

The article primarily aims to delineate the essential elements required for ensuring compliance in medical device labeling. It underscores the necessity of adhering to specific regulatory requirements, including:
Such compliance is paramount for ensuring patient safety and facilitating market access. Non-compliance poses substantial legal and financial risks for manufacturers, making it imperative to prioritize these elements.
The landscape of medical device labeling compliance is increasingly intricate, driven by evolving regulations and heightened scrutiny from global health authorities. Manufacturers face the dual challenge of ensuring their products meet stringent labeling requirements while also maintaining patient safety and market access. This article explores the essential elements of medical device labeling compliance, offering insights into best practices and strategies that can help manufacturers navigate this complex terrain.
How can companies effectively balance regulatory adherence with the need for speed in bringing innovative medical devices to market?
bioaccess® excels in accelerating compliance with medical device labeling by leveraging its deep understanding of governance structures across Latin America, the Balkans, and Australia. By offering customized solutions, bioaccess® empowers medical product manufacturers to adeptly navigate the complex medical device labeling requirements, securing ethical approvals in as few as 4-6 weeks. This expedited process not only enhances market access but also significantly accelerates patient enrollment, enabling innovators to bring their products to market much faster than traditional methods allow.
As the medical device clinical trial market experienced a 14% increase globally in early 2022, the importance of effective medical device labeling adherence is underscored, particularly as producers face heightened competition and scrutiny. Moreover, with the OECD advocating for harmonization in clinical trial approval processes, bioaccess® positions itself at the forefront, ensuring clients can capitalize on these advancements for successful market entry.
Anticipated updates to governance structures in 2025 are expected to further streamline adherence, making it crucial for manufacturers to align with these changes. By prioritizing efficient tagging strategies, including FDA/EMA/MDR-prepared datasets and centralized monitoring, bioaccess® not only assists in regulatory compliance but also supports medical device labeling and fosters innovation within the Medtech sector.
Given that compliance rates in medical device labeling for clinical studies can vary significantly, with some studies reporting rates as low as 60%, bioaccess®'s role in ensuring comprehensive and efficient adherence to regulations is increasingly vital. Furthermore, the collaboration with Welwaze Medical Inc. for the Celbrea® medical product launch in Colombia exemplifies bioaccess's expertise in navigating regulatory pathways and facilitating market access in the region.

21 CFR Part 801 delineates the critical marking requirements for medical devices in the United States. These essential provisions dictate that labels must prominently feature:
Compliance with these regulations is not merely advisable; it is imperative, as non-compliance can result in severe enforcement actions, including product recalls and financial penalties. The FDA has a history of taking decisive action against manufacturers whose labels fall short of these stringent guidelines, underscoring the necessity for accuracy and clarity in product information.
Manufacturers are strongly urged to implement robust quality management systems to ensure their labels are not only informative but also fully compliant with FDA standards, thereby mitigating risks associated with inaccuracies in product information. Noteworthy instances of compliance illustrate the effectiveness of proactive identification strategies, demonstrating that adherence to these regulations not only safeguards consumers but also enhances market access and bolsters brand reputation.

The Unique Identification (UDI) system serves as a pivotal element in medical device labeling, designed to enhance traceability and improve patient safety. Under UDI requirements, each medical instrument must possess a unique identifier that facilitates tracking throughout its lifecycle—from manufacturing to post-market surveillance. This system not only aids in identifying equipment during recalls but also ensures compliance with regulatory standards, thereby fostering greater accountability within the healthcare system.
As we approach 2025, the importance of UDI in medical device labeling and equipment traceability cannot be overstated, especially with the U.S. Medical Equipment Manufacturing market projected to reach $56.4 billion. The UDI system streamlines inventory management and enhances patient safety by enabling efficient recall management and supporting adverse event reporting. For example, the successful implementation of UDI has resulted in faster identification of defective devices, underscoring its critical role in healthcare.
