


This article delineates ten essential features of electronic Case Report Forms (eCRFs) that are vital for research directors in clinical trials. Key attributes include:
Collectively, these features significantly enhance the efficiency, accuracy, and overall effectiveness of clinical research. By streamlining data management processes and facilitating faster patient enrollment, eCRFs contribute to reducing costs and improving study outcomes. The integration of these features is not merely advantageous; it is imperative for advancing clinical research in an increasingly competitive landscape.
The evolving landscape of clinical trials demands innovative solutions that streamline processes and enhance data integrity. As research directors navigate the complexities of managing trials, the implementation of electronic Case Report Forms (eCRFs) emerges as a pivotal strategy. This article delves into ten essential features of eCRFs that not only facilitate rapid implementation but also optimize trial management, ultimately leading to improved outcomes.
How can these features transform the way clinical studies are conducted, and what challenges do they address in the pursuit of efficiency and accuracy?
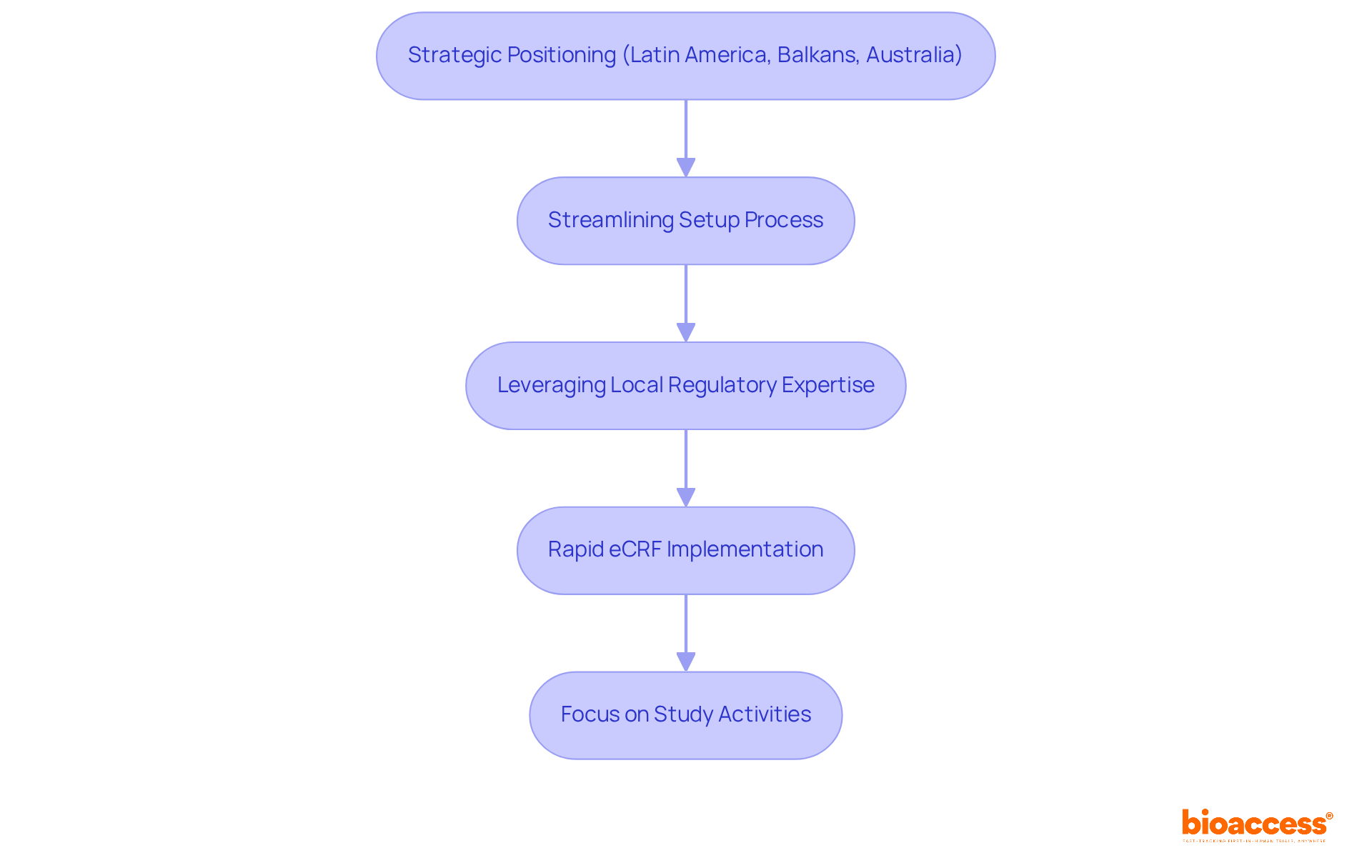
The company strategically positions itself in Latin America, the Balkans, and Australia to implement electronic Case Report Forms (eCRFs) promptly for the eCRF clinical trial, ensuring that clinical studies can commence without unnecessary delays.
