


The article articulates essential insights for designing clinical trial case report forms (CRFs), underscoring the significance of clarity, standardization, and technological integration. It illustrates how effective CRF design enhances data accuracy and compliance, ultimately leading to improved efficiency in clinical trials and expedited product approvals. This focus on CRF design is not merely procedural; it is a strategic imperative that influences the overall success of clinical research initiatives.
The design of clinical trial case report forms (CRFs) is crucial to the success of medical research; however, many still grapple with the complexities involved. As the demand for efficient data collection escalates, grasping the nuances of CRF design can unlock substantial improvements in trial outcomes.
What essential strategies can transform CRF development from a cumbersome task into a streamlined process that enhances accuracy and compliance? This article delves into ten critical insights that not only highlight best practices but also explore the future of CRF design within the ever-evolving landscape of clinical trials.
bioaccess® leverages its extensive experience in early-stage research to enhance the design and execution of clinical trial case report forms (CRFs). By integrating regulatory efficiency with diverse patient populations, bioaccess® guarantees that the clinical trial case report forms (CRFs) are not only compliant but also specifically tailored to the unique demands of Medtech, Biopharma, and Radiopharma innovators. This strategic focus accelerates the research process, facilitating faster information gathering and evaluation, which ultimately leads to quicker product approvals and expedited market entry.
A clinical trial case report form (CRF) is a crucial document in research studies, meticulously designed to collect information from each participating patient. Its primary objective is to guarantee that all essential data is recorded systematically and consistently, thereby facilitating accurate information analysis and regulatory submissions. Acting as the bedrock of trial information management, the clinical trial case report form provides a structured format for documenting patient outcomes, adverse events, and other critical details necessary for evaluating the safety and effectiveness of medical interventions. Research indicates that 90% of medical trials in China have relied on manual data collection and submission, underscoring the vital role of CRFs in ensuring accuracy and efficiency.
