

This article highlights the essential features of clinical trial data software that are pivotal for the success of clinical trials. It underscores the significance of functionalities such as:
These elements are crucial for enhancing the efficiency, integrity, and overall outcomes of clinical research.
In the ever-evolving Medtech landscape, the role of robust clinical trial data software cannot be overstated. As clinical trials face increasing scrutiny and complexity, having the right tools at your disposal is vital. Consider how regulatory compliance ensures that trials meet necessary standards, while data analytics provides insights that drive informed decision-making.
Moreover, a user-friendly interface allows researchers to navigate the software with ease, reducing the learning curve and enabling teams to focus on what truly matters—delivering results. Real-time access to information empowers stakeholders to make timely adjustments, ultimately leading to more successful trial outcomes.
In conclusion, collaboration among stakeholders is essential in navigating the challenges of clinical research. By leveraging advanced clinical trial data software, organizations can enhance their operational efficiency and improve the integrity of their trials. The next steps involve evaluating your current systems and considering how these essential features can be integrated into your clinical research strategy.
In the dynamic realm of clinical research, the success of medical innovations relies heavily on effective data management. As trials grow increasingly complex and regulatory compliance becomes more critical, grasping the essential features of clinical trial data software is vital for Medtech innovators.
What key components can streamline processes, foster collaboration, and ultimately enhance patient outcomes? This article explores ten indispensable features of clinical trial data software, offering insights that empower organizations to tackle challenges and achieve success in their research endeavors.
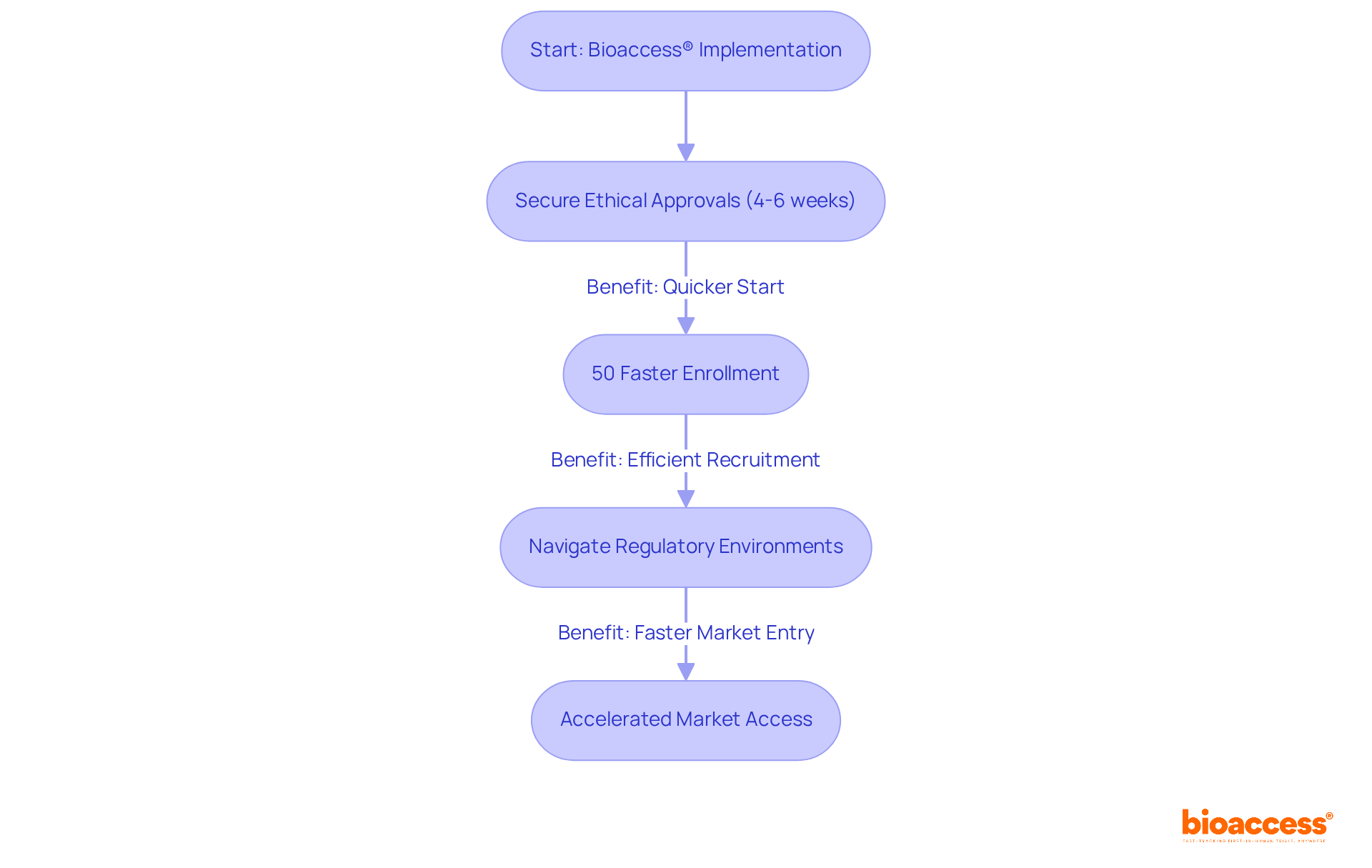
bioaccess® distinguishes itself in the research landscape by providing enhanced information management solutions tailored specifically for Medtech innovators. With the capability to secure ethical approvals in just 4-6 weeks and achieve enrollment rates that are 50% faster than traditional markets, bioaccess® adeptly navigates the complex regulatory environments of Latin America, the Balkans, and Australia. This operational agility not only accelerates the research process but also facilitates quicker market access for innovative medical technologies, ultimately improving patient care and health outcomes.
Industry leaders emphasize that such rapid ethical authorizations are crucial for maintaining momentum in research, highlighting the importance of clinical trial data software for effective information management in achieving successful study results. As the demand for innovative solutions grows, bioaccess® remains at the forefront by continually updating its clinical trial data software and clinical research tools to meet the evolving needs of Medtech innovators.
