


The insights regarding bioburden testing for clinical research underscore its vital importance in ensuring product safety, adhering to regulatory standards, and preventing expensive recalls. This article emphasizes that comprehensive evaluations of microbial load are crucial for protecting patient health. Neglecting these tests can result in dire outcomes, such as infections and substantial financial repercussions stemming from product recalls.
The landscape of clinical research is increasingly defined by the critical necessity of bioburden testing, a process that quantifies viable microorganisms in products and samples. As the stakes rise with stringent regulatory demands and the ever-present risk of contamination, understanding the nuances of bioburden testing becomes essential for researchers aiming to safeguard patient safety and ensure compliance.
With rapid advancements in testing methodologies and the growing complexity of regulations, researchers face significant challenges. How can they effectively navigate these complexities to optimize their clinical trials? This question underscores the urgency and importance of mastering bioburden testing in today's research environment.
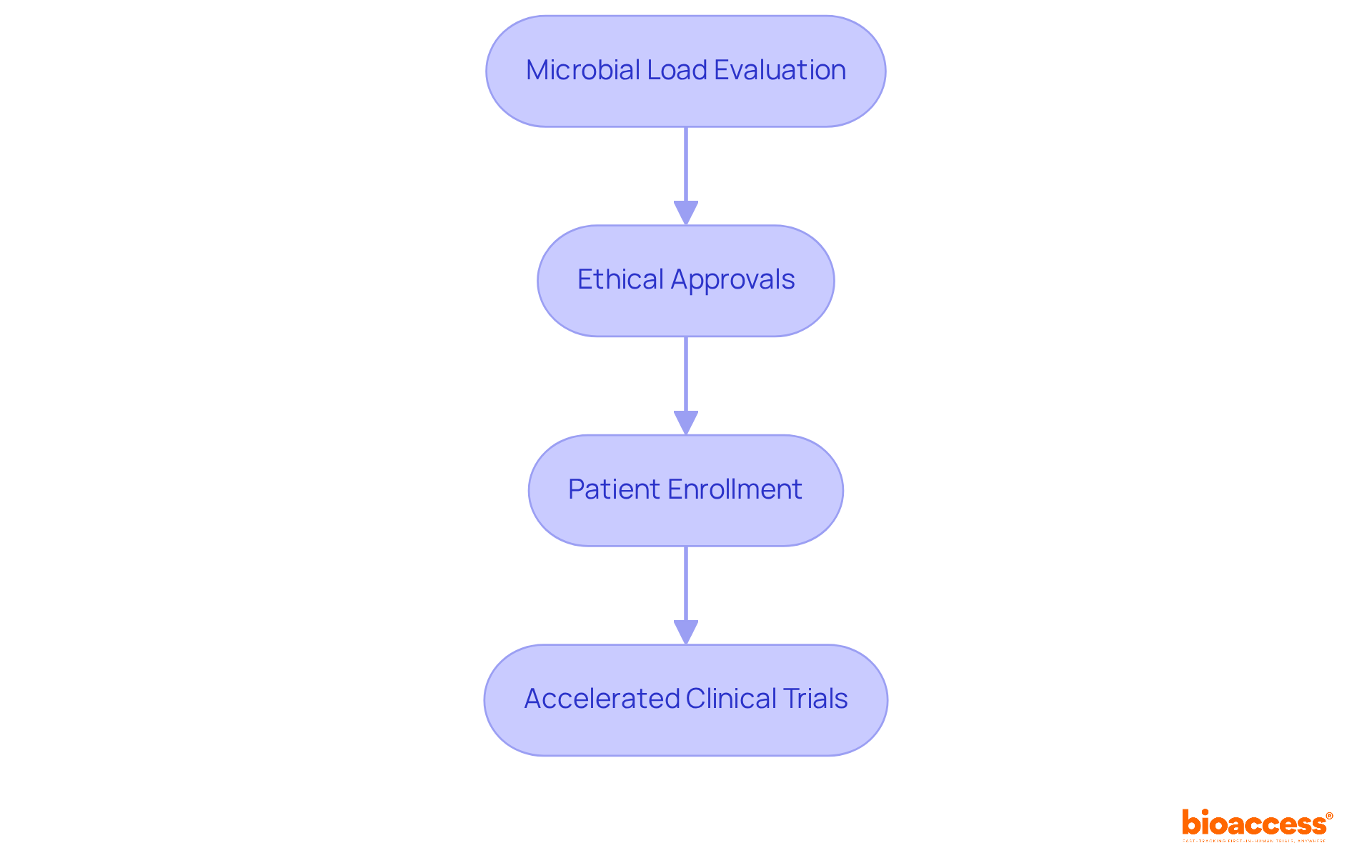
bioaccess® leverages its extensive expertise in clinical research to streamline microbial load evaluation procedures within its comprehensive acceleration services. By utilizing advanced methodologies, regulatory knowledge, and centralized monitoring, bioaccess® guarantees that bioburden assessments are executed efficiently. This efficiency facilitates faster ethical approvals and expedites patient enrollment.
With over 50 pre-qualified sites activated in less than eight weeks and FDA/EMA/MDR-ready datasets, this service proves particularly advantageous for Medtech, Biopharma, and Radiopharma innovators. These innovators can accelerate their clinical trials without compromising quality or compliance, addressing key challenges in the industry effectively.
