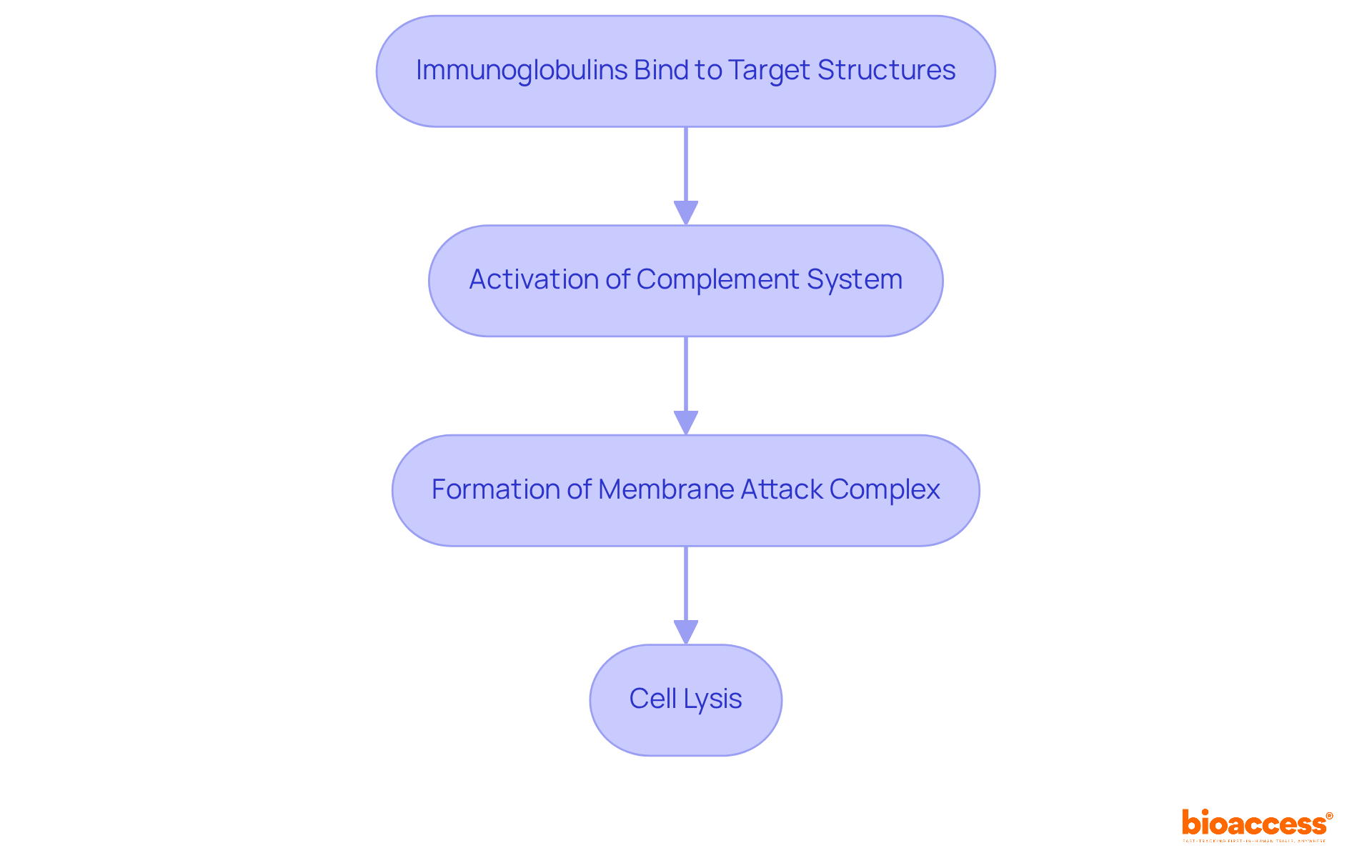
Complement-dependent cytotoxicity (CDC) represents a pivotal immune response mechanism wherein immunoglobulins bind to target structures, subsequently activating the complement system and leading to cell lysis. This intricate process encompasses the formation of the membrane attack complex, which effectively disrupts the integrity of the target cell's membrane. Understanding CDC is essential for the advancement of therapeutic proteins that rely on this mechanism to enhance their efficacy.
Pharmacokinetics (PK) is the study of how an immune protein is absorbed, distributed, metabolized, and excreted in the body. Factors such as molecular size, charge, and glycosylation significantly influence the PK profile of monoclonal immunoglobulins. Understanding PK is essential for optimizing dosing regimens, thereby improving therapeutic outcomes in clinical settings. This knowledge is not merely academic; it has practical implications that can enhance patient care and treatment efficacy.