In this dynamic landscape, collaboration is key. By partnering with bioaccess®, Medtech companies can navigate challenges more effectively, ensuring that their groundbreaking technologies reach the market swiftly and efficiently.
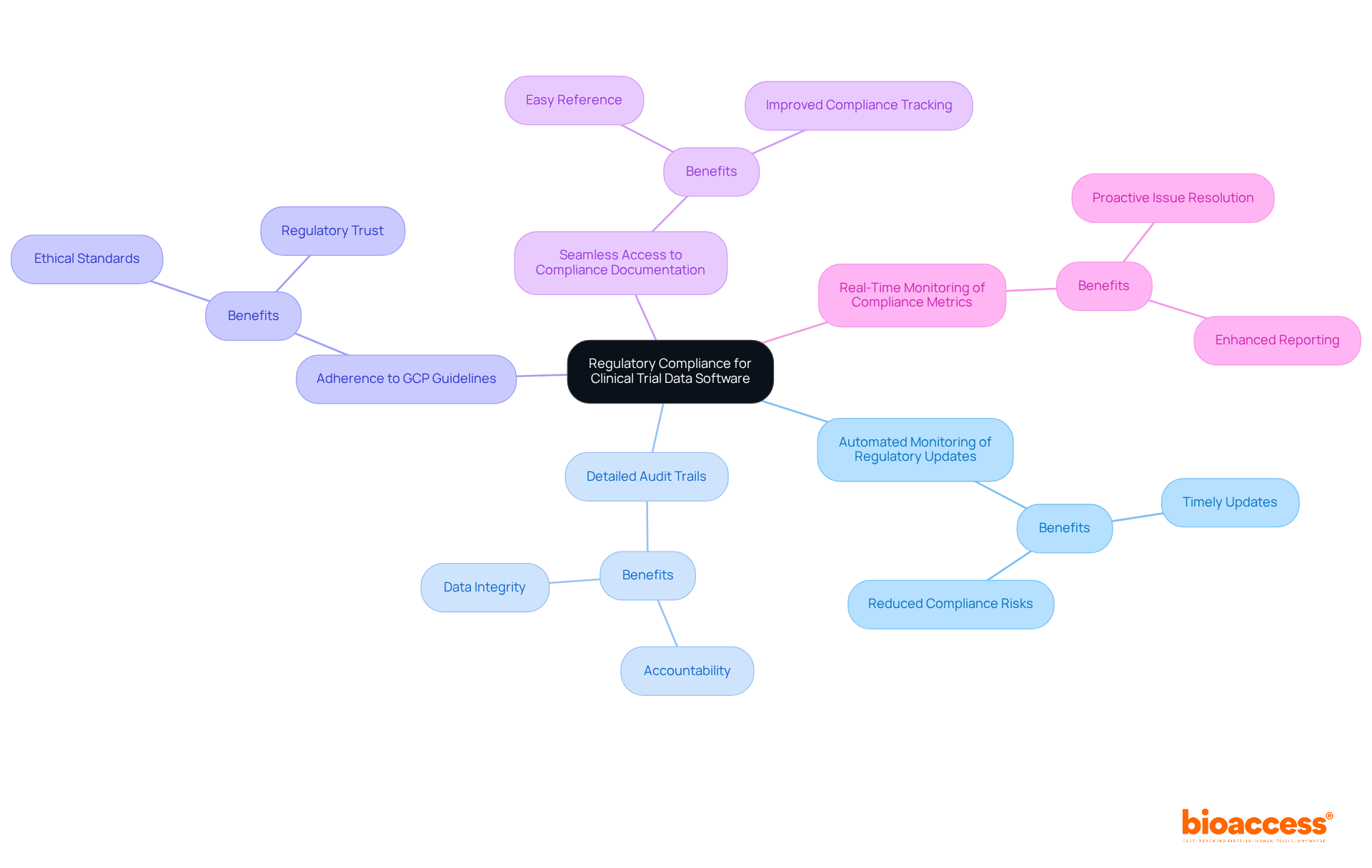
Clinical trial data software plays a pivotal role in clinical trial management tools to ensure regulatory compliance, which is essential for the integrity of clinical research. These tools must include:
Such functionalities are not just beneficial; they are crucial for validating the reliability of collected data, a key factor for successful regulatory submissions using clinical trial data software.
Moreover, seamless access to compliance documentation and real-time monitoring of compliance metrics are vital features that these tools should offer. This proactive approach significantly reduces the risk of non-adherence, a major concern in the field. Research indicates that a substantial number of studies fail due to non-compliance with GCP standards. For instance, while adherence rates for timely reporting of research outcomes have improved since the implementation of the 2017 FDAAA Final Rule, many studies still fall short of these requirements.
Platforms that excel in GCP compliance often provide integrated monitoring features and support for electronic consent procedures, ensuring that ethical standards are upheld throughout the study lifecycle with the use of clinical trial data software. By prioritizing these compliance features, organizations can enhance the overall quality of their research studies, fostering greater trust with regulatory bodies and participants alike. This commitment to compliance not only safeguards the integrity of the research but also positions organizations as leaders in the Medtech landscape.