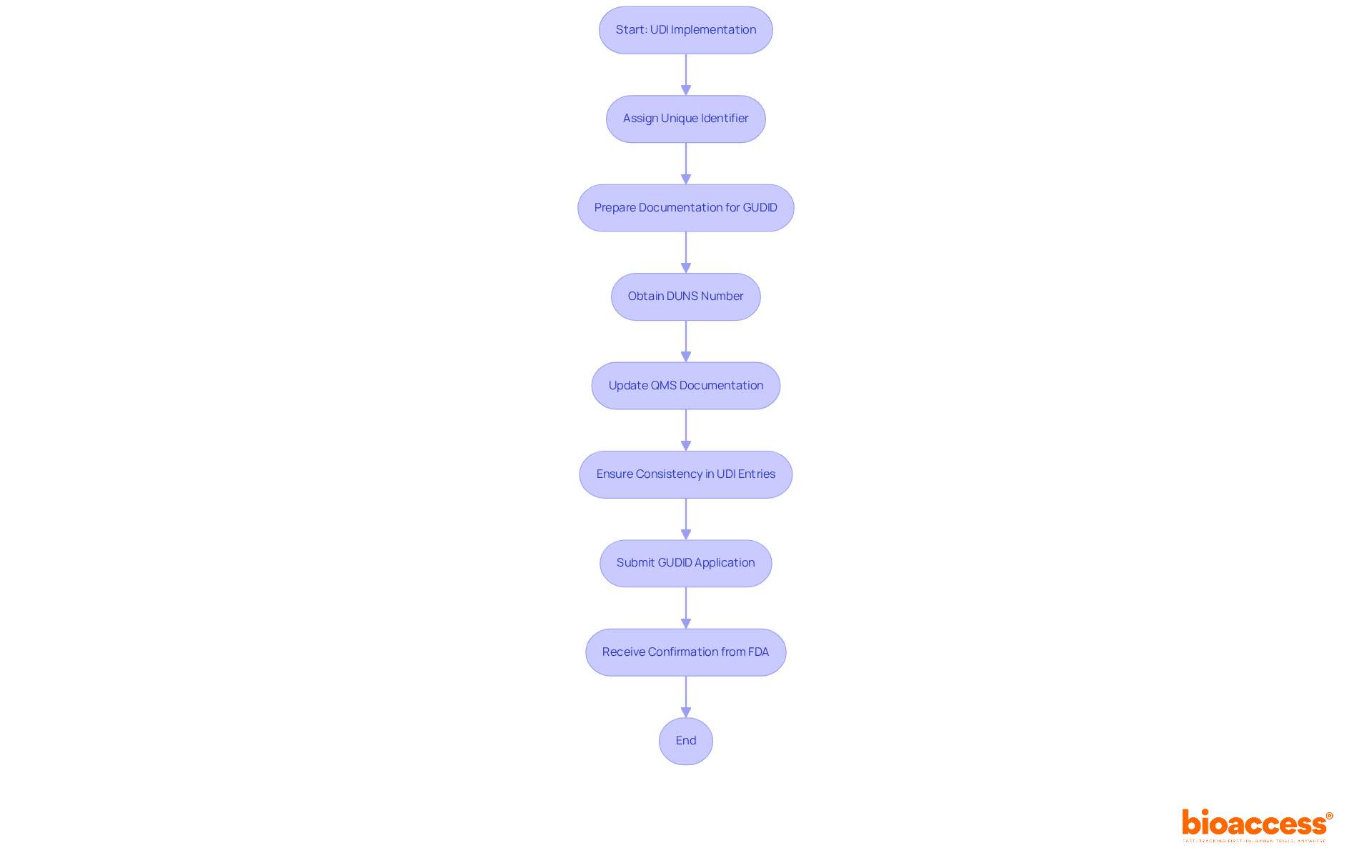
Manufacturers are required to prepare specific documentation to access the Global Unique Device Identification Database (GUDID), which is essential for regulatory compliance. This preparation includes obtaining a DUNS number and ensuring that Quality Management System (QMS) documentation is up-to-date. By comprehending GUDID requirements, manufacturers can navigate common obstacles that impede approval, as thorough preparation is vital for meeting standards.
Industry leaders stress that consistency in UDI entries is crucial for regulatory adherence. As the FDA articulates, "Consistency is essential for adherence, particularly as the FDA emphasizes the significance of precise and uniform information in the database." With the FDA's ongoing enforcement of UDI requirements, manufacturers are encouraged to develop internal strategies for compliance, ensuring that all necessary fields are accurately completed to prevent complications. The UDI system transcends mere compliance; it is integral to enhancing patient outcomes and safety within the medical equipment sector.