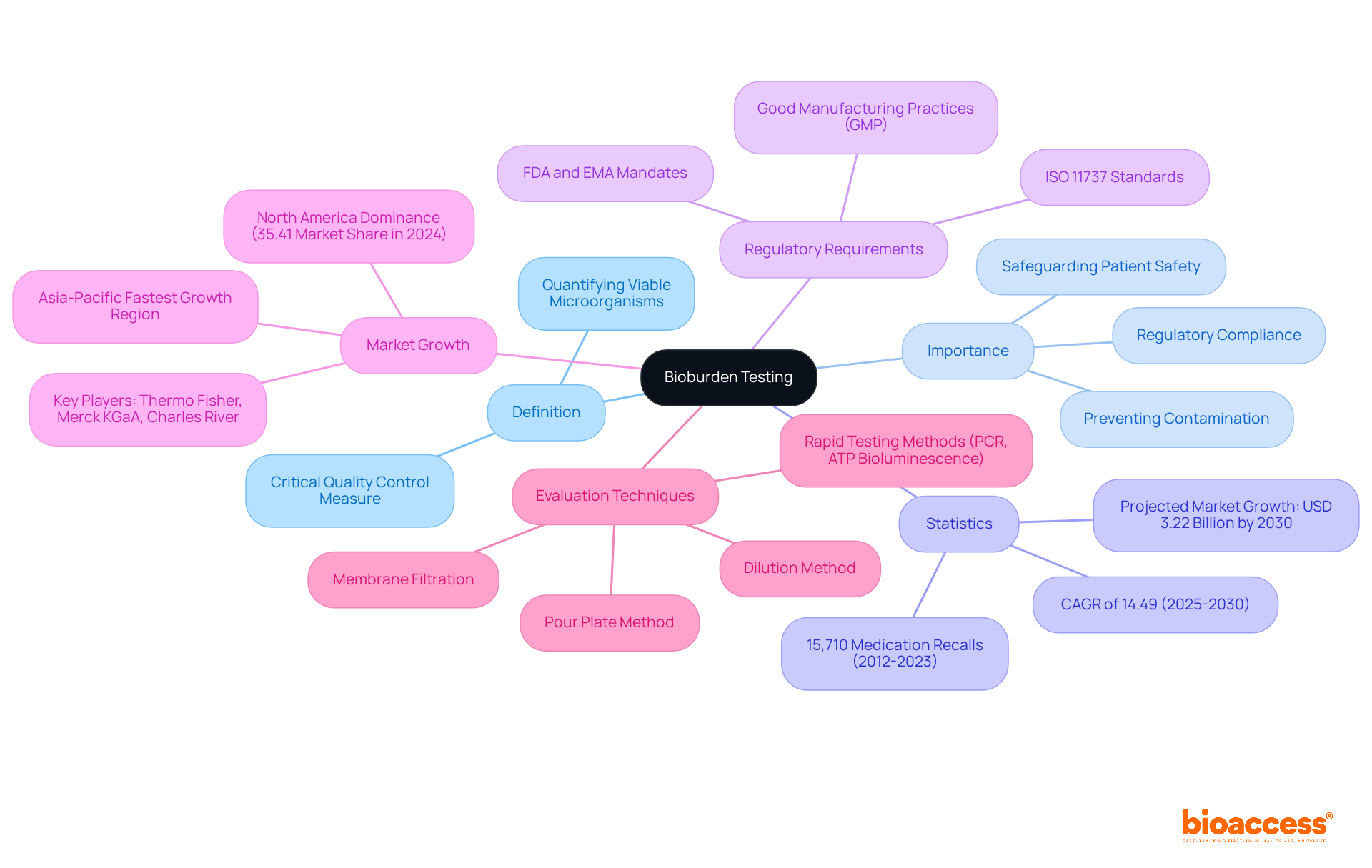
Bioburden testing is the critical process of quantifying viable microorganisms present on a product or within a sample, serving as a cornerstone in clinical research. This evaluation is essential for ensuring that medical devices and pharmaceuticals are free from harmful contamination levels through bioburden testing. By accurately identifying microbial presence, researchers can significantly mitigate infection risks, thereby safeguarding patient safety.
The significance of microbial load evaluation is underscored by alarming statistics; from 2012 to 2023, over 15,710 medication recalls occurred due to contamination issues. Furthermore, regulatory agencies such as the FDA and EMA mandate thorough bioburden testing and microbial load assessments in accordance with Good Manufacturing Practices (GMP) and ISO 11737, ensuring that products meet stringent safety standards.
As the microbial load assessment market is projected to expand at a compound annual growth rate of 14.49% from 2025 to 2030, reaching an anticipated market size of USD 3.22 billion by 2030, the importance of this evaluation method in the clinical trial process cannot be overstated. Various microbial load evaluation techniques, including membrane filtration and the pour plate method, are employed to ensure comprehensive analysis and effective contamination management.
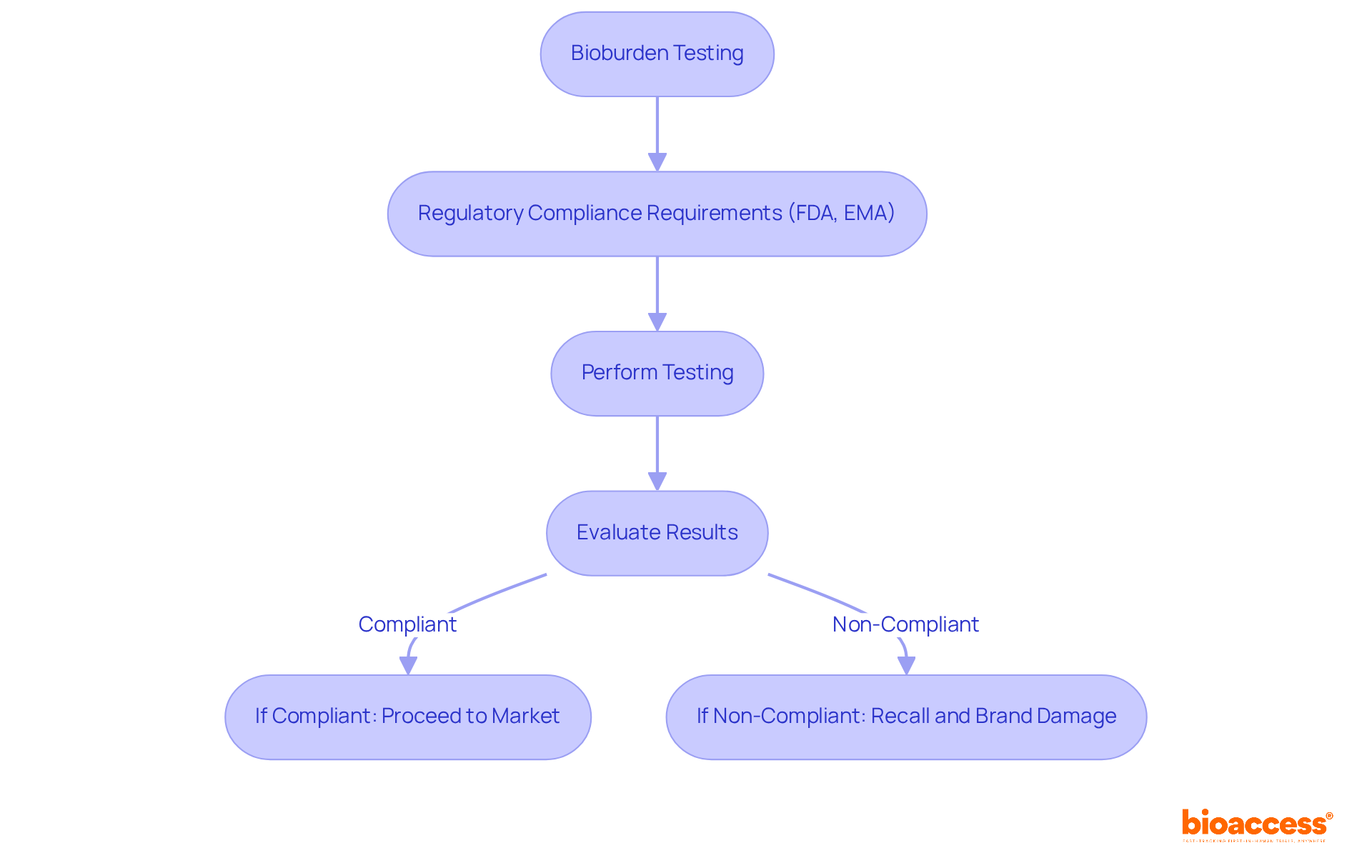
Bioburden evaluation is crucial in ensuring compliance with regulatory standards established by organizations such as the FDA and EMA. These regulations require pharmaceutical manufacturers to perform bioburden testing to prove that their products meet safety and quality standards. By following these guidelines, companies can avert costly recalls and legal complications, thereby protecting their reputation and ensuring patient safety.