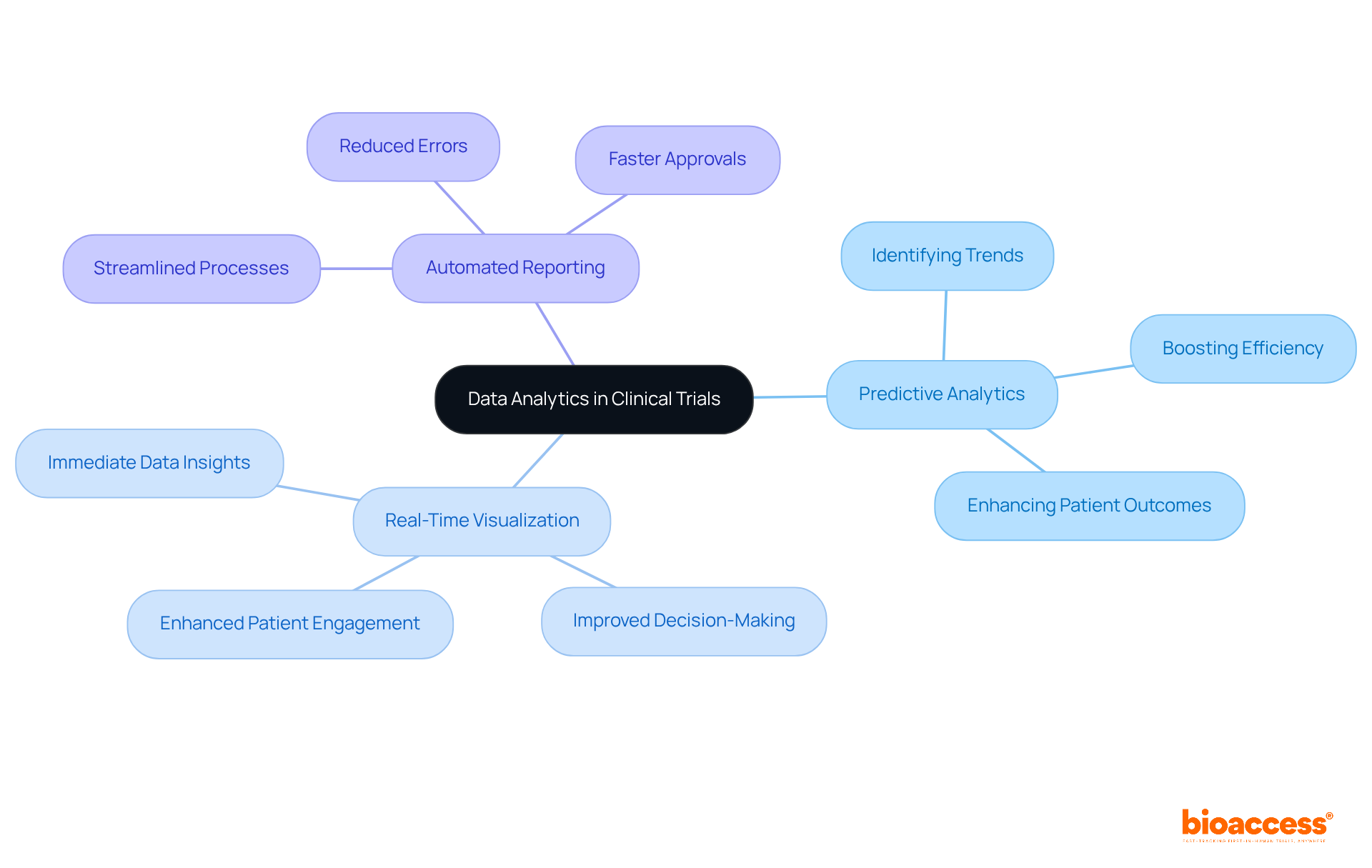
Enhanced analytical features in research software empower scientists to transform unrefined information into actionable insights. With attributes like predictive analytics, real-time information visualization, and automated reporting, these tools help identify trends and patterns that can inform adjustments and enhance patient outcomes. By leveraging these analytics capabilities, healthcare teams can make informed decisions that boost the efficiency and effectiveness of their studies, leading to quicker approvals and better patient care. Notably, with bioaccess®'s advanced solutions, studies can achieve patient enrollment 50% faster and realize $25K savings per patient through FDA-ready information. This ensures a streamlined process that supports timely and effective research results.
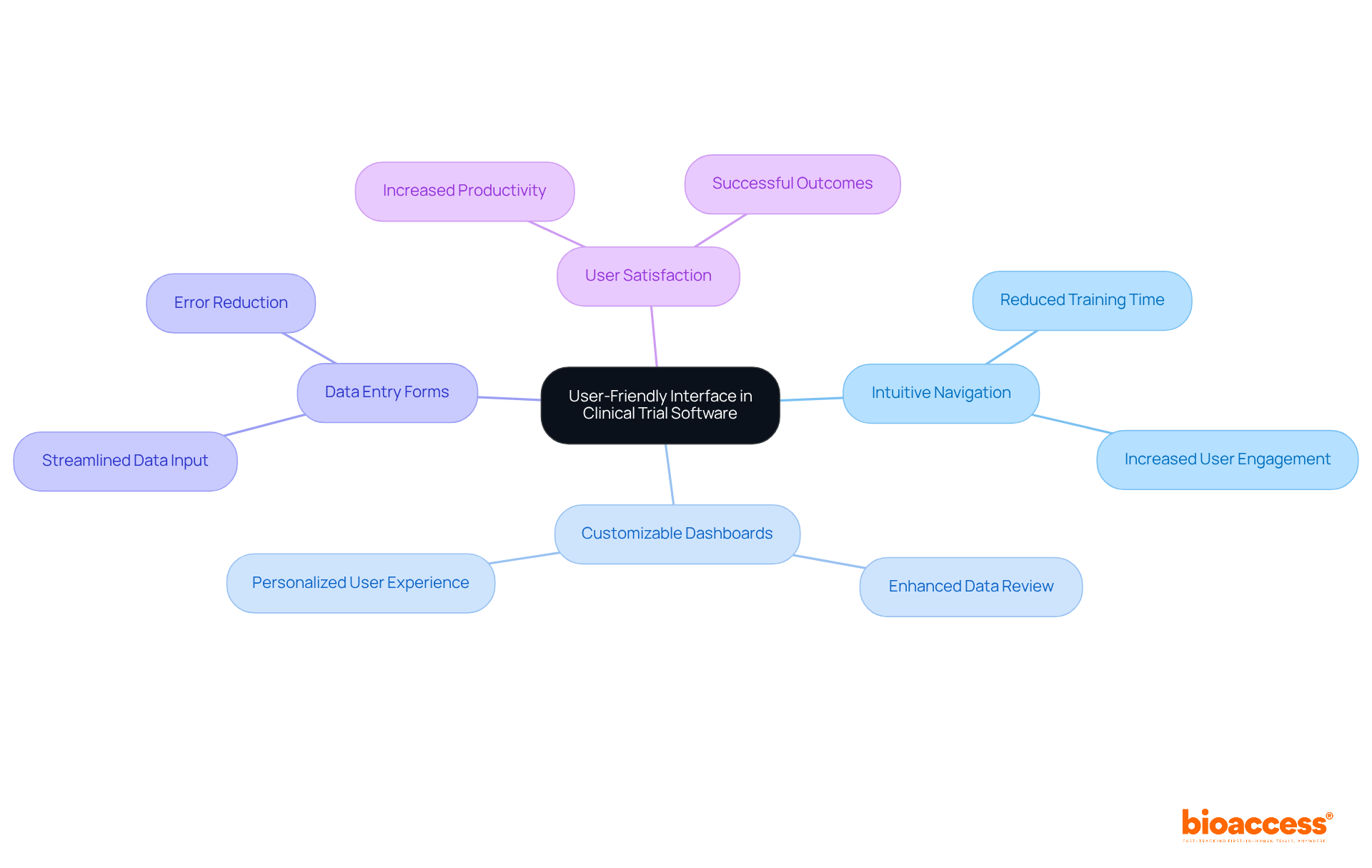
An intuitive interface in research project management tools is crucial for enhancing usability across diverse teams. Intuitive navigation, customizable dashboards, and straightforward data entry forms can significantly cut down the average training time for new users—sometimes by as much as 40%. By prioritizing user experience, clinical trial data software developers enable clinical study teams to spend less time on training and more time on essential research tasks. This shift not only boosts productivity but also leads to more effective management of experiments.