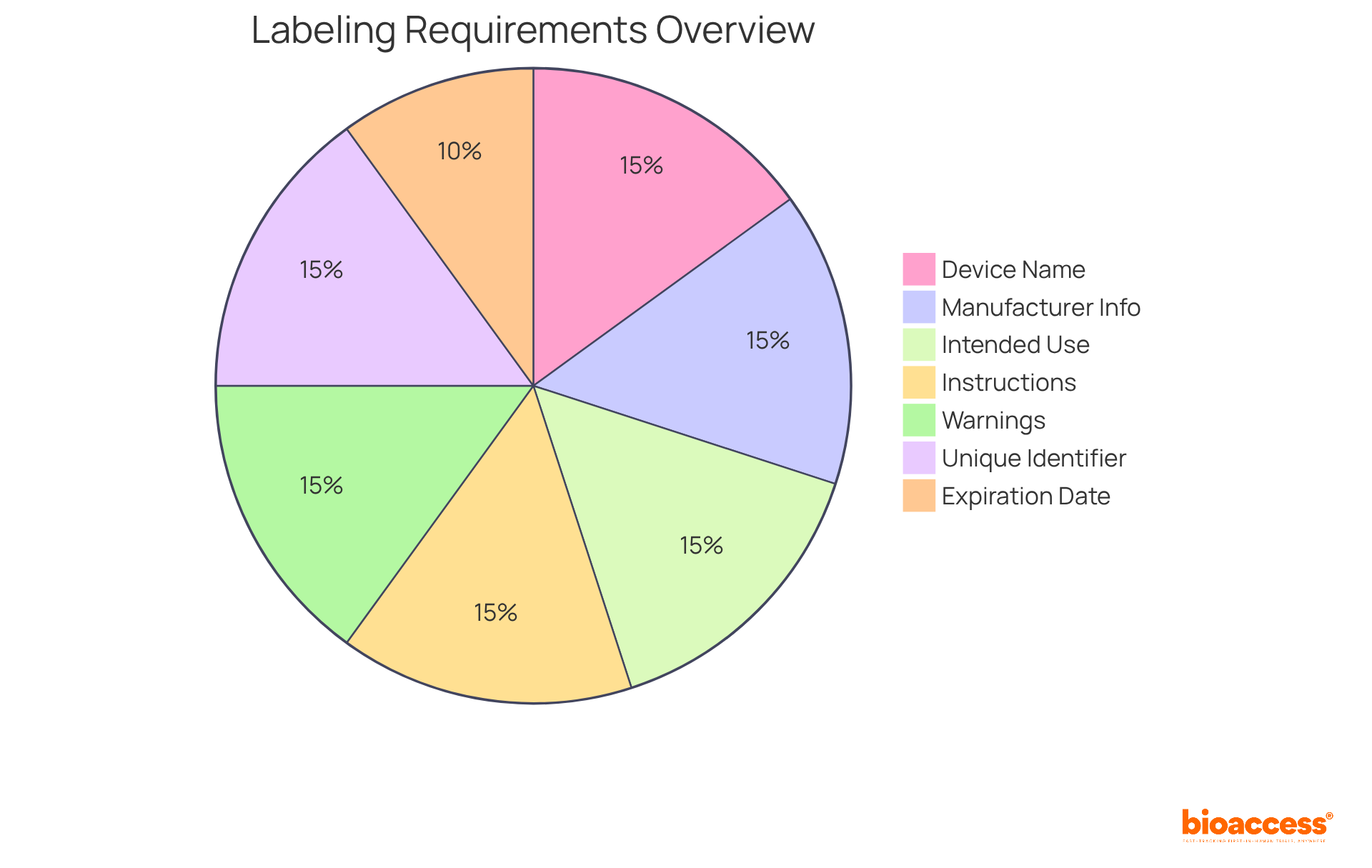
To ensure compliance and safety, medical device labeling must incorporate several essential elements. These elements typically include:
Incorporating these elements into medical device labeling not only fulfills regulatory requirements but also delivers vital information to healthcare professionals and patients, thereby enhancing safety and efficacy.
In Colombia, the regulatory environment is monitored by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which plays a vital role in inspecting and overseeing the marketing and production of health products, including medical equipment. INVIMA is responsible for identifying and evaluating violations of health standards and implementing best practices to ensure compliance. As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA ensures that medical devices meet stringent safety, efficacy, and quality standards.