By streamlining the setup process and leveraging local regulatory expertise, the platform achieves eCRF clinical trial implementation in record time.
This efficiency enables research directors to focus on essential study activities rather than administrative hurdles, ultimately enhancing the overall effectiveness of clinical research.
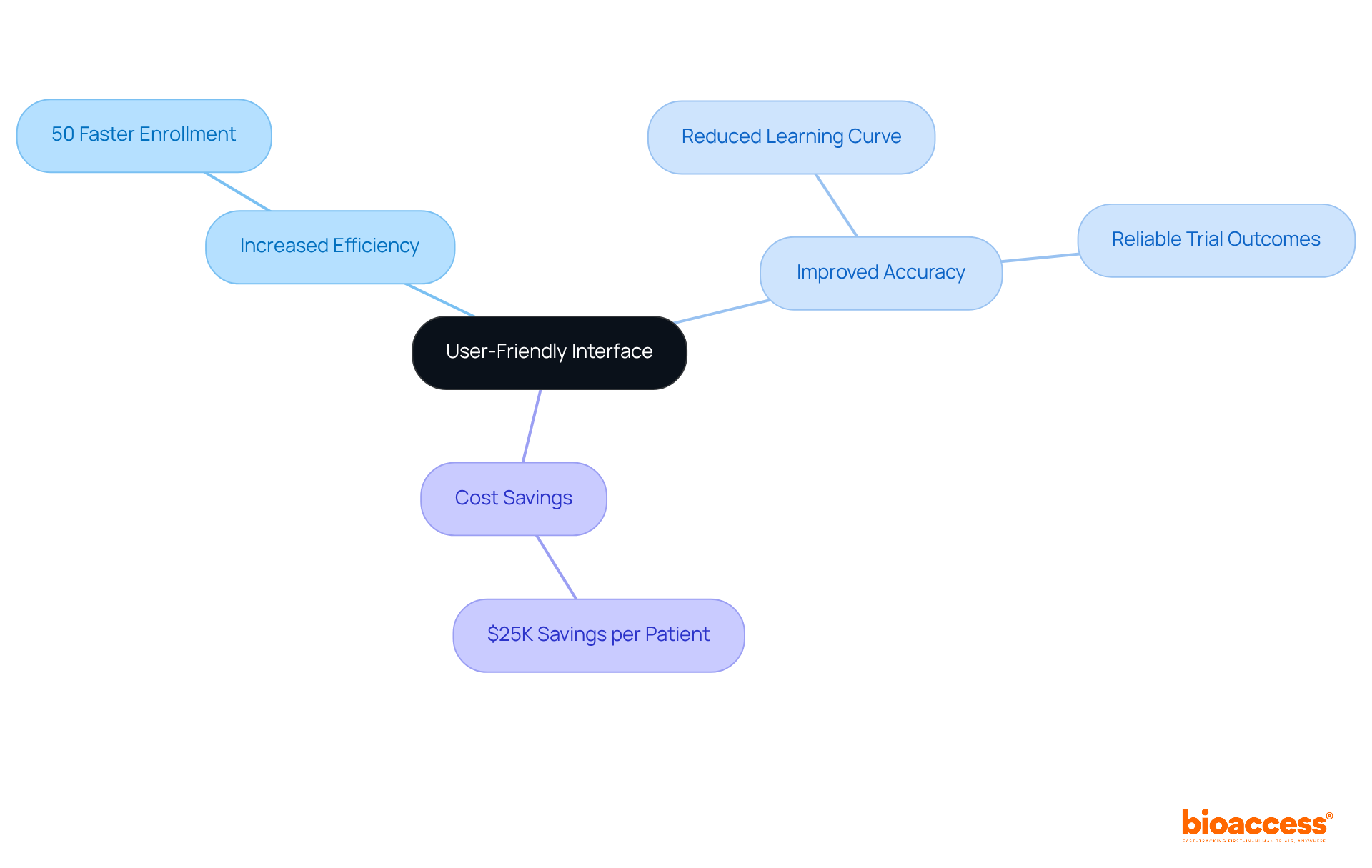
The system for the eCRF clinical trial offered by bioaccess® features a user-friendly interface designed to enhance entry efficiency, enabling research teams to enroll treatment-naive cardiology or neurology cohorts 50% faster than their Western counterparts. This intuitive design significantly reduces the learning curve for new users, facilitating quicker information input and yielding improved accuracy and more reliable trial outcomes. By streamlining information entry, research leaders can anticipate substantial savings of $25K per patient in the eCRF clinical trial with FDA-ready data—effectively eliminating rework and delays.