Moreover, effective clinical information management is indispensable for safeguarding patient safety, ensuring regulatory compliance, and optimizing cost efficiency in healthcare. The quality of data collected on the clinical trial case report form directly influences the integrity of the database and the validity of study outcomes, emphasizing the need for meticulous design and management of the clinical trial case report form. Furthermore, the potential for errors in data entry due to transcription mistakes accentuates the necessity for implementing precision protocols in CRF management. The adoption of electronic Case Report Forms (eCRFs) can significantly enhance data quality and streamline management processes, establishing them as an essential asset in modern research studies. Ultimately, clear and accurate research findings are paramount for statistical evaluation and regulatory filings, reinforcing the essential role CRFs play in the overall success of medical research.
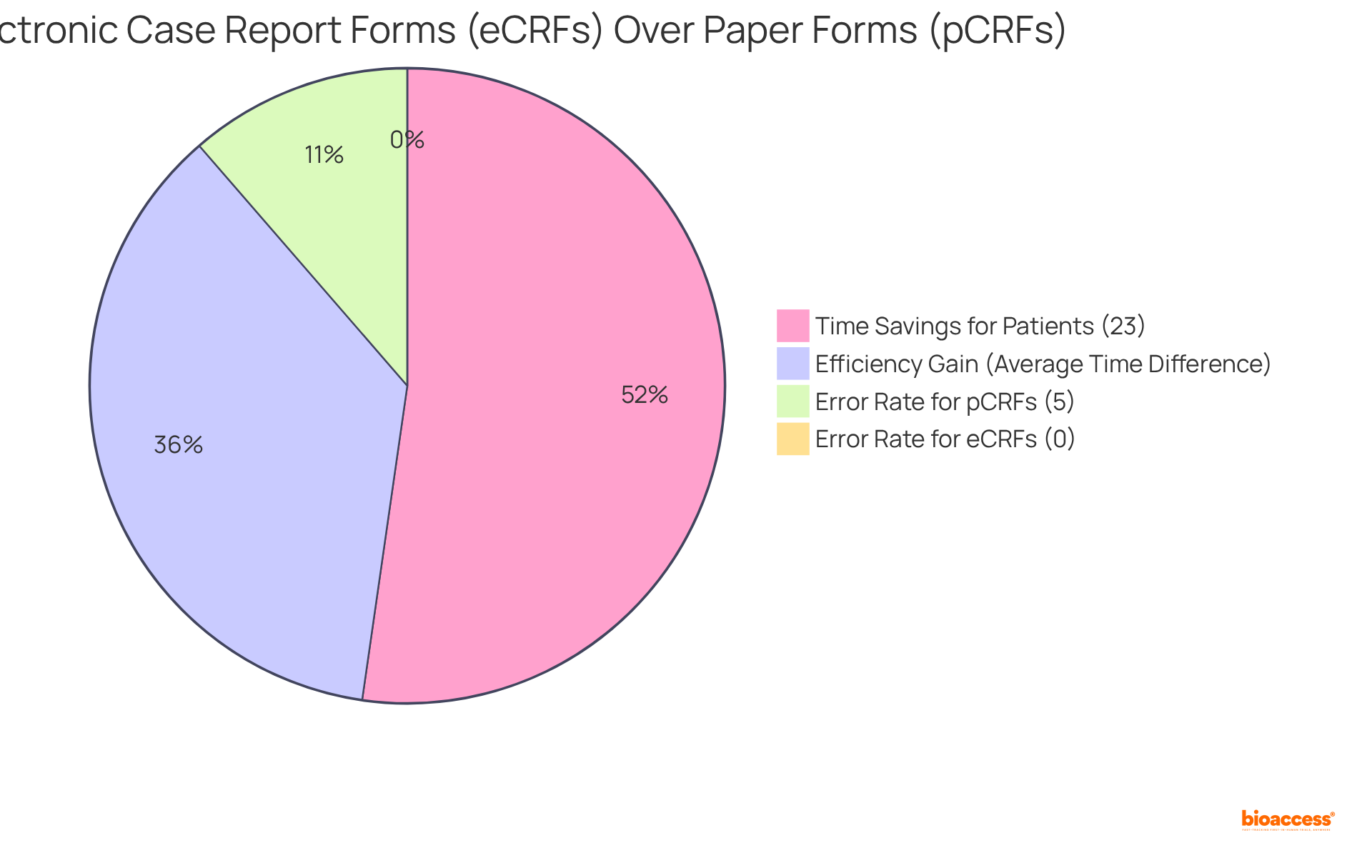
The transition from conventional paper-based Case Report Forms (pCRFs) to electronic Case Report Forms (eCRFs) has fundamentally transformed the information collection in the clinical trial case report form. eCRFs present a multitude of advantages, including real-time data entry and automated validation checks, which enhance both accuracy and security. Notably, studies indicate that completing an eCRF averages 8.29 minutes, in contrast to 10.54 minutes for pCRFs, underscoring a significant efficiency gain.
Furthermore, eCRFs are associated with a remarkable reduction in entry errors, with no mistakes reported in eCRF conditions compared to a 5% error rate for pCRFs. Patients also experience a 23% time savings when utilizing eCRFs versus pCRFs, further highlighting their effectiveness. Innovations such as cloud-based platforms enable seamless collaboration among research teams, facilitating faster data access and analysis.
Prominent eCRF platforms, including Medidata Rave and ClinPlus EDC, enhance efficiency and minimize errors, exemplifying the technological advancements in this domain. This progress not only simplifies the regulatory submission process but also accelerates the overall timeline for trials, ultimately leading to quicker delivery of new therapies to patients. As the industry increasingly embraces the clinical trial case report form, the advantages become clear, establishing it as an essential component in contemporary clinical research.