Regulatory experts stress that efficient marking practices can greatly diminish frequent mistakes, which often result in recalls and safety concerns. For example, the withdrawal of Elekta's biopsy needle kits due to contamination highlights the significance of precise identification in avoiding patient injury. By adhering to the medical device labeling requirements, manufacturers can reduce risks and ensure their products are both compliant and safe for use. To enhance compliance, manufacturers should regularly review and update their packaging practices to align with the latest regulatory changes.
Clear medical equipment markings are essential for patient safety. Labels that are easy to read and understand empower healthcare providers and patients to use devices correctly, thereby reducing the risk of misuse and associated complications. Insufficient identification can lead to serious medical errors, such as improper usage or dosage, which can have dire repercussions for patient health.
Studies indicate that marking and packaging contribute to 33% of medication errors, including 30% of fatalities, underscoring the critical nature of clear marking practices. Furthermore, the EU Medical Equipment Regulation (MDR) mandates enhanced information on traceability and specific warning symbols, emphasizing the regulatory context surrounding medical device labeling.
Comprehensive Instructions for Use (IFUs) are also crucial for equipment operation and risk management, ensuring safe usage. Experts in the field assert that clear and user-friendly labels not only enhance comprehension but also foster confidence in medical instruments. Therefore, manufacturers must prioritize comprehensive and transparent identification practices to safeguard patient well-being and improve overall health outcomes.

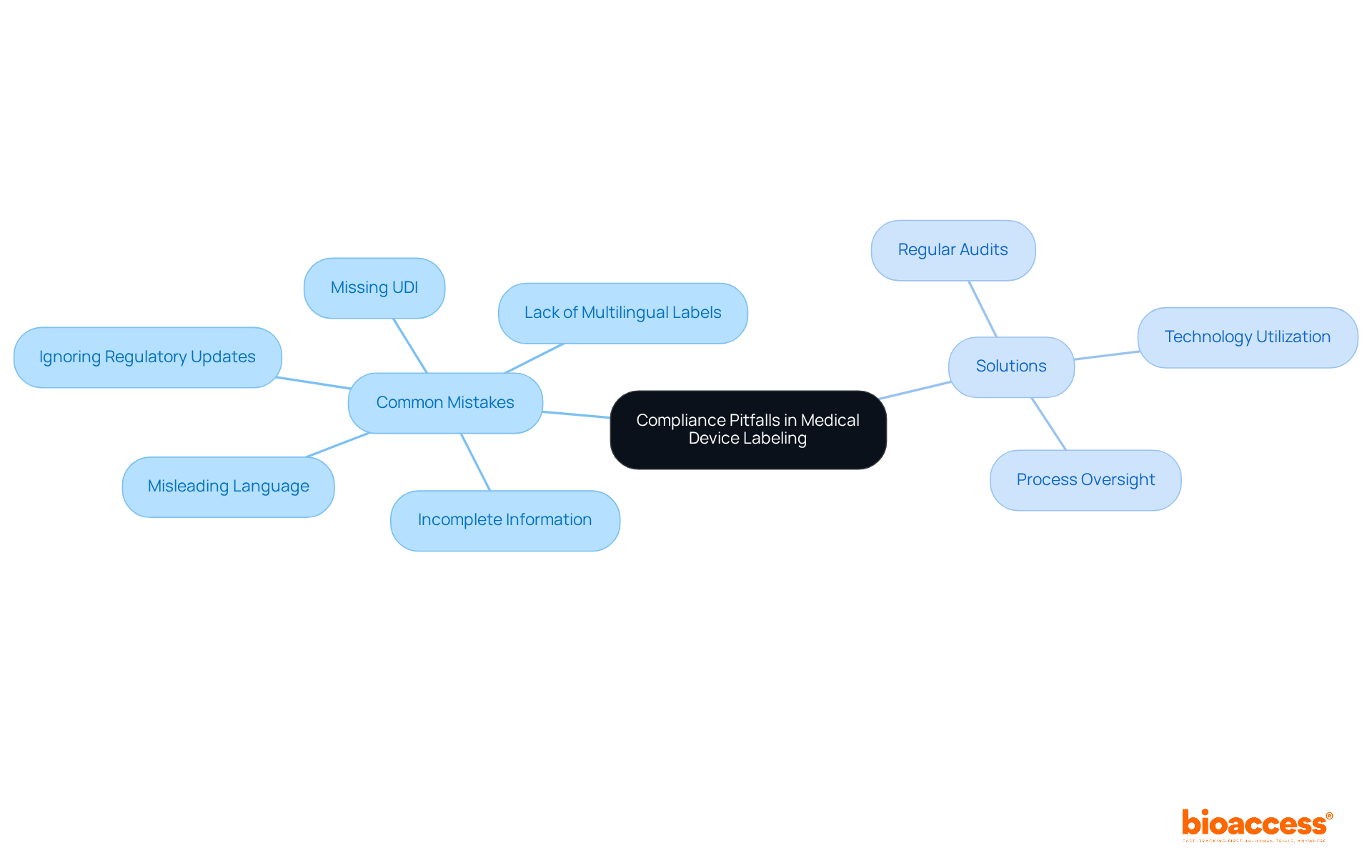
Common compliance pitfalls in medical device labeling can significantly impact market access and patient safety. Key issues in medical device labeling include:
Statistics show that classification mistakes are among the top five most frequent Form 483 citations issued to medical equipment companies, highlighting the significance of careful attention to detail. Effective approaches to evade these challenges involve:
Advisors in oversight, such as specialists like Ana Criado, who possesses significant expertise in oversight matters for medical instruments in Colombia, stress that attaining complete adherence in packaging and marking is a necessity and a crucial aspect of providing safe and effective medical instruments to patients. By following best practices and remaining updated on compliance changes, manufacturers can reduce risks and enhance their medical device labeling processes.
Inspections of medical device labeling are essential for ensuring regulatory compliance in the medical equipment sector. Regulatory authorities meticulously evaluate whether medical device labeling adheres to established standards and effectively communicates the equipment's intended use. Recent data indicates that approximately 85% of devices pass inspection on their first attempt, highlighting the critical nature of thorough preparation.