Usability specialists assert that a well-designed interface enhances user satisfaction and engagement, ultimately increasing the likelihood of successful outcomes. For example, tools that feature clear visual cues and logical workflows have been proven to improve user efficiency, allowing teams to concentrate on critical tasks rather than grappling with complex systems. By enhancing usability in research management with clinical trial data software, organizations can foster a more efficient environment, paving the way for improved results in medical studies.
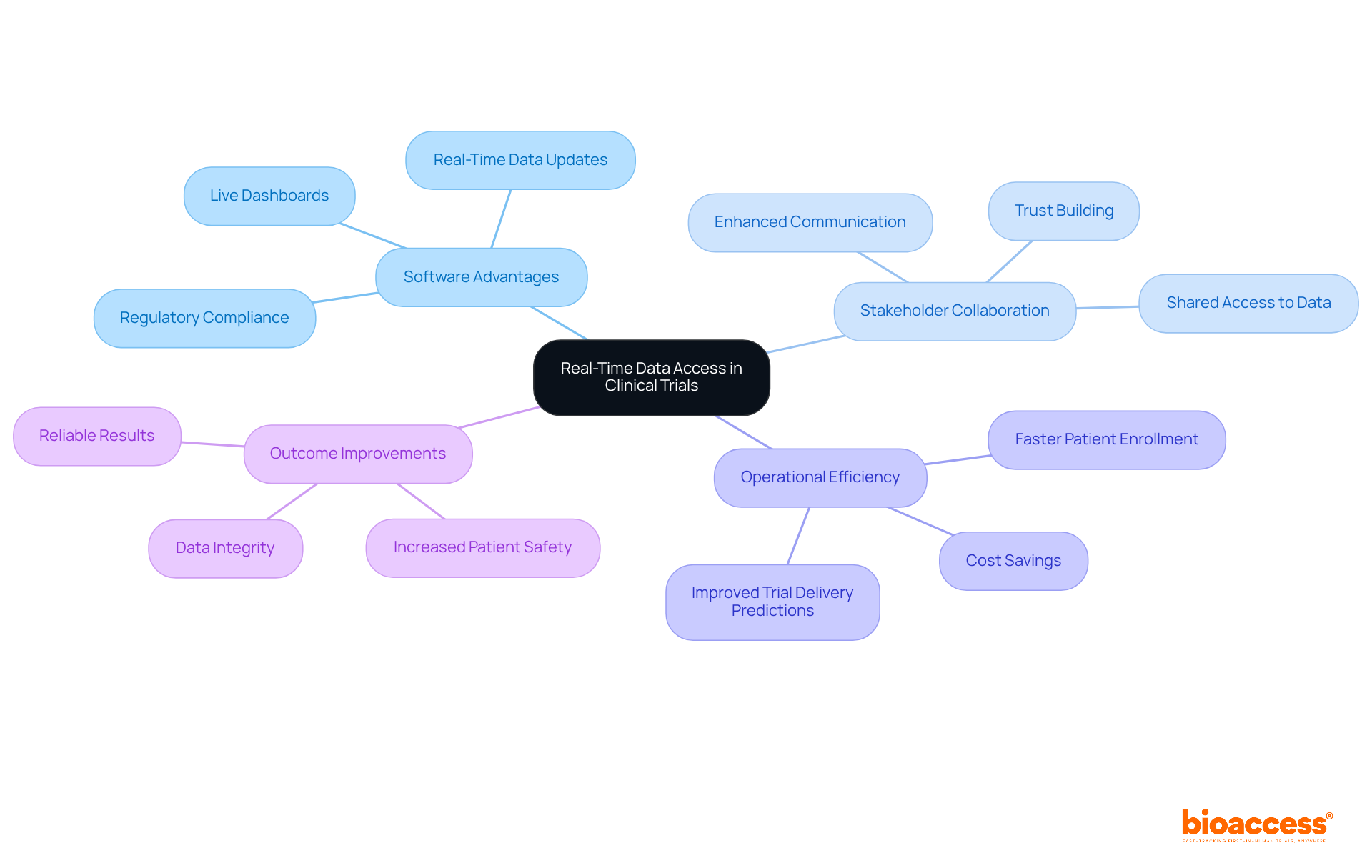
Instant information access, facilitated by clinical trial data software, is revolutionizing clinical research management by enabling continuous observation of study progress. The implementation of clinical trial data software, featuring live dashboards and real-time data updates, allows stakeholders to swiftly identify and address issues, ensuring timely interventions. This capability significantly enhances oversight, as all team members operate with the latest information, which is vital for maintaining integrity and regulatory compliance. Under the updated ICH E6(R3) guidance, clinical trial data software has made real-time data access a regulatory necessity, underscoring its essential role in compliance.