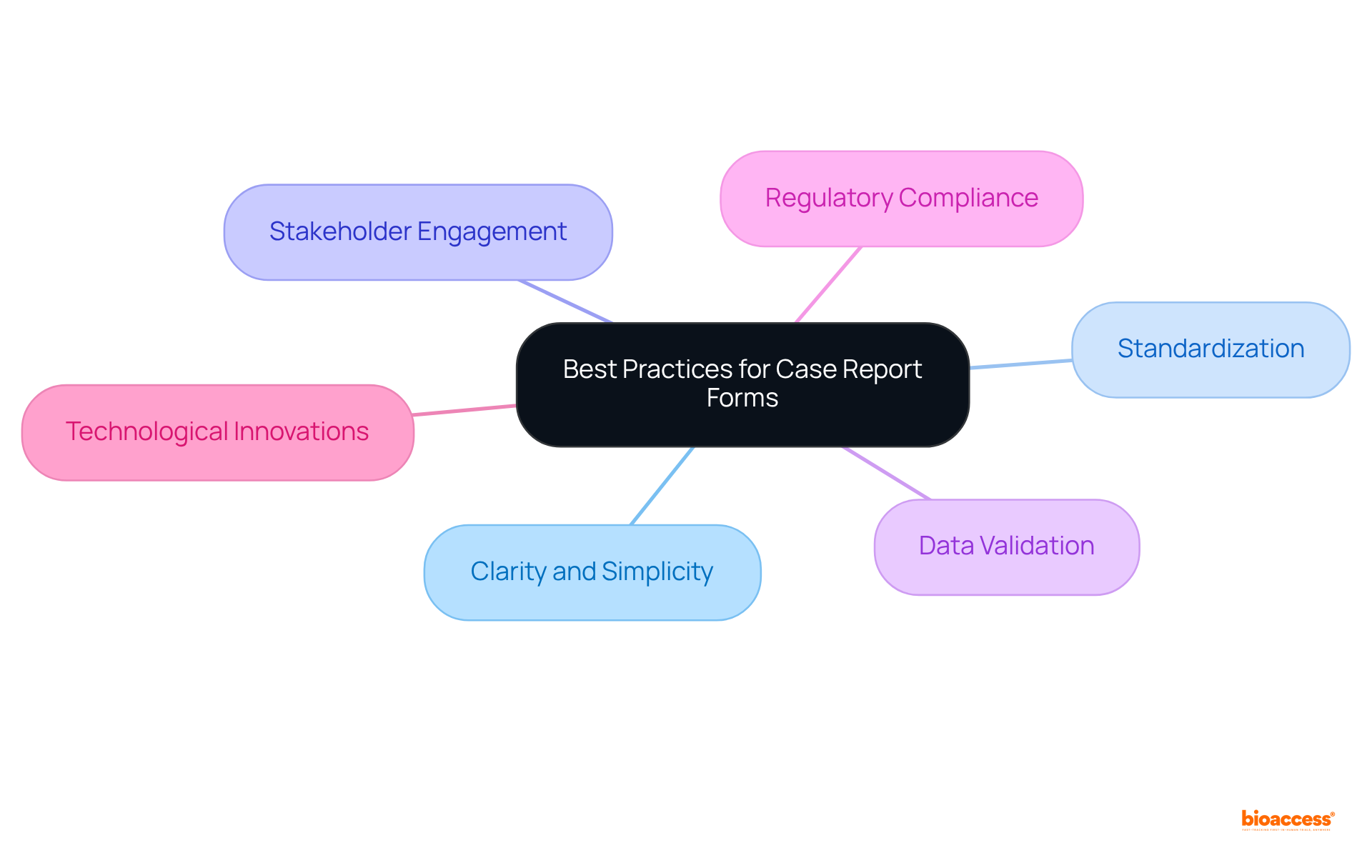
Designing effective clinical trial case report forms is founded on several fundamental principles: clarity, simplicity, and relevance. An accessible CRF features clear instructions and a logical progression, significantly reducing confusion for information gatherers. Each item included must align with the study objectives, ensuring that only essential information is collected.
Integrating user feedback during the design stage is crucial; research indicates that iterative testing can enhance usability and adherence, leading to superior quality information gathering. For instance, five users can identify 85% of usability problems when testing continuously, underscoring the effectiveness of small user testing groups.
Well-organized clinical trial case report forms not only facilitate simpler monitoring and auditing but also enhance efficiency by conserving time on information entry and adjustments, ultimately boosting participant involvement. By emphasizing these design principles, researchers can develop systems that are not only functional but also intuitive, fostering a more effective information collection process.
As noted by industry professionals, 'Ease of use is identified as the most crucial attribute for mobile applications by 97% of users,' which highlights the significance of user-centered design in clinical research forms.