As Mohamed Ashraf Al-Nagar emphasizes, unauthorized items arising from cross-contamination can result in recalls, severely damaging the reputation of pharmaceutical firms. Furthermore, the financial repercussions of non-compliance can be significant; companies may face substantial losses, including costs associated with recalls and damage to their brand image. Notably, 21% of consumers abandon a manufacturer following a recall, underscoring the necessity of maintaining product integrity.
A prominent example is the FDA's withdrawal of over 80 drugs in 2019 due to contamination issues, highlighting the essential need for stringent bioburden testing procedures. As the microbial load evaluation market continues to grow, propelled by increasing regulatory scrutiny and a heightened focus on product safety, it becomes imperative for producers to prioritize these evaluation protocols to safeguard both their products and their consumers.
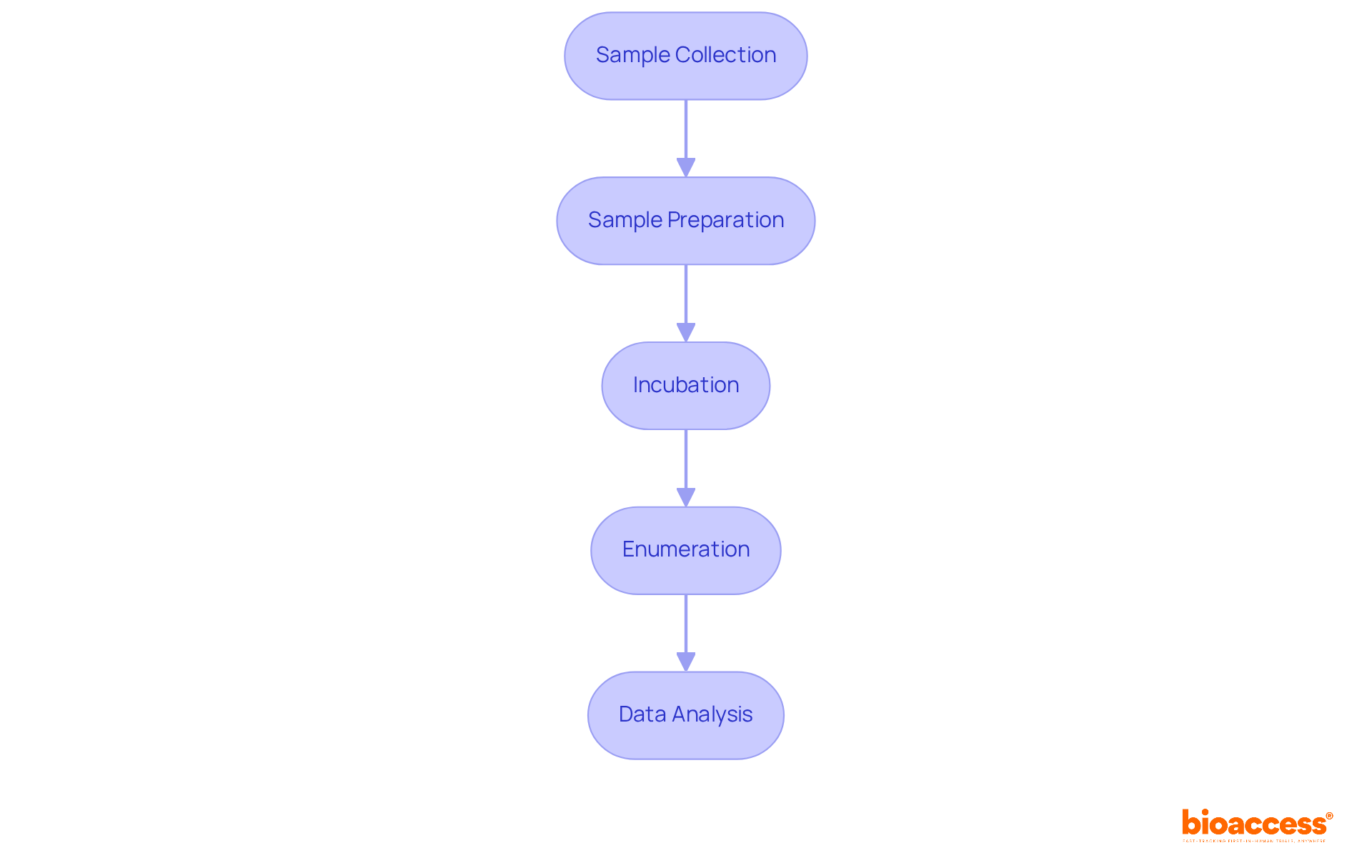
The procedure for bioburden testing encompasses several essential steps that ensure the accuracy and reliability of results.