Modern testing dashboards have evolved to seamlessly integrate with various management systems, providing tailored insights that foster collaboration among sponsors, CROs, and service providers. These dashboards not only enhance operational efficiency but also improve delivery predictions. As clinical studies become increasingly complex, the demand for clinical trial data software as adaptive tools has intensified, making real-time monitoring crucial for effective study management in 2025. Clinical study managers, like Meri Beckwith, Co-CEO, have noted that these dashboards simplify processes while promoting transparency and trust among stakeholders, ultimately leading to more reliable outcomes.
Beckwith emphasizes, "Real-time monitoring acts as a method to uphold information integrity, providing a level of assurance that the studies will produce trustworthy outcomes." Furthermore, with bioaccess®'s capabilities, clinical studies can achieve patient enrollment 50% faster and save $25K per patient through FDA-ready data, addressing common recruitment challenges faced by Medtech and biopharma startups.
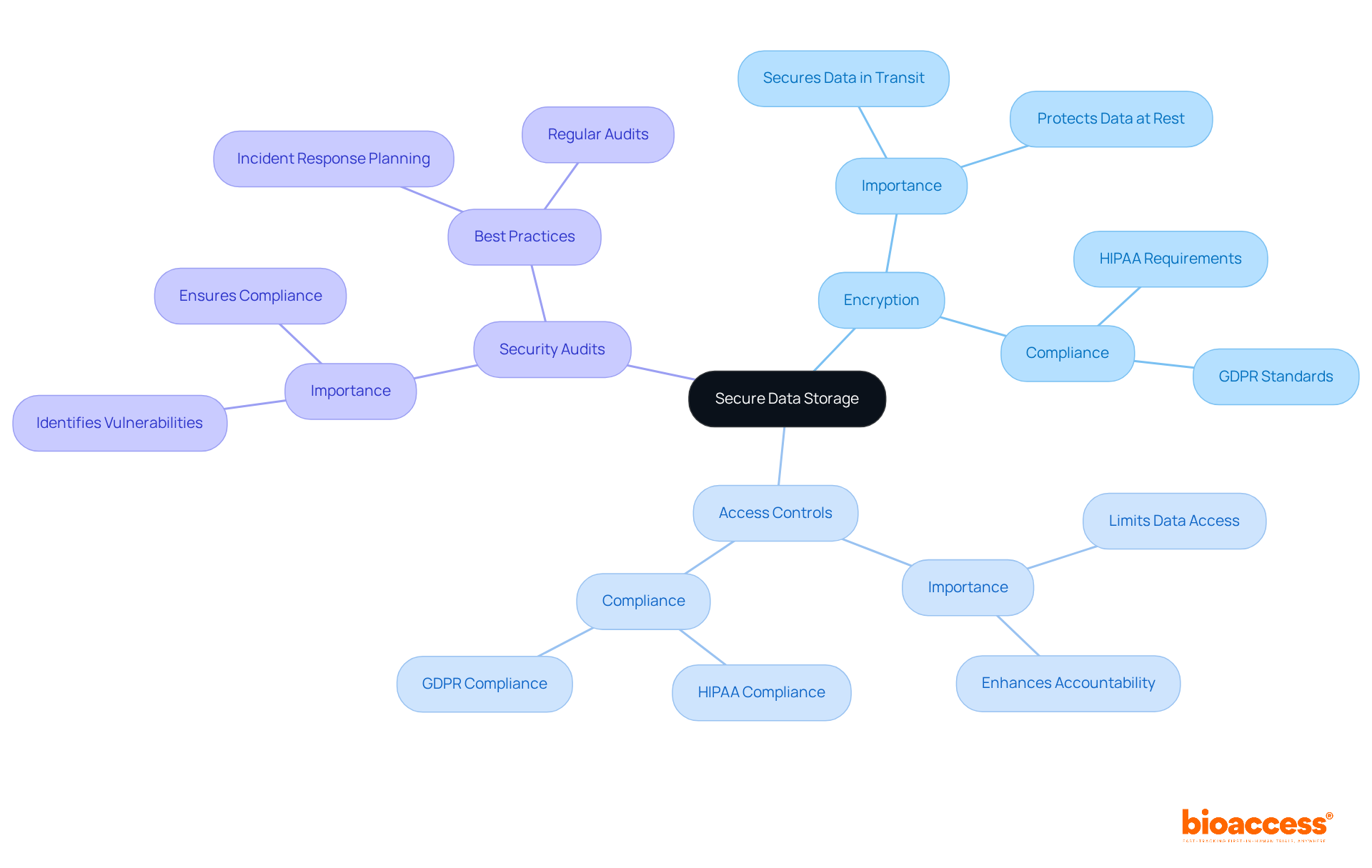
Reliable clinical trial data software is crucial for safeguarding patient details in clinical trials. By implementing robust features such as encryption, stringent access controls, and regular security audits, organizations can effectively protect sensitive information from breaches and unauthorized access. Compliance with regulations like HIPAA and GDPR is not merely a legal obligation; it stands as a cornerstone of ethical research practices. Current compliance rates indicate that many organizations still grapple with meeting these standards, underscoring the urgent need for enhanced security measures.
Clinical trial data software must provide detailed logs of information access and modifications, ensuring accountability and transparency. This level of oversight not only reinforces compliance but also builds trust among stakeholders. By prioritizing information security, study sponsors can cultivate patient confidence and uphold the credibility of their research. Ultimately, this commitment to safeguarding data plays a vital role in advancing medical science.