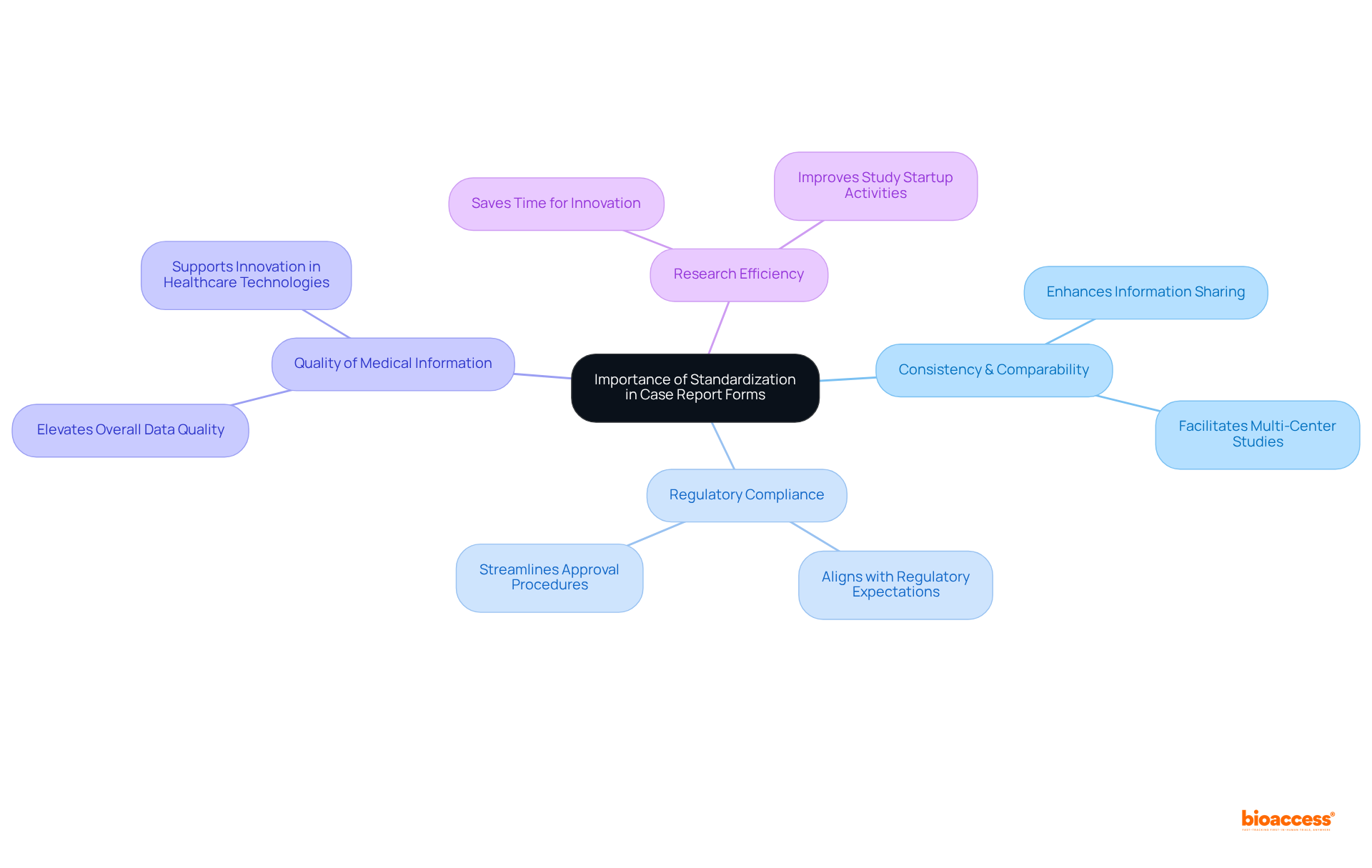
Standardization in the clinical trial case report form is crucial for preserving consistency and comparability of information across various clinical trials. By adhering to established protocols and employing standardized templates, researchers can significantly enhance information sharing and integration, which is vital for multi-center studies. This approach not only improves the comparability of information but also aligns with the expectations of regulatory authorities, thereby facilitating regulatory compliance. As a result, the approval procedure becomes more efficient, minimizing the risk of inconsistencies and fostering a more effective research environment.
Furthermore, standardized CRFs elevate the overall quality of medical information, enabling sponsors to focus on innovation and the advancement of healthcare technologies. According to Roche Diagnostics, standardization empowers teams to allocate more time to innovation and idea development, reinforcing the efficiencies gained through these practices.