Manufacturers must maintain comprehensive documentation and ensure their practices regarding medical device labeling align with regulatory requirements to facilitate seamless inspections. Furthermore, implementing regular internal audits can proactively identify potential issues, thereby enhancing product integrity and compliance.
Effective identification methods, such as:
have proven to significantly improve inspection outcomes. By prioritizing these strategies, manufacturers can adeptly navigate the complexities of inspection processes and ensure their products meet the required standards.
Navigating global medical equipment marking standards necessitates a comprehensive understanding of various regulatory frameworks, including those set forth by the FDA, EU MDR, and other international entities. Alarmingly, about 70% of manufacturers lack full awareness of the specific international marking requirements, which can result in compliance challenges. Each market presents unique stipulations regarding language, format, and content, underscoring the importance for manufacturers to grasp these nuances to facilitate effective international promotion of their products.
Engaging with local regulatory experts can yield invaluable insights, enabling manufacturers to adeptly navigate these requirements and circumvent potential pitfalls. Recent updates in global medical device labeling regulations emphasize the critical need for clear and precise information, reinforcing the importance of compliance in maintaining market access and consumer trust.
Effective storage and operational practices for labeling are crucial for ensuring adherence and maximizing efficiency in medical device labeling. To prevent damage or deterioration that can jeopardize their clarity and compliance with regulatory standards, medical device labeling must be maintained in regulated conditions.
It is essential to conduct inspections of medical device labeling at least quarterly for any signs of wear, damage, or fading, as regulations mandate that labels remain readable under all circumstances. The implementation of standardized procedures for medical device labeling and verification significantly mitigates the risk of errors during the manufacturing process.
Furthermore, continuous education for personnel on marking standards and best practices enhances both adherence and operational efficiency. As Amanda Cox asserts, 'Establishing an appropriate tagging system provides various advantages, such as better inventory management, heightened productivity, and enhanced safety and adherence to regulations.'
Additionally, incorporating identification early in product design and risk management, as recommended by FDA guidance, is vital for ensuring that tags are effective and user-friendly. The use of larger font sizes and high-contrast colors is also advised to guarantee that labels are legible and effective across various environments, thereby contributing to patient safety and compliance.