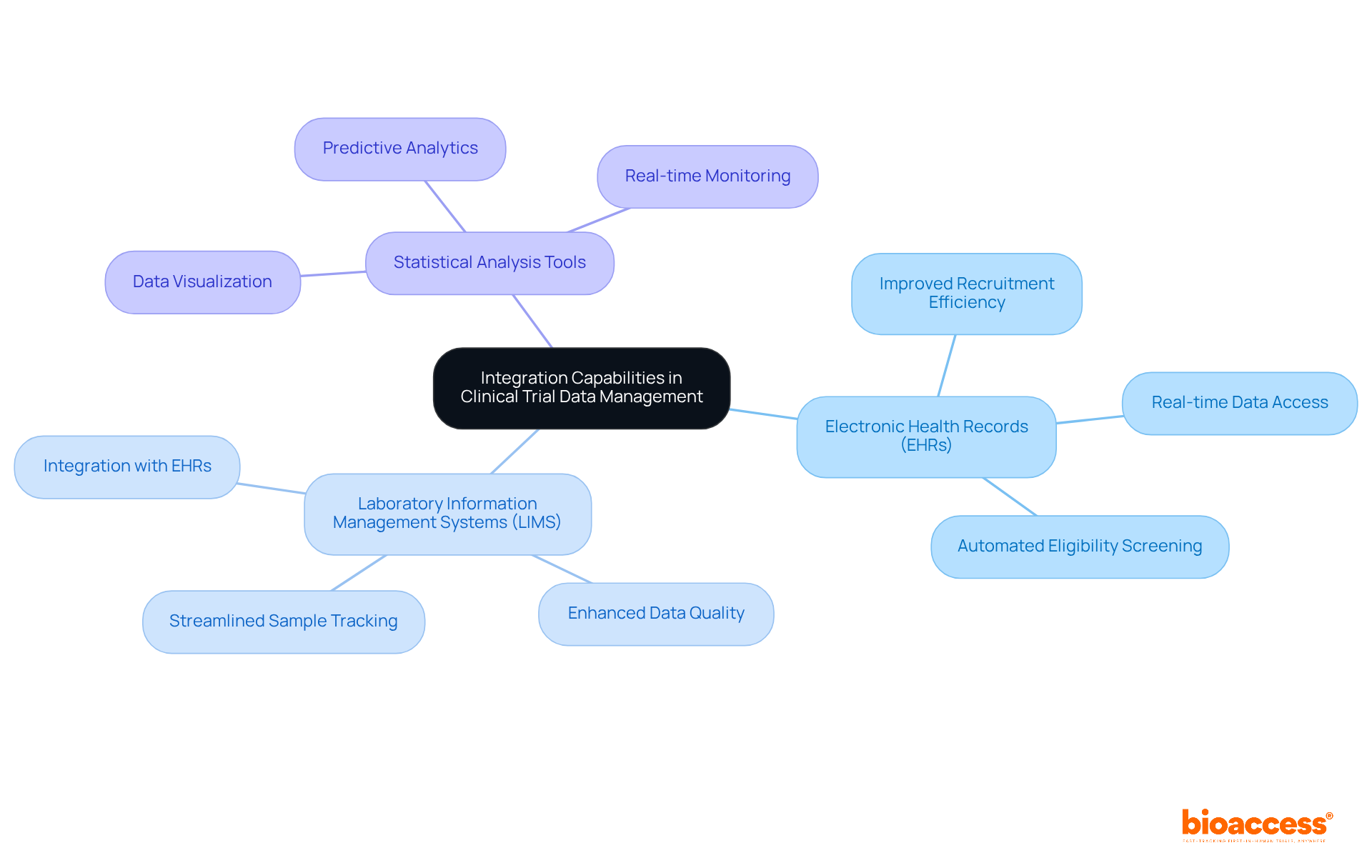
Integration features in research software play a pivotal role in ensuring seamless information flow between various systems, such as electronic health records (EHRs), laboratory information management systems (LIMS), and statistical analysis tools. This interoperability is essential for reducing information silos that can hinder effective management processes. By facilitating real-time information sharing and collaboration across platforms, these integration features streamline workflows and significantly enhance the overall quality of research findings.
Research has shown that EHR integration can improve feasibility and efficiency in recruitment, screening, and information gathering, ultimately leading to faster and more reliable outcomes. Moreover, the implementation of unified dashboards empowers stakeholders to monitor enrollment metrics and safety signals in real time, allowing for quicker interventions when necessary. As organizations increasingly recognize the importance of interoperability, the integration of EHRs into research systems continues to evolve, driving advancements in information management and patient safety.
Customizable reporting features in research software are crucial for generating tailored insights that meet the diverse needs of stakeholders, including sponsors, regulatory bodies, and research teams. By enabling users to select relevant metrics, formats, and visualizations, these features significantly enhance communication and facilitate informed decision-making. This flexibility becomes increasingly vital as research studies progress; notably, 72% of sponsors believe that improved training would greatly enhance site performance.
Furthermore, the shift towards information ownership and transparency underscores the necessity for stakeholders to access real-time insights, empowering them to respond swiftly to emerging challenges. As we look ahead to 2025, the demand for customized reporting will only intensify, making it imperative for research project tools to provide adaptable solutions that cater to the specific needs of all stakeholders.