Each of these steps plays a crucial part in the overall success of microbial load assessment, ensuring that products satisfy safety and quality standards. Routine bioburden testing assessments could have saved firms millions by preventing recalls, emphasizing the significance of these procedures.
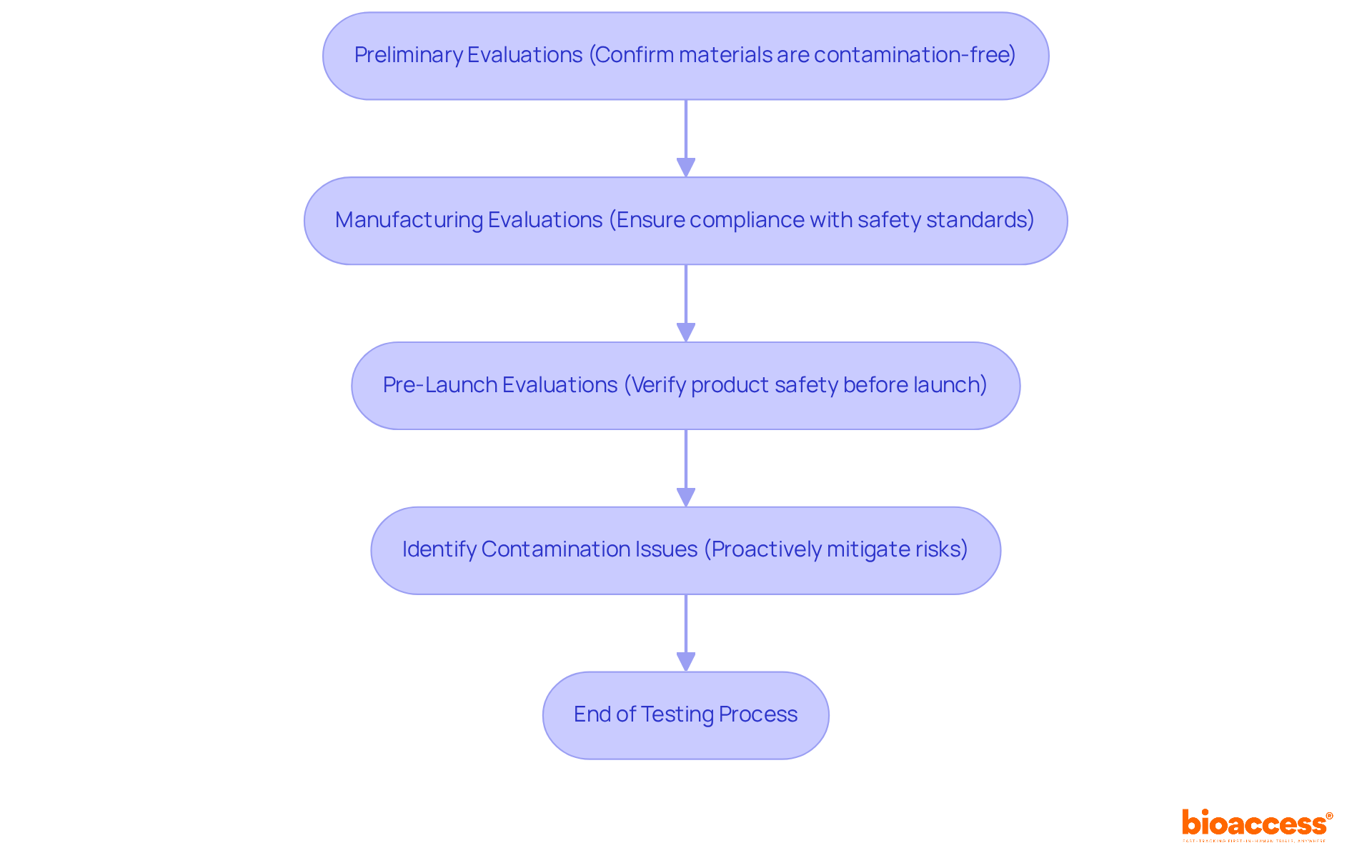
Bioburden testing is crucial at multiple stages of the clinical research process. Preliminary evaluations during the development stage are vital to confirm that materials and components are free from contamination. Subsequently, evaluations should be conducted during manufacturing and just before launch to ensure compliance with safety standards. By strategically timing these tests, researchers can proactively identify and mitigate potential contamination issues, significantly reducing the risk of delays in the clinical trial process.
For instance, from 2012 to 2023, there were 15,710 medication recalls due to contamination and other issues, highlighting the critical role of bioburden testing in preventing such costly recalls, which average approximately USD 10 million in the food sector. Furthermore, the FDA emphasizes preventive measures, underscoring the importance of early detection in maintaining integrity and safety.
Bioburden testing is conducted on each batch prior to sterilization in medical device production, ensuring that items meet the stringent standards set by agencies such as the FDA and EMA. Routine assessments, including bioburden testing, help in identifying common pollutants such as bacteria, fungi, spores, and viruses, further reinforcing the necessity of comprehensive evaluations throughout the clinical research process.