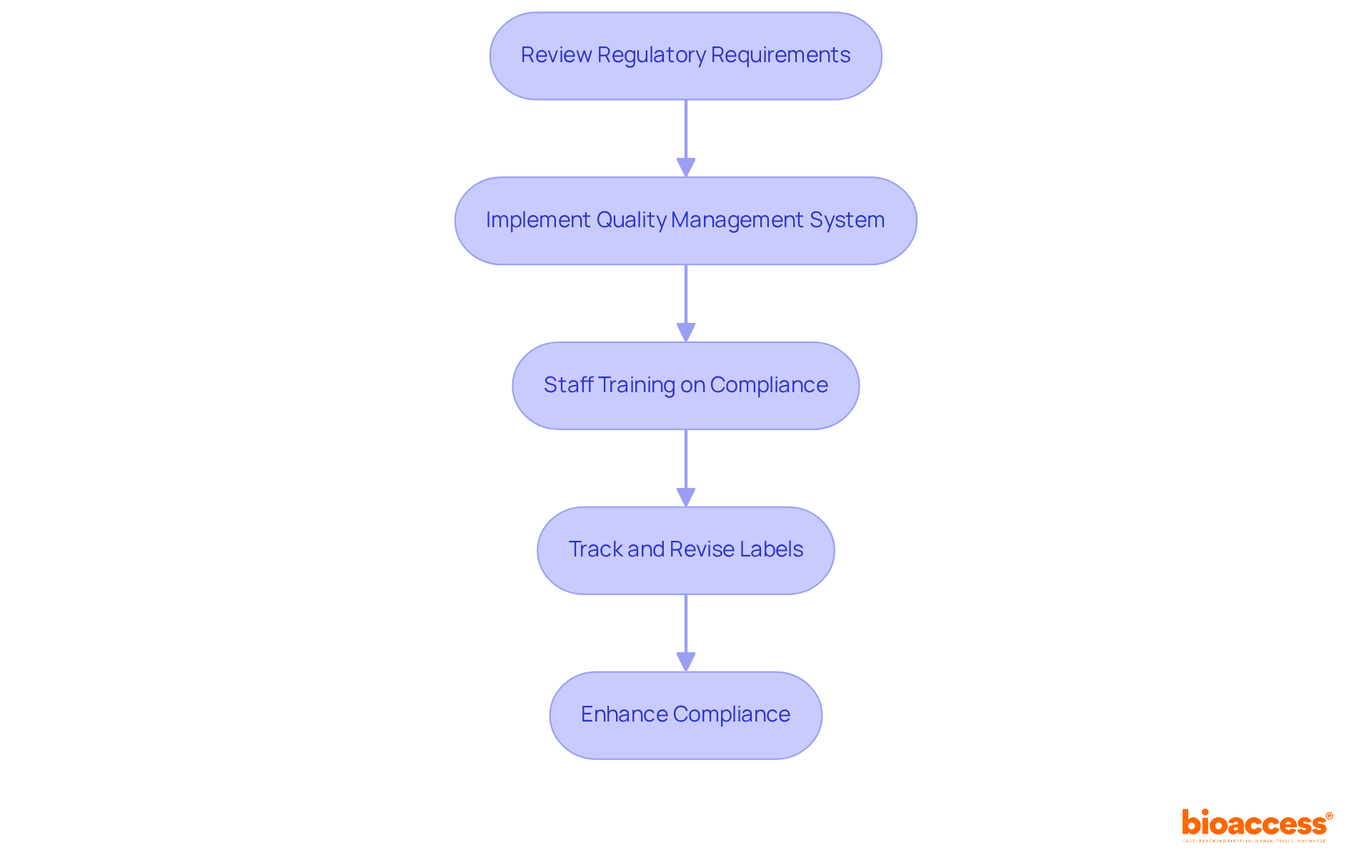
To establish a compliant device labeling program, manufacturers must adopt effective strategies. First, conducting a comprehensive review of regulatory requirements is essential to ensure that all necessary labeling elements—such as device name, intended use, and instructions for use—are included. This process involves submitting draft labels, instructions for use (IFUs), and promotional materials as part of the initial submission for FDA regulatory review.
Next, implementing a robust quality management system (QMS) is critical. This system should integrate marking processes and controls, ensuring that marking practices align with the most stringent applicable standards. Additionally, maintaining control numbers for devices subject to traceability is mandated, as outlined in 21 CFR 820.65.
Regular training for staff on compliance standards and labeling best practices is also vital. This training fosters a culture of quality and accountability. Specialists like Ana Criado, Director of Compliance Affairs and a professor in biomedical engineering, emphasize the significance of ongoing education in navigating intricate oversight environments.