With over 15 years of expertise, bioaccess® underscores the importance of adhering to clinical trial case report form Completion Guidelines to ensure precision and timeliness in the design of clinical trial case report forms, ultimately enhancing the reliability and efficacy of research studies.
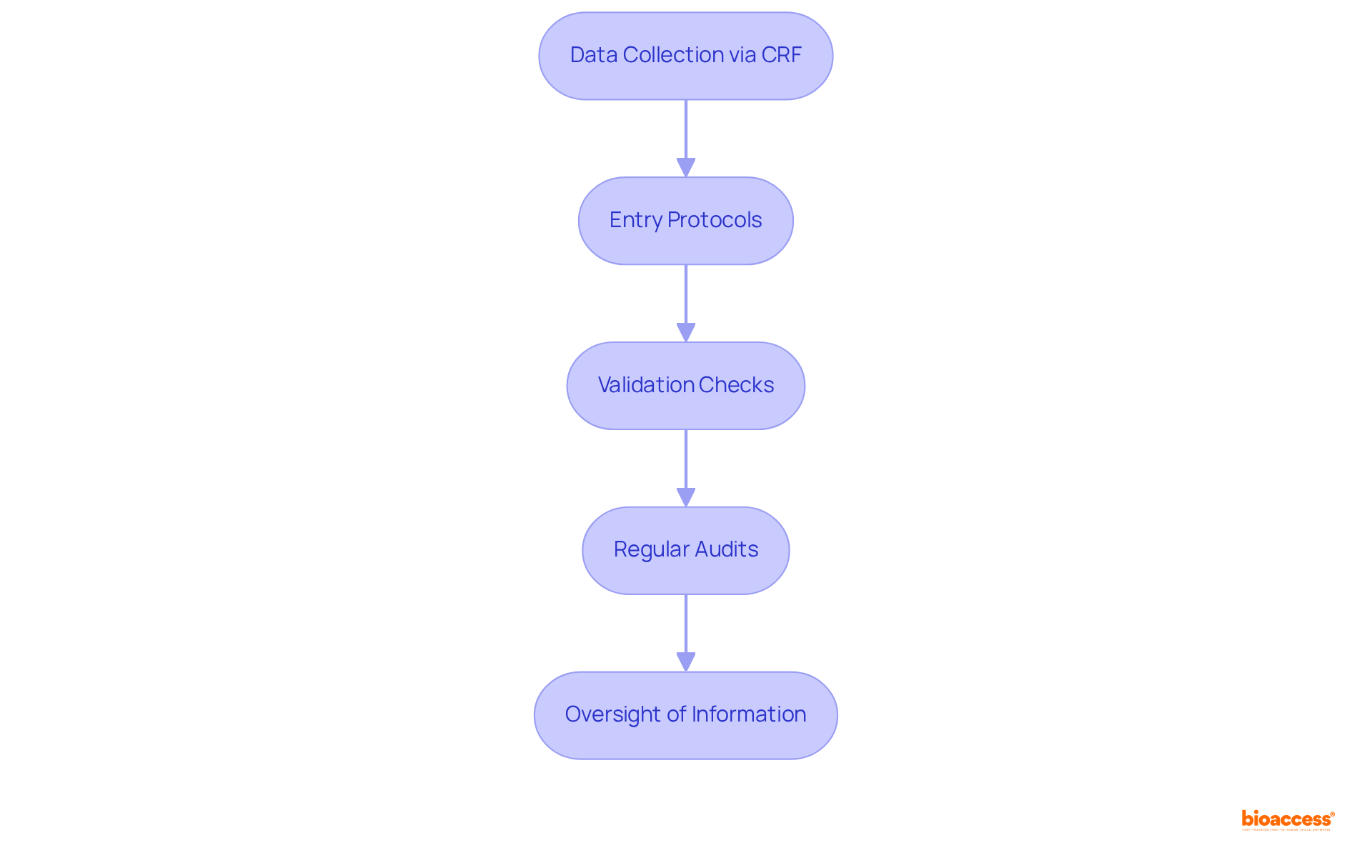
The clinical trial case report form (CRF) serves as a cornerstone in information management, guaranteeing the accuracy and integrity of data collected during clinical trials. By adhering to rigorous entry protocols and implementing validation checks, researchers significantly minimize errors and discrepancies.