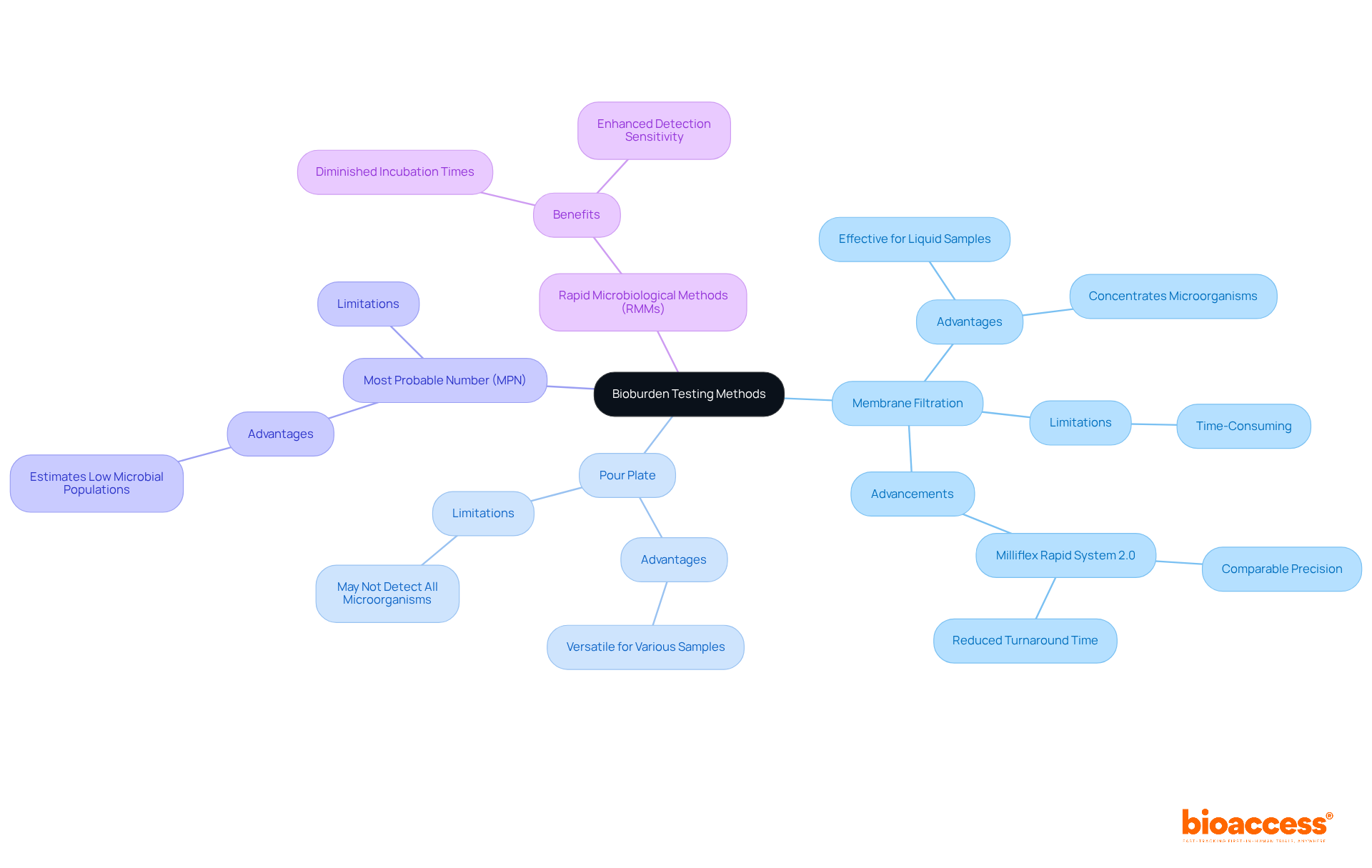
Selecting the appropriate method for microbial load evaluation, such as bioburden testing, is essential and depends on factors such as item category, anticipated microbial levels, and compliance with regulatory standards. The most commonly employed methods include membrane filtration, pour plate, and most probable number (MPN) techniques.
Recent advancements in bioburden testing have led to the development of rapid microbiological methods (RMMs), which significantly diminish incubation times and enhance detection sensitivity. For instance, the Milliflex Rapid System 2.0 has demonstrated comparable precision to traditional methods while expediting the evaluation process, facilitating item release in as few as four days compared to the standard 14-day timeline.
Successful applications of these methods underscore their critical role in ensuring safety and regulatory compliance, especially with the incorporation of bioburden testing. A notable example involves a pharmaceutical firm that implemented stringent contamination assessment protocols, enabling early identification of impurities and averting an expensive recall, thus ensuring that only safe items reached the market.
Experts advocate for researchers to thoroughly assess their specific needs and consult certified laboratories to determine the most suitable method for bioburden testing in contamination assessment. This collaboration can effectively navigate the complexities of bioburden evaluation, ensuring the chosen approach aligns with both product characteristics and compliance expectations.
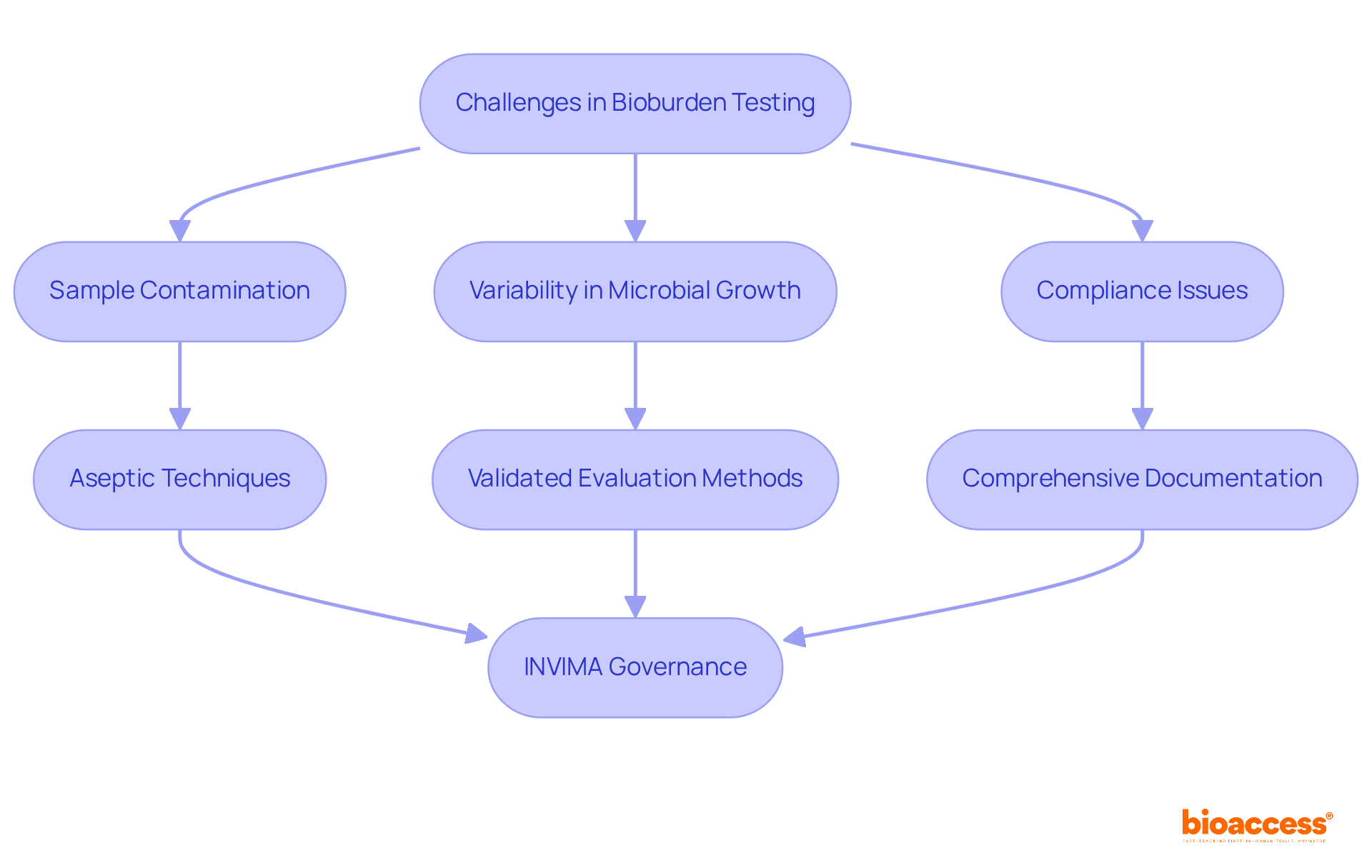
Bioburden testing presents several challenges, including sample contamination, variability in microbial growth, and compliance issues. To effectively address these challenges, researchers must implement stringent aseptic techniques during sample collection, utilize validated evaluation methods including bioburden testing, and maintain comprehensive documentation throughout the examination process.