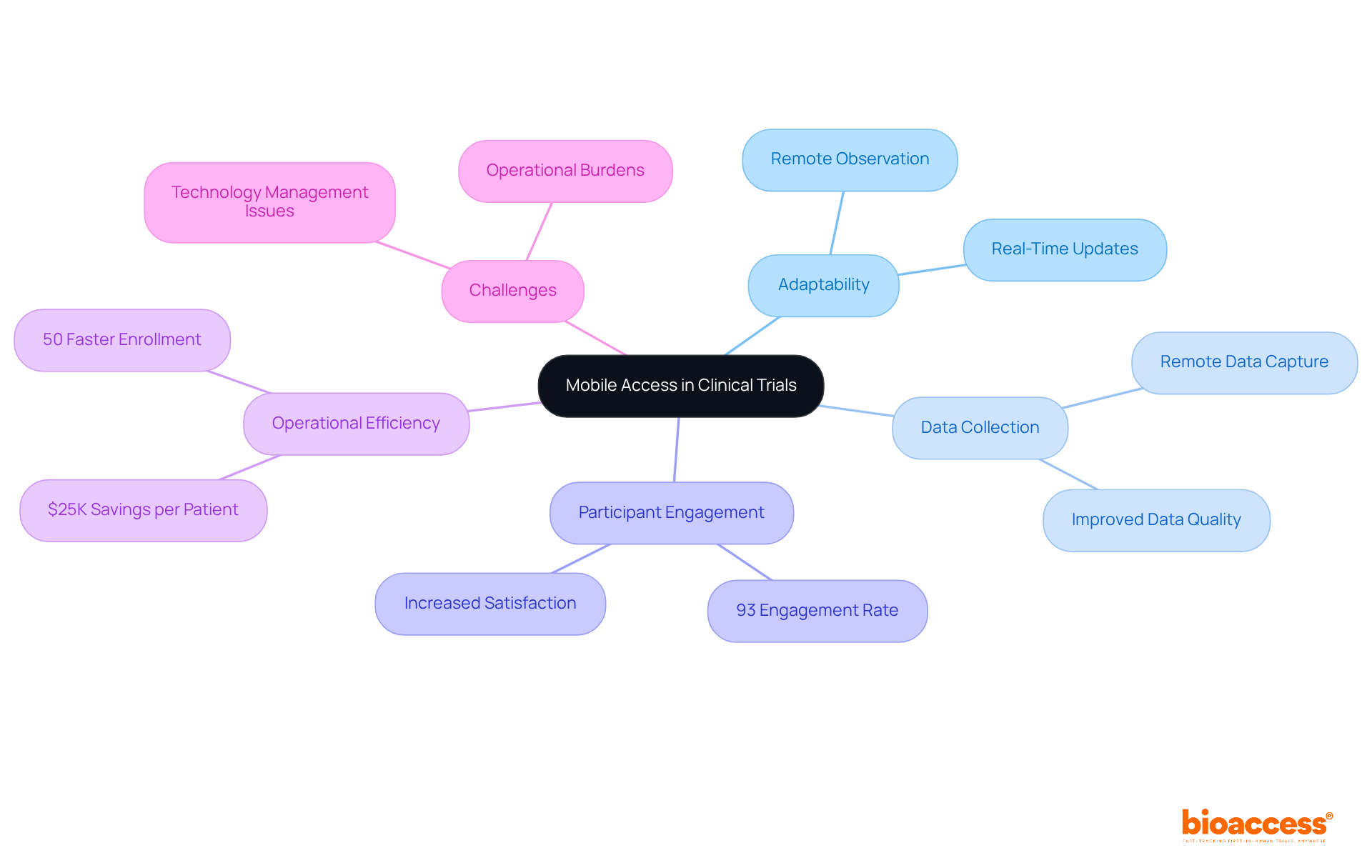
Mobile access to research study software empowers investigators and coordinators to manage studies from virtually anywhere, significantly enhancing adaptability and responsiveness. This capability is crucial in the Medtech landscape, where bioaccess features—such as mobile data entry, remote observation, and participant engagement tools—facilitate real-time updates and interactions. These elements are essential for improving data collection and participant adherence.
For instance, mobile health studies (MCTs) have demonstrated enhanced operations and superior data quality. Researchers have noted that remote data collection leads to better compliance management and increased participant satisfaction. A striking study found that 93% of participants engaged with the app at least weekly after one year, underscoring the effectiveness of mobile solutions in sustaining participant involvement.
By leveraging mobile technology, research teams can streamline operations, reduce logistical challenges, and ultimately boost the overall efficiency of their studies, paving the way for more successful outcomes. With bioaccess® capabilities, treatment-naive cardiology or neurology cohorts can be enrolled 50% faster than traditional methods, translating to $25K savings per patient through the use of FDA-ready data—eliminating rework and delays.
However, it is important to acknowledge that challenges such as operational burdens and technology management issues persist in mobile health studies.
As the clinical research landscape evolves, collaboration becomes increasingly vital. By embracing mobile solutions, research teams can not only enhance participant engagement but also drive forward the efficiency and effectiveness of their studies.