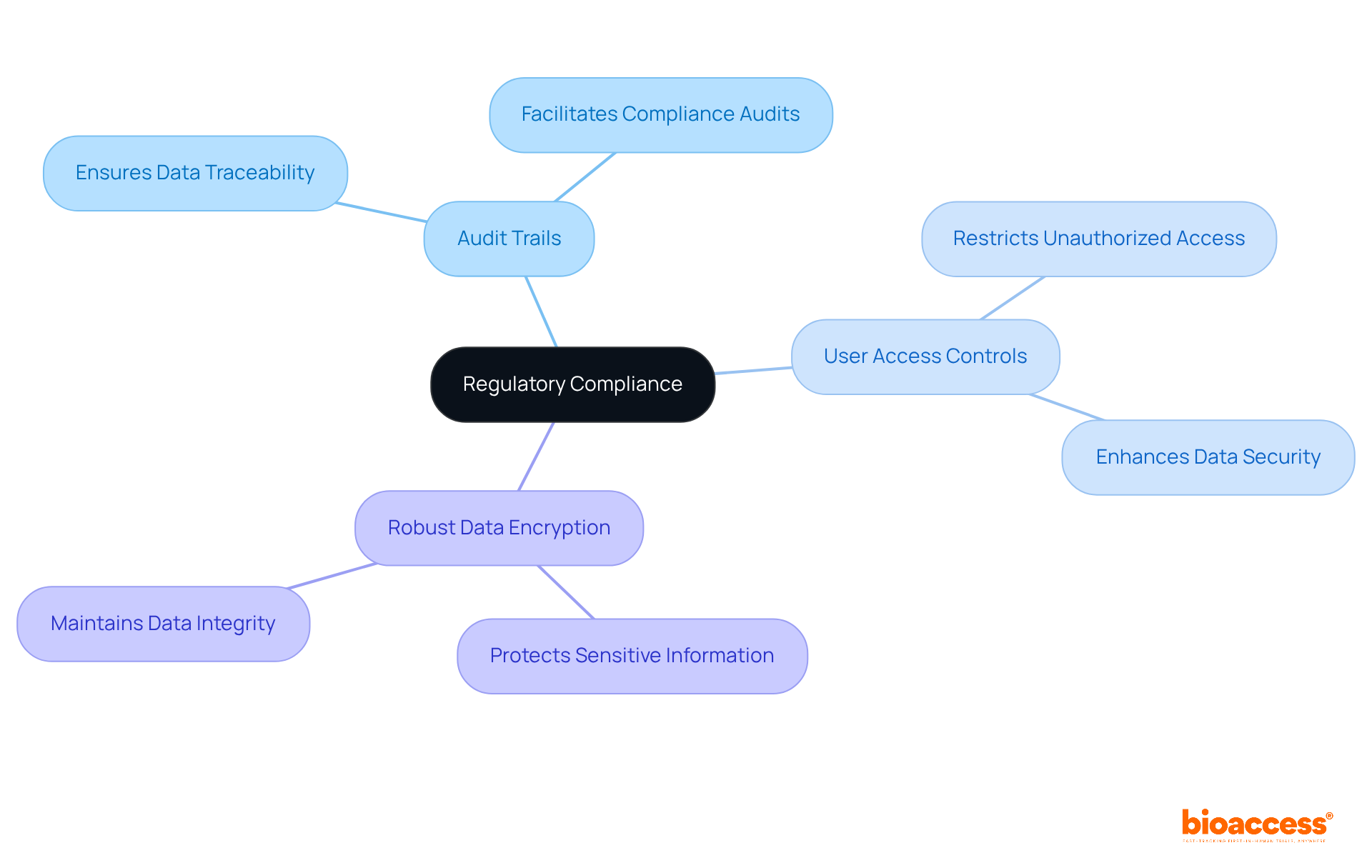
The company places a strong emphasis on regulatory compliance within its eCRF clinical trial systems, ensuring that all collected information adheres to rigorous industry standards. With an accelerated regulatory approval timeline of just 6-8 weeks—compared to the typical 6-12 months in the US/EU—bioaccess® enables research directors to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites. Key features such as:
are seamlessly integrated to uphold data integrity and security. These measures not only safeguard sensitive information but also improve the overall dependability of clinical studies. Research leaders can be assured that their studies will adhere to all required regulations, thereby protecting both the integrity of the research and the welfare of participants. As Seema Verma, Executive Vice President and General Manager at Oracle Health and Life Sciences, states, "Our EDC enhancements underscore Oracle's commitment to delivering AI-driven capabilities and aligning clinical research and clinical care by creating a more open healthcare ecosystem that fuels medical breakthroughs for the patients who desperately need them." This statement emphasizes the essential function of advanced eCRF clinical trial systems in preserving information integrity while addressing regulatory challenges in clinical studies.