Understanding INVIMA's specific governance functions, particularly its designation as a Level 4 health authority by PAHO/WHO, is essential. This classification highlights INVIMA's capability in ensuring compliance with health standards for medical devices. The Directorate for Medical Devices within INVIMA is pivotal in monitoring and controlling medical devices, suggesting technical standards, and ensuring adherence to established protocols.
Collaborating with skilled laboratories can significantly aid in tackling these challenges efficiently, ensuring that all evaluations align with the rigorous standards set by INVIMA and incorporate best practices in bioburden testing.
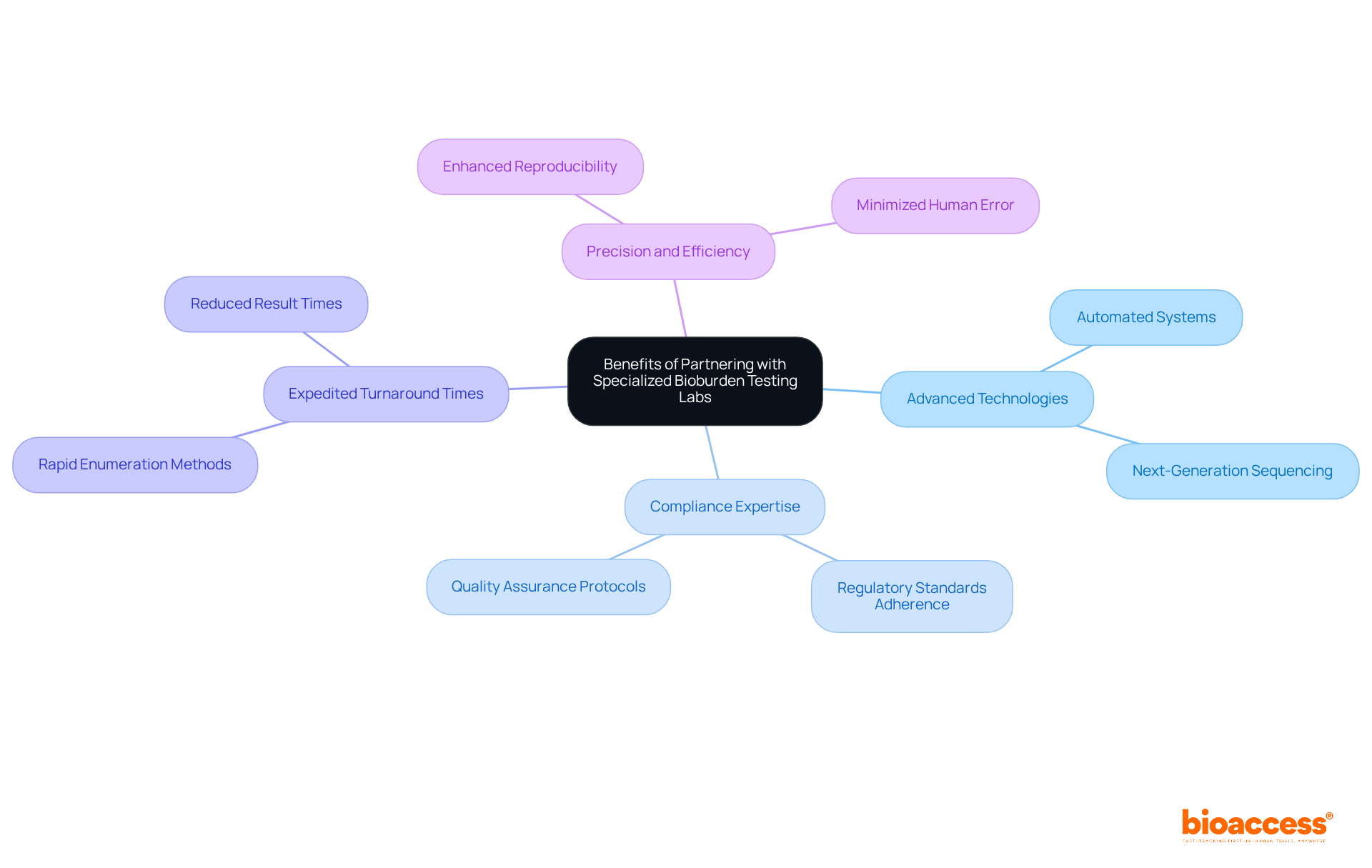
Partnering with specialized contamination analysis laboratories presents significant advantages, including access to advanced technologies, compliance expertise, and remarkably expedited turnaround times for results. These laboratories utilize cutting-edge equipment and are staffed by experts well-versed in the complexities of bioburden testing.
For example, rapid enumeration methods can shorten result times from days to just hours, a critical factor for maintaining the momentum of clinical trials. By collaborating with these specialists, researchers can ensure that their evaluations, including bioburden testing, adhere to stringent regulatory standards, thereby enhancing the quality and reliability of their clinical studies.