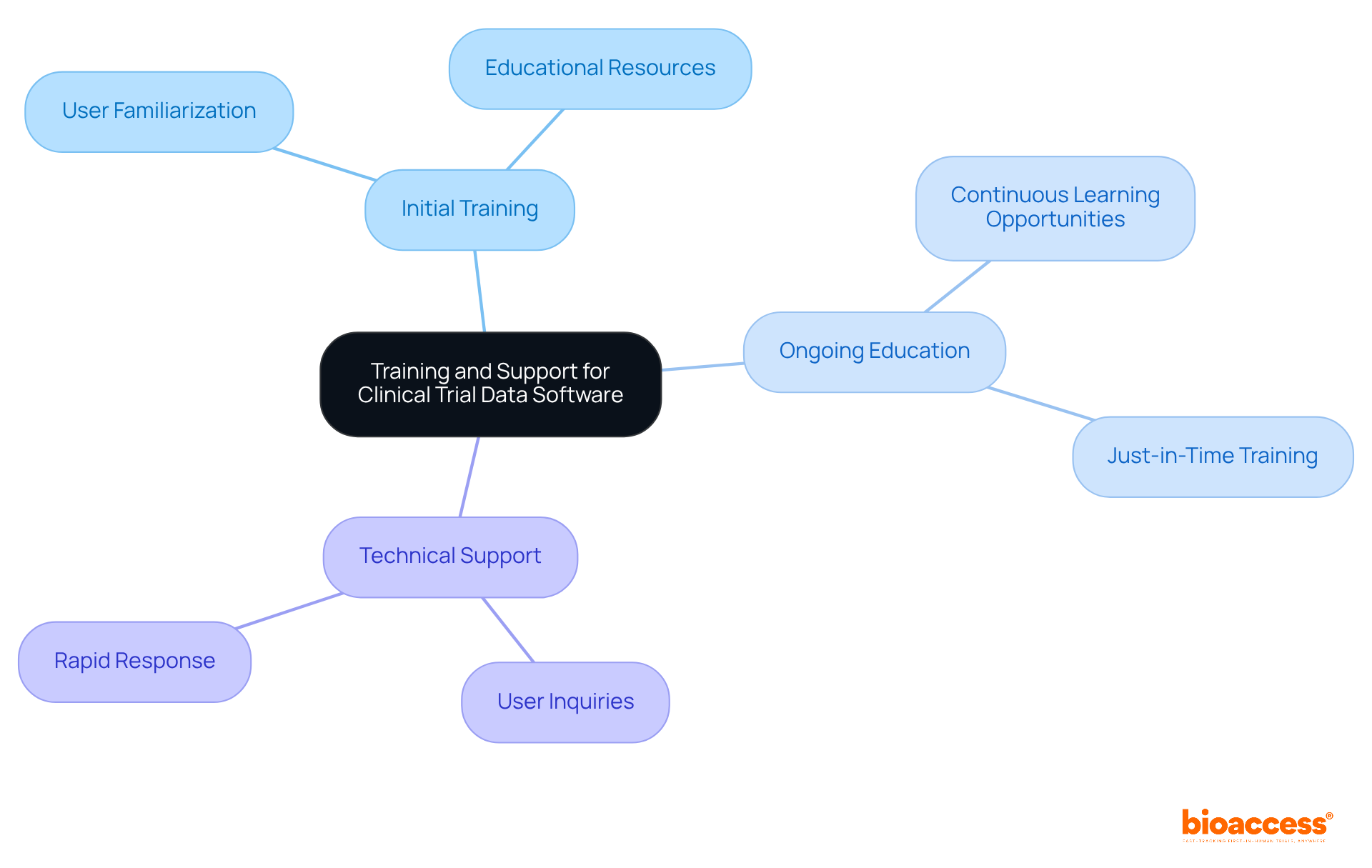
Enhancing the efficiency of clinical trial management tools hinges on robust training and support systems. Organizations must implement initial training sessions that familiarize users with the system's features, complemented by ongoing educational resources to reinforce learning. Moreover, responsive technical support is crucial for addressing user inquiries and challenges promptly. Investing in these training initiatives not only boosts user confidence but also significantly reduces errors, leading to enhanced information integrity and improved research outcomes.
As the research landscape evolves, staying aware of current trends in technical support requirements—such as the need for just-in-time training options and user-friendly access—will further enable teams to utilize updates effectively. This ensures they remain at the forefront of innovation in medical research. Notably, 37% of US workers report being extremely satisfied with the training opportunities available at their jobs, underscoring the importance of effective training programs. Furthermore, organizations that overlook training may incur substantial expenses, with an estimated $5.2 million in revenue lost due to untapped information.
As Dr. Jennifer Priestley emphasizes, successful data analysts must bridge the gap between technical skills and business acumen, underscoring the critical role of comprehensive training in utilizing clinical trial data software. This is not just a matter of efficiency; it’s about ensuring that teams are equipped to meet the challenges of modern clinical research head-on.
The landscape of clinical trial data management is evolving rapidly, making the features of clinical trial data software crucial for the success of Medtech innovators. By concentrating on essential functionalities—such as regulatory compliance, data analytics, user-friendly interfaces, and secure data storage—organizations can significantly enhance their research capabilities and streamline processes. Integrating these features not only accelerates trial phases but also fosters improved patient outcomes and builds trust among stakeholders.
Key arguments throughout this article highlight the importance of these software capabilities. From automated compliance monitoring and real-time data access to customizable reporting and mobile management, each feature plays a vital role in boosting the efficiency and effectiveness of clinical trials. The ability to adapt to emerging challenges while maintaining high standards of data integrity is essential for organizations aiming to lead in the competitive Medtech environment.
As the demand for innovative medical solutions continues to grow, embracing advanced clinical trial data software becomes increasingly significant. Organizations are urged to invest in these essential features to ensure they remain at the forefront of clinical research advancements. By prioritizing robust training and support systems, Medtech companies can empower their teams to fully harness the potential of these tools, ultimately contributing to the success of their research endeavors and enhancing patient care.
What is bioaccess® and how does it benefit Medtech innovators?
bioaccess® is an information management solution designed specifically for Medtech innovators. It offers enhanced capabilities to secure ethical approvals in 4-6 weeks and achieve enrollment rates that are 50% faster than traditional markets, facilitating quicker market access for innovative medical technologies and improving patient care.
What are the essential features of clinical trial data software for regulatory compliance?
Essential features include automated monitoring of regulatory updates, detailed audit trails, strict adherence to Good Clinical Practice (GCP) guidelines, seamless access to compliance documentation, and real-time monitoring of compliance metrics. These features are crucial for validating the reliability of collected data and ensuring successful regulatory submissions.
Why is regulatory compliance important in clinical trials?
Regulatory compliance is vital for the integrity of clinical research. Non-compliance can lead to study failures, and adhering to GCP standards fosters greater trust with regulatory bodies and participants. It safeguards the integrity of research and positions organizations as leaders in the Medtech landscape.
How does data analytics enhance clinical trial outcomes?
Enhanced analytical features in research software allow scientists to transform unrefined information into actionable insights. Tools like predictive analytics, real-time information visualization, and automated reporting help identify trends that can inform study adjustments, leading to quicker approvals and improved patient care.
What are the financial benefits of using bioaccess® solutions in clinical trials?
Using bioaccess®'s advanced solutions can lead to patient enrollment being achieved 50% faster and result in savings of $25K per patient through the provision of FDA-ready information, streamlining the research process.
How does collaboration with bioaccess® benefit Medtech companies?
Partnering with bioaccess® allows Medtech companies to navigate regulatory challenges more effectively, ensuring that their innovative technologies reach the market swiftly and efficiently, thus maintaining momentum in research.