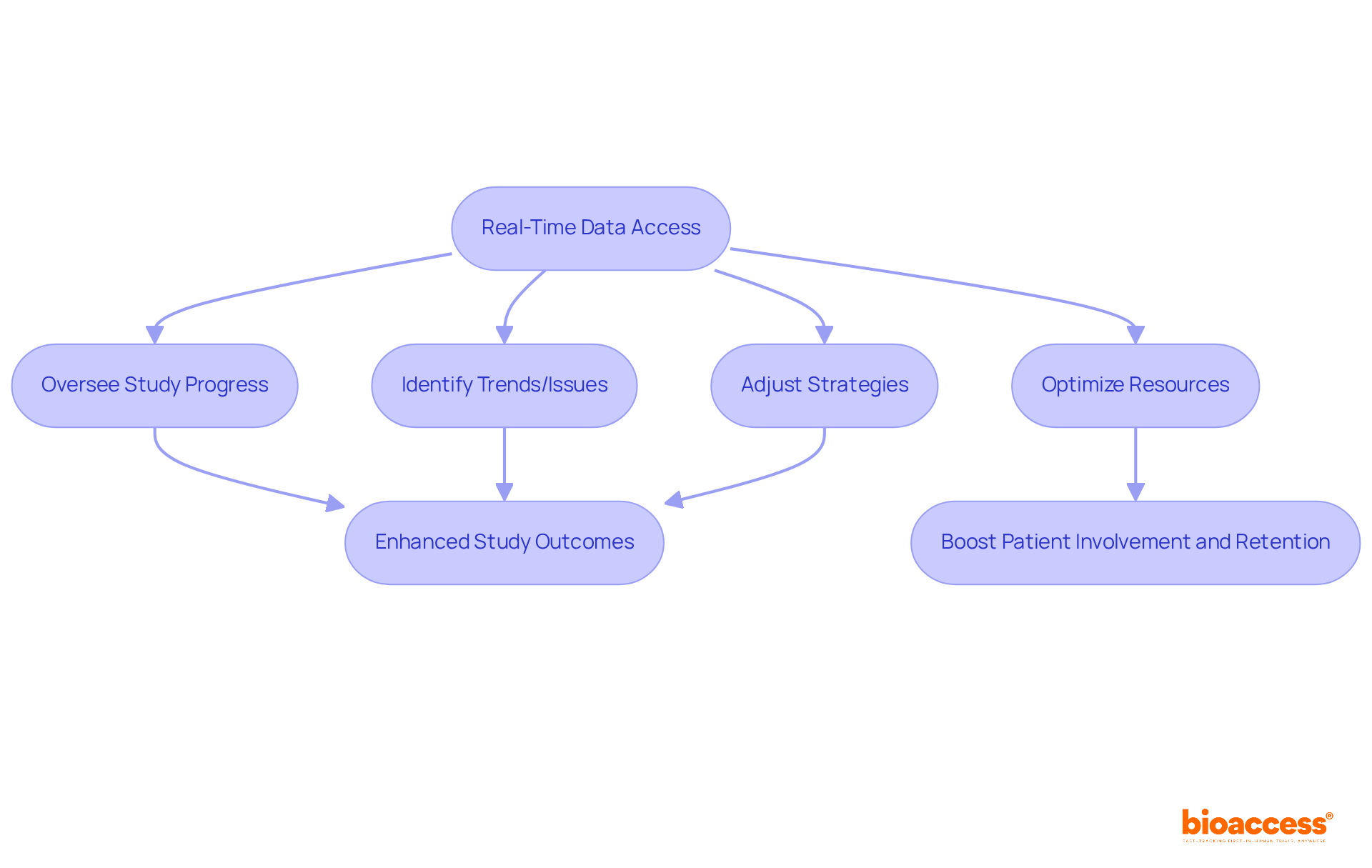
The eCRF clinical trial system from bioaccess® empowers research leaders with real-time data access, enabling them to oversee study progress and make informed decisions swiftly. This capability facilitates the prompt identification of trends or issues, allowing for proactive management throughout the study. With real-time insights, leaders can dynamically optimize resources and adjust strategies, ultimately enhancing the outcomes of the tests.