Recent advancements in automated systems further improve the precision and efficiency of evaluation processes, making such collaborations indispensable for the success of clinical research initiatives.
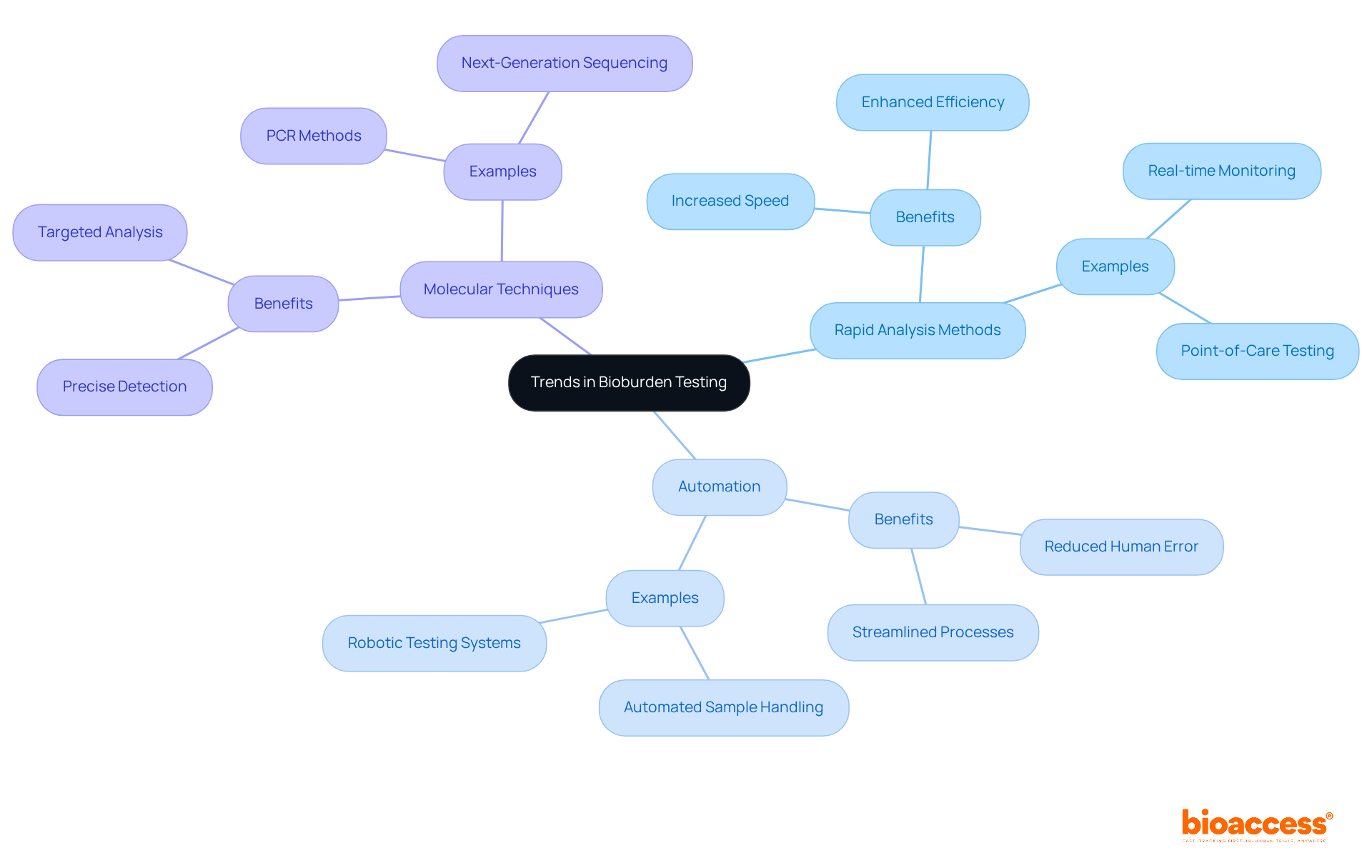
Recent advancements in bioburden testing underscore the increasing adoption of rapid analysis methods, the automation of procedures, and the integration of molecular techniques for precise microorganism detection. These innovations markedly enhance the speed and efficiency of evaluations, while simultaneously bolstering the reliability of results.
For instance, the U.S. Biomonitoring and bioburden testing market was valued at USD 1.2 billion in 2020 and is projected to grow at a CAGR of 9.8% from 2026 to 2033, reflecting a robust demand for stringent quality control in pharmaceutical and medical device manufacturing.
As oversight bodies impose rigorous criteria for managing microbial contamination, companies such as Merck and Becton Dickinson are prioritizing sophisticated evaluation methods, such as bioburden testing, to ensure compliance. Staying informed about these developments empowers researchers to leverage new technologies that can significantly enhance their contamination assessment procedures.
Additionally, as one expert noted, 'The rising incidence of hospital-acquired infections and the need to prevent microbial contamination in healthcare settings are encouraging the adoption of biomonitoring solutions.