Furthermore, regular audits and oversight of CRF information are essential in maintaining high standards of quality. Ultimately, precise and trustworthy information is vital for drawing valid conclusions and making informed decisions regarding the safety and efficacy of medical interventions.
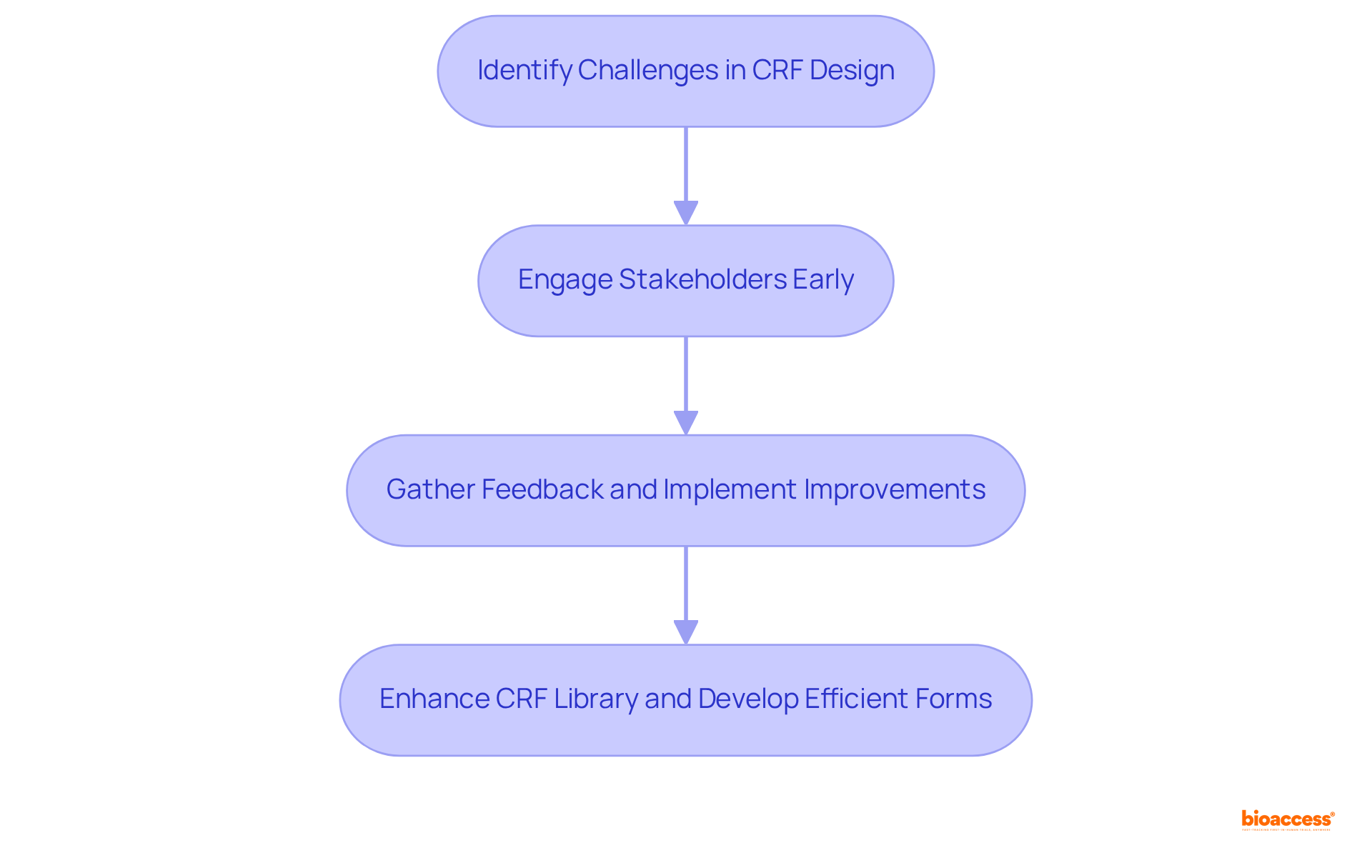
The creation of a clinical trial case report form presents several challenges that can significantly impede the efficiency of clinical trials. A primary obstacle lies in the necessity to balance extensive information gathering with simplicity and user-friendliness. This complexity is further exacerbated by the diverse regulatory requirements across different regions, complicating the design process.
To effectively navigate these challenges