By leveraging real-time analytics, research leaders can significantly boost patient involvement and retention, which are crucial for achieving successful study outcomes. The collaboration between GlobalCare Clinical Trials and bioaccess™ exemplifies the effectiveness of these strategies, having achieved over a 50% reduction in recruitment time and a retention rate exceeding 95% in Colombia.
This underscores the transformative potential of real-time data in the eCRF clinical trial process, establishing it as an essential tool for effective trial management in 2025.
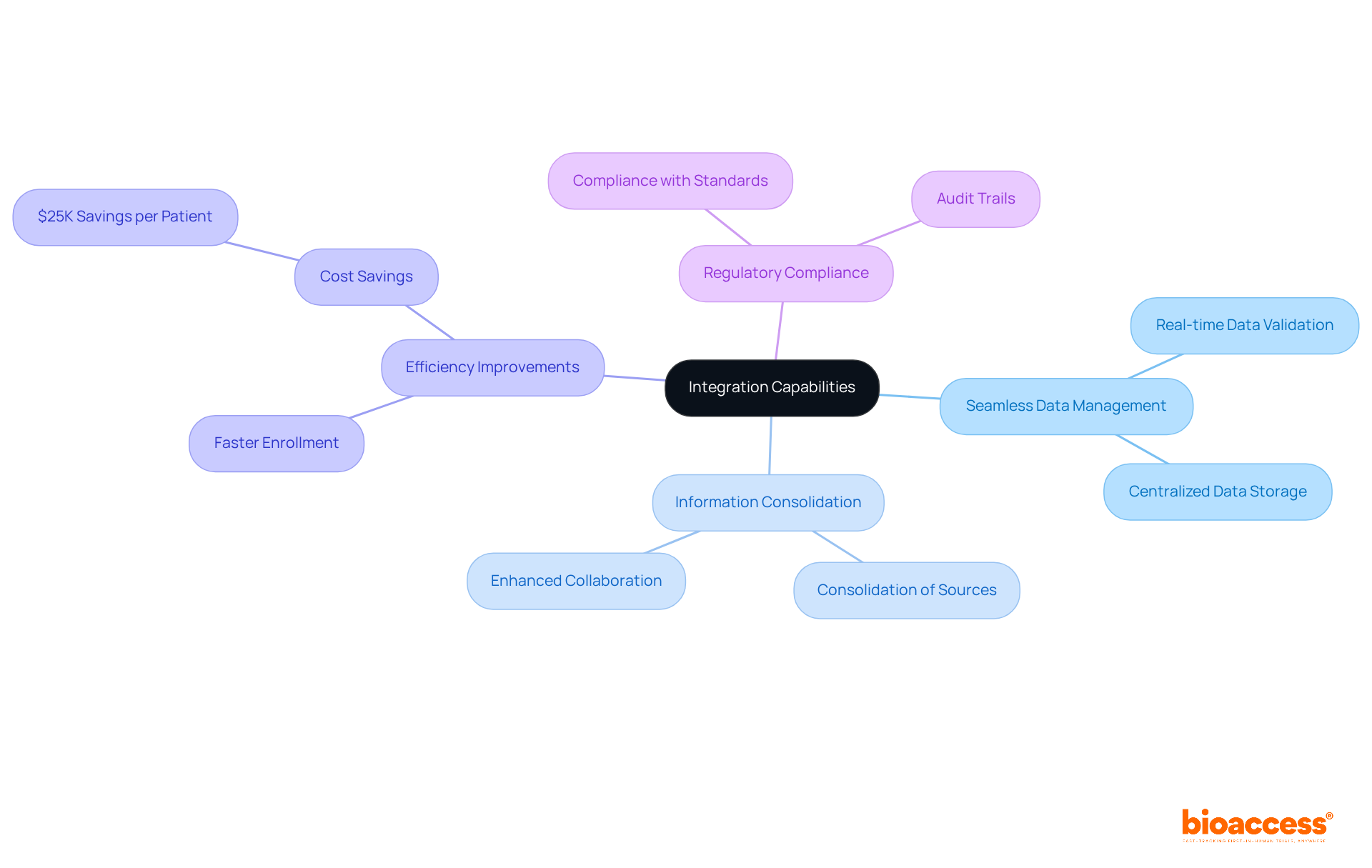
bioaccess®’s eCRF clinical trial system showcases advanced integration capabilities that facilitate seamless data management across multiple platforms. This powerful feature empowers research leaders to consolidate various information sources, including laboratory results and patient records, into a cohesive dataset. By streamlining information management processes, directors can significantly enhance the accuracy of their analyses and improve the overall efficiency of the eCRF clinical trial.
In 2025, the emphasis on information consolidation becomes crucial, as it not only minimizes the time spent on entry and validation but also mitigates errors associated with manual processes. Remarkably, bioaccess® allows treatment-naive cardiology or neurology cohorts to be enrolled 50% faster than Western sites, resulting in $25K savings per patient with FDA-ready data—no rework, no delays.