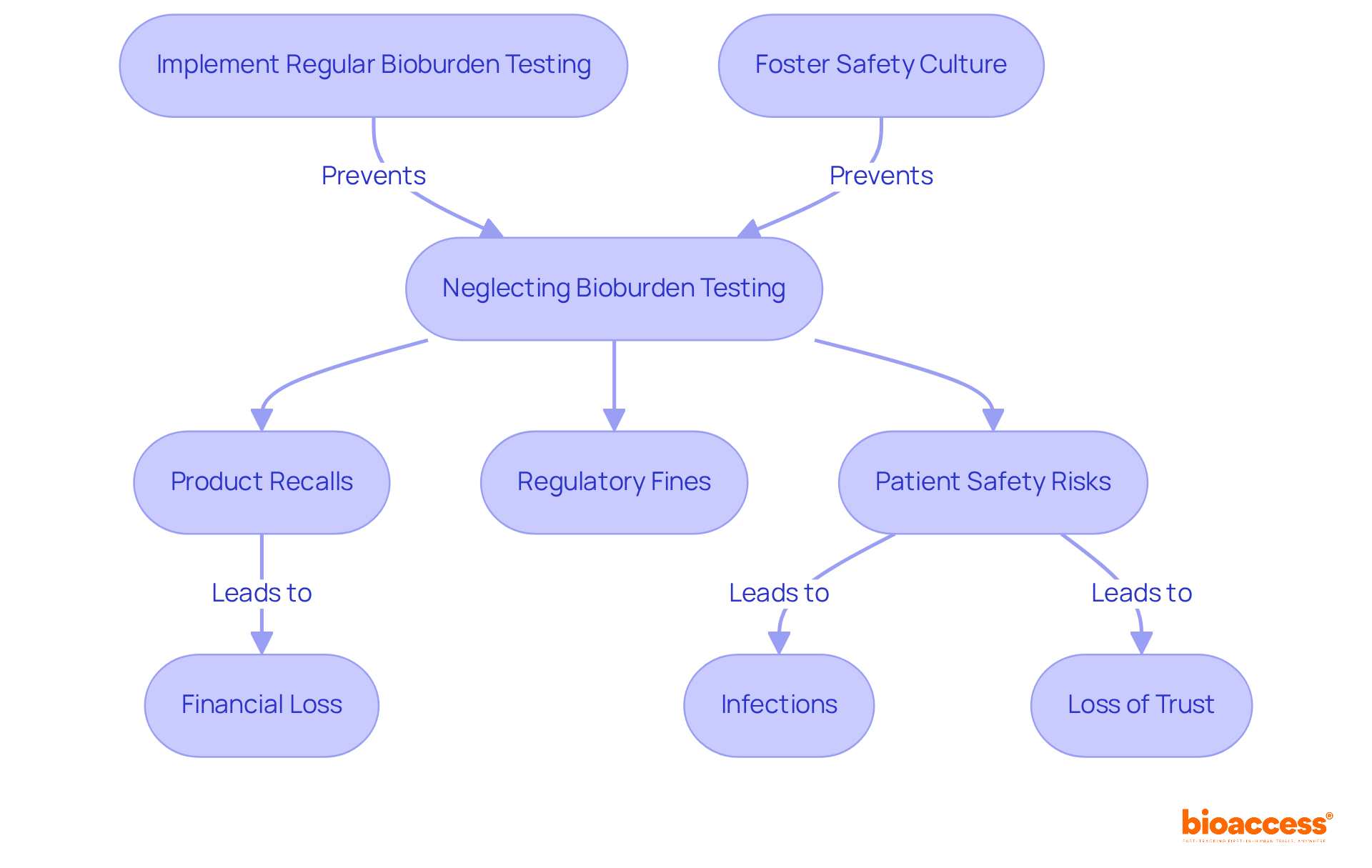
Ignoring microbial load assessment can lead to severe consequences, including product recalls, regulatory fines, and jeopardized patient safety. Contaminated medical devices pose risks of infections, adverse reactions, and a significant loss of trust among stakeholders. A notable case involves a U.S.-based orthopedic implant firm that faced a $5.4 million recall due to microbial contamination in a batch of spinal cages. This incident underscores the financial and reputational dangers associated with insufficient microbial load assessment.
Regulatory organizations, such as the FDA and ISO, mandate that all medical devices undergo thorough sterilization, followed by bioburden testing and sterility evaluations to ensure safety. Elevated levels of microbial contamination can compromise sterilization procedures, allowing harmful pathogens to persist and potentially endangering patients.
Experts emphasize that regular microbial load assessments should be conducted at various stages of the development process to monitor cleanliness in production environments. Failures to identify contamination sources can lead to significant health hazards, as contamination can originate from personnel, manufacturing equipment, and raw materials.
By prioritizing bioburden testing and fostering a robust safety culture, researchers can effectively mitigate risks, ensuring the safety and efficacy of their products while adhering to stringent regulatory standards.
The critical role of bioburden testing in clinical research is paramount. This essential process safeguards patient safety by identifying microbial contamination and ensures compliance with stringent regulatory standards. By prioritizing bioburden assessments, researchers can effectively mitigate risks associated with contamination, ultimately enhancing the integrity and success of clinical trials.
Key insights highlighted throughout the article emphasize:
Each of these elements significantly contributes to maintaining high safety standards and avoiding costly regulatory repercussions. Furthermore, advancements in technology and methodologies are making bioburden testing more efficient, allowing for quicker results and improved accuracy.
Ultimately, embracing best practices in bioburden testing is vital for both researchers and pharmaceutical manufacturers. By fostering a culture of safety and compliance, and staying informed about the latest trends and innovations, stakeholders can ensure their products meet the highest standards of quality. This commitment not only protects patient safety but also enhances trust and credibility within the industry, paving the way for successful clinical outcomes and a healthier future for all.
What is bioaccess® and what services does it provide?
bioaccess® is a service that leverages expertise in clinical research to streamline microbial load evaluation procedures. It offers comprehensive acceleration services, utilizing advanced methodologies and centralized monitoring to ensure efficient bioburden assessments, which facilitate faster ethical approvals and patient enrollment.
Why is bioburden testing important in clinical research?
Bioburden testing is critical for quantifying viable microorganisms in products or samples. It ensures that medical devices and pharmaceuticals are free from harmful contamination, thereby safeguarding patient safety and mitigating infection risks.
What are the regulatory requirements for bioburden testing?
Regulatory agencies such as the FDA and EMA require thorough bioburden testing and microbial load assessments in accordance with Good Manufacturing Practices (GMP) and ISO 11737, ensuring that products meet stringent safety standards.
What techniques are used for microbial load evaluation?
Techniques for microbial load evaluation include membrane filtration and the pour plate method, which are employed to ensure comprehensive analysis and effective contamination management.
What are the consequences of failing to comply with bioburden testing regulations?
Non-compliance with bioburden testing regulations can lead to costly recalls, legal complications, and damage to a company's reputation. It can also result in significant financial losses and a loss of consumer trust, as 21% of consumers abandon a manufacturer following a recall.
How significant has the microbial load assessment market become?
The microbial load assessment market is projected to grow at a compound annual growth rate of 14.49% from 2025 to 2030, reaching an anticipated market size of USD 3.22 billion by 2030, highlighting the increasing importance of bioburden testing in clinical trials.
What examples illustrate the necessity of stringent bioburden testing?
A notable example is the FDA's withdrawal of over 80 drugs in 2019 due to contamination issues, emphasizing the essential need for rigorous bioburden testing procedures to ensure product safety and compliance.