The enzyme-linked immunosorbent assay (ELISA) stands as a pivotal laboratory method for identifying and quantifying specific proteins, immunoglobulins, or antigens within a sample. This assay relies on the binding of an antigen to a specific immune protein, followed by the detection of this complex via enzyme-linked secondary proteins. ELISA plays a crucial role in assessing levels of the abbreviation for antibodies in various research and healthcare applications.
Historically, ELISA has proven indispensable in numerous medical contexts. Initially applied in 1945 for malaria diagnosis, it highlighted its early significance in managing infectious diseases. By 1974, the method found utility in parasitology for identifying trichinosis, further cementing its status in clinical diagnostics. As of 2025, the relevance of ELISA continues to expand, particularly in immune system studies, where it is vital for understanding immune responses and developing therapeutic interventions.
Recent advancements in ELISA methodologies have significantly enhanced its sensitivity and specificity, establishing it as the preferred technique for detecting the abbreviation for antibodies. Innovations, including the integration of nanotechnology and refined enzyme conjugates, have yielded more precise results, with some methods demonstrating diagnostic sensitivities exceeding those of traditional assays—such as NaSRED's sensitivity being over 10 times greater. These developments not only streamline the detection process but also expand the potential applications of ELISA in both clinical and experimental environments, ensuring its sustained importance in the evolving field of biomedical research.
As Peter Perlman noted, 'The ELISA method has changed the field of laboratory diagnostics, offering researchers a dependable tool for detection and quantification, which is essential for understanding the abbreviation for antibodies.' This statement underscores the critical role of ELISA in ongoing antibody research and highlights the importance of understanding the abbreviation for antibodies in the advancement of medical science.
Understanding essential abbreviations for antibodies is crucial for any research director navigating the complex landscape of immunological studies. This article highlights ten key abbreviations that encapsulate significant concepts in antibody research and underscore the transformative role these proteins play in diagnostics and therapeutics. Familiarity with these terms enhances communication within the scientific community and aids in the efficient development of innovative therapies.
The discussion covers various abbreviations, including:
Each abbreviation represents a vital aspect of antibody function and application, from mechanisms of action like ADCC and CDC to methodologies such as ELISA that facilitate research and clinical diagnostics. These insights illustrate the dynamic nature of antibody research and the importance of these abbreviations in shaping future therapeutic strategies.
As the field of antibody research continues to evolve, staying informed about these key terms and their implications will empower researchers to make informed decisions and drive advancements in medical science. Embracing this knowledge not only enhances individual expertise but also contributes to collective progress in developing effective treatments that harness the power of the immune system.
What is bioaccess® and what services does it provide?
bioaccess® is a company that offers expedited medical study services specifically designed for immune response investigations, facilitating clinical research for antibody studies.
How quickly can bioaccess® secure ethical approvals for clinical trials?
bioaccess® can secure ethical approvals in a remarkable timeframe of just 4-6 weeks.
What is the significance of the 50 locations activated by bioaccess®?
In under 8 weeks, bioaccess® has activated over 50 locations to ensure that clinical trials can commence swiftly and effectively.
What kind of datasets does bioaccess® deliver?
bioaccess® delivers FDA/EMA/MDR-ready datasets and provides centralized monitoring as part of its comprehensive services.
What is the collaboration between bioaccess® and Caribbean Health Group about?
The collaboration aims to position Barranquilla, Colombia, as a premier site for clinical trials in Latin America, supported by the Colombian Minister of Health.
What is the role of monoclonal antibodies (mAbs) in medicine?
Monoclonal antibodies are laboratory-engineered molecules designed to attach specifically to targeted antigens, playing a pivotal role in diagnostics and therapeutics, particularly in oncology and autoimmune diseases.
How many mAbs have received marketing approval?
Approximately 80 monoclonal antibodies have received marketing approval, with 12 approved by the FDA in 2018.
What market segment accounted for the largest share of mAb applications in 2022?
The oncology segment accounted for 49.2% of the applications market for monoclonal antibodies in 2022.
What are the five primary types of immunoglobulins (Ig)?
The five primary types of immunoglobulins are IgG, IgA, IgM, IgD, and IgE.
What is the most prevalent immunoglobulin and its primary role?
IgG is the most prevalent immunoglobulin, comprising 70-85% of the total immunoglobulin pool, and is essential for long-term immunity and neutralizing pathogens.
What is the role of IgA in the immune response?
IgA accounts for about 5-15% of the immune protein pool and is crucial for mucosal immunity, found in regions like saliva, tears, and breast milk.
What is the function of IgM during an immune response?
IgM is the first antibody produced during an immune response, playing a key role in the initial defense against infections.
What is the role of IgE in the immune system?
IgE mediates allergic reactions and defends against parasitic infections by binding to allergens and triggering histamine release from mast cells.