Clinical researchers have noted that effective information management practices are essential for preserving integrity and ensuring compliance with regulatory standards in an eCRF clinical trial. As one researcher stated, 'The effectiveness achieved from optimized information management is priceless in speeding up the progress of clinical studies.'
This integration not only enables real-time information validation but also enhances collaboration among research groups, ultimately leading to more successful study outcomes.
The eCRF clinical trial system developed by a leading company offers customizable templates that can be tailored to meet the specific needs of each clinical study. This adaptability ensures that the information collected is relevant and aligned with the study's objectives, thereby enhancing information quality and enabling patient enrollment 50% faster. Furthermore, with FDA-ready information, eCRF clinical trials can realize savings of $25K per patient, significantly improving the overall clinical trial process.

bioaccess® delivers comprehensive training and support for its eCRF clinical trial system, empowering users with the essential skills to navigate the eCRF clinical trial platform with precision. This training encompasses all facets of the system, including:
Research indicates that well-structured training initiatives significantly enhance information quality and reduce entry errors, leading to improved management and outcomes. For instance, studies implementing stringent guidelines for the eCRF clinical trial completion report up to 30% fewer regulatory inquiries during audits. Furthermore, expert insights highlight that fostering an environment conducive to user confidence and accuracy in information entry is crucial for achieving optimal results. By leveraging extensive support services, research leaders can elevate their team's expertise, ultimately facilitating faster participant enrollment and more effective clinical studies.

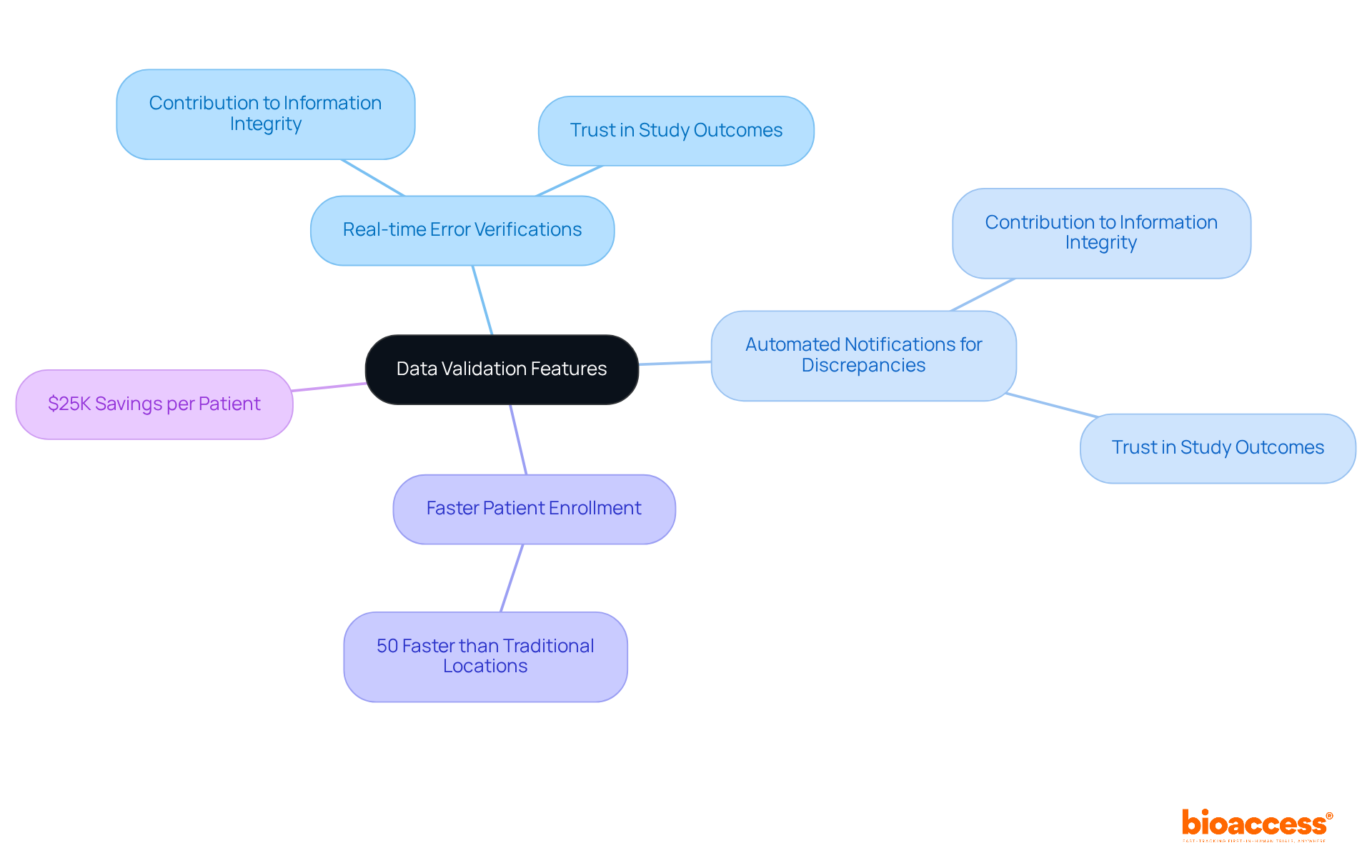
The eCRF clinical trial system from bioaccess® captures attention with its advanced information validation features, meticulously designed to ensure the quality and precision of collected data. These features include:
These empower research leaders to maintain high information integrity throughout their studies. By prioritizing information validation, directors can confidently trust their study outcomes and make informed decisions based on reliable data. Moreover, this cutting-edge technology enables eCRF clinical trials to achieve patient enrollment 50% faster than traditional Western locations, resulting in $25K savings per patient, all while utilizing FDA-compliant information that eliminates rework and delays.