Furthermore, manufacturers should create a methodical strategy for tracking and revising labels in response to legal changes. This ensures that all materials remain current and compliant. Collaboration with regulatory specialists, such as Katherine Ruiz, who focuses on regulatory matters for medical products and in vitro diagnostics in Colombia, is crucial for navigating complex requirements and proactively addressing potential adherence challenges. As Marco Theobold, a specialist in medical device and drug regulations, states, 'Clear, accurate, and comprehensive device information is essential to the safety and effectiveness of medical devices.'
By adopting these strategies, manufacturers can significantly enhance their compliance with regulations, ultimately contributing to improved patient safety and successful market access. The risks associated with labeling errors, which can lead to FDA warning letters, further underscore the importance of effective labeling practices.
In the realm of medical devices, compliance with labeling regulations is not merely a bureaucratic necessity; it is a fundamental aspect that directly impacts patient safety and market access. This article has explored the essential elements of medical device labeling compliance, emphasizing the importance of adhering to regulatory standards such as 21 CFR Part 801 and the Unique Device Identification (UDI) system. By understanding and implementing these requirements, manufacturers can navigate the complexities of the medical device landscape more effectively.
Key arguments presented include:
Additionally, the role of organizations like bioaccess® in accelerating compliance processes and ensuring manufacturers meet evolving regulatory standards has been highlighted, showcasing the importance of expert guidance in this field.
Ultimately, the stakes are high in medical device labeling compliance. Manufacturers are urged to prioritize accurate and comprehensive labeling practices, not only to fulfill legal obligations but to enhance patient safety and trust. By adopting best practices and remaining vigilant about regulatory changes, companies can foster a culture of compliance that not only safeguards their products but also contributes to the overall integrity of the healthcare system. Embracing these strategies will not only mitigate risks but also pave the way for successful market entry and innovation within the Medtech sector.
What is bioaccess® and how does it assist medical device manufacturers?
bioaccess® specializes in accelerating compliance with medical device labeling by providing customized solutions that help manufacturers navigate complex labeling requirements, securing ethical approvals in as few as 4-6 weeks.
Why is effective medical device labeling important?
Effective medical device labeling is crucial as it enhances market access, accelerates patient enrollment, and helps manufacturers stay competitive, especially in a growing market where compliance rates can vary significantly.
What are the anticipated updates to governance structures in 2025?
Updates to governance structures in 2025 are expected to streamline adherence to medical device labeling requirements, making it essential for manufacturers to align with these changes for successful market entry.
What are the key requirements outlined in 21 CFR Part 801 for medical device labels?
21 CFR Part 801 requires that medical device labels prominently feature the manufacturer's name and address, intended use, and comprehensive instructions for use.
What can happen if manufacturers do not comply with labeling regulations?
Non-compliance with labeling regulations can lead to severe enforcement actions, including product recalls and financial penalties, highlighting the necessity for accuracy and clarity in product information.
What is the Unique Device Identification (UDI) system?
The UDI system is designed to enhance traceability and improve patient safety by assigning a unique identifier to each medical device, facilitating tracking throughout its lifecycle and aiding in recalls.
How does the UDI system benefit manufacturers and the healthcare system?
The UDI system improves inventory management, enhances patient safety through efficient recall management, and supports adverse event reporting, ultimately fostering greater accountability within the healthcare system.
What documentation is required for manufacturers to access the Global Unique Device Identification Database (GUDID)?
Manufacturers must prepare specific documentation, including obtaining a DUNS number and ensuring that their Quality Management System (QMS) documentation is up-to-date, to access the GUDID.
Why is consistency in UDI entries important?
Consistency in UDI entries is crucial for regulatory adherence, as it ensures precise and uniform information in the database, which is essential for compliance with FDA requirements.
How does bioaccess® demonstrate its expertise in regulatory pathways?
bioaccess® exemplifies its expertise through collaborations, such as with Welwaze Medical Inc. for the Celbrea® medical product launch in Colombia, facilitating market access in the region.
A Guide to Medical Device Labeling Requirements - Dot Compliance (https://dotcompliance.com/blog/medical-device-manufacturing/a-guide-to-medical-device-labeling-requirements)
FDA Medical Device Labeling: Requirements, Content, and Compliance Strategy (https://registrarcorp.com/blog/medical-devices/medical-device-registration/medical-device-labeling)
Best Practices
Case Studies (https://ors.od.nih.gov/OD/OQM/benchmarking/bestpractice/Pages/case_studies.aspx)