bioaccess®'s eCRF clinical trial system offers mobile accessibility, empowering researchers to collect information in real-time from various locations. This flexibility significantly enhances participant involvement and retention, as data can be gathered during visits or remotely. Research directors can leverage this capability to ensure comprehensive information collection, ultimately leading to more robust study outcomes.
Remarkably, bioaccess facilitates the enrollment of treatment-naive cardiology or neurology cohorts at a rate 50% faster than Western sites, resulting in a $25K savings per patient with FDA-ready data—no rework, no delays. The emphasis on real-time data collection is growing, with studies indicating that 70% of EDC users report improved information quality in the context of eCRF clinical trials compared to traditional methods.
This shift not only streamlines the data gathering process but also fosters a more responsive and engaging environment for participants, enhancing retention rates and overall study success. As one research leader noted, 'The capacity to gather information on-the-go has revolutionized our method, making experiments more efficient and accommodating for participants.

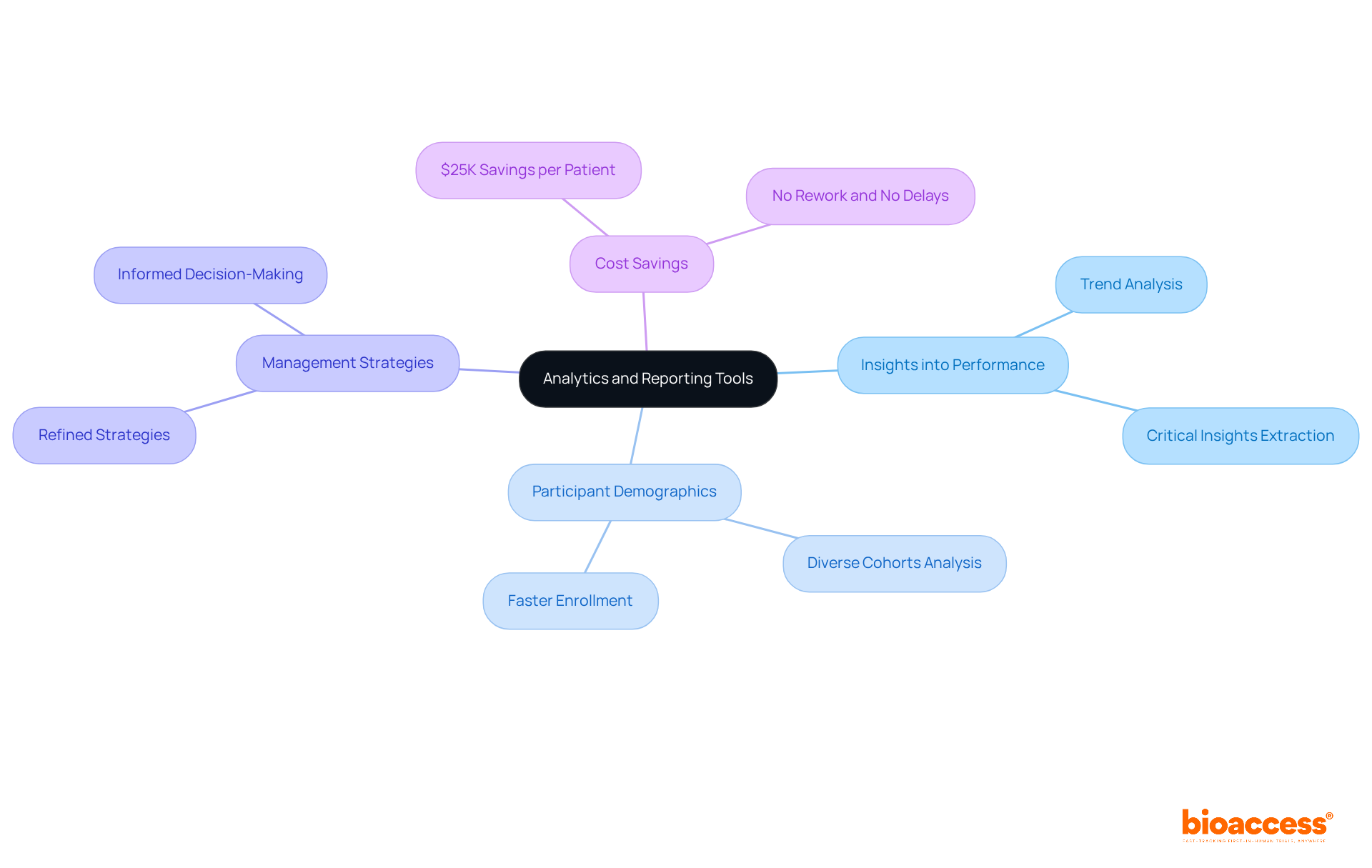
The eCRF clinical trial system from bioaccess® stands out with its advanced analytics and reporting tools, empowering research leaders to extract critical insights into performance. These tools facilitate the examination of trends, participant demographics, and other essential metrics, fostering informed decision-making. By leveraging these analytics, leaders can refine management strategies, ultimately driving improved results.
Remarkably, bioaccess® allows treatment-naive cardiology or neurology cohorts to be enrolled 50% faster than Western sites, translating to $25K savings per patient with FDA-ready data—no rework and no delays.
As the landscape of eCRF clinical trial studies evolves, the integration of data analytics is becoming increasingly vital, enabling precise adjustments and enhancements in study execution. To fully capitalize on these tools, research directors must routinely review analytics reports and adapt trial strategies based on the insights acquired.
The significance of incorporating essential eCRF features into clinical trials is paramount. By integrating advanced systems like bioaccess®, research directors can streamline their processes, enhance data integrity, and ultimately improve the overall effectiveness of clinical studies. The focus on rapid implementation, user-friendly interfaces, and real-time data access positions these eCRF systems as critical tools for modern clinical research.
Throughout this discussion, key features such as regulatory compliance, data validation, and mobile accessibility emerge as vital components. These features not only facilitate efficient data management but also ensure the quality and accuracy of the information collected. The ability to customize templates and leverage analytics further empowers research leaders to adapt to specific trial needs and drive informed decision-making.
As the landscape of clinical trials continues to evolve, embracing these eCRF features will be essential for research directors aiming to enhance study outcomes and participant engagement. The call to action is clear: prioritize the adoption of comprehensive eCRF systems to navigate the complexities of clinical trials effectively and pave the way for successful research endeavors.
What is bioaccess® and its role in clinical trials?
bioaccess® is a company that implements electronic Case Report Forms (eCRFs) for clinical trials in regions such as Latin America, the Balkans, and Australia, ensuring that clinical studies can begin promptly without unnecessary delays.
How does bioaccess® improve the implementation speed of eCRFs?
The company streamlines the setup process and leverages local regulatory expertise to achieve rapid eCRF clinical trial implementation, allowing research directors to concentrate on essential study activities instead of administrative tasks.
What advantages does the user-friendly interface of bioaccess® provide?
The user-friendly interface enhances data entry efficiency, enabling research teams to enroll treatment-naive cardiology or neurology cohorts 50% faster than their Western counterparts, while also reducing the learning curve for new users.
How does the eCRF system by bioaccess® impact cost savings in clinical trials?
By streamlining information entry and yielding improved accuracy, research leaders can anticipate savings of $25K per patient in the eCRF clinical trial, effectively eliminating rework and delays.
What regulatory compliance measures does bioaccess® implement?
bioaccess® emphasizes regulatory compliance by ensuring that all collected information meets rigorous industry standards, featuring audit trails, user access controls, and robust data encryption to maintain data integrity and security.
What is the typical timeline for regulatory approval with bioaccess® compared to the US/EU?
bioaccess® offers an accelerated regulatory approval timeline of just 6-8 weeks, compared to the typical 6-12 months in the US/EU.
How does bioaccess® ensure the integrity and security of data in clinical trials?
The integration of features like audit trails, user access controls, and robust data encryption safeguards sensitive information and enhances the overall reliability of clinical studies, ensuring compliance with all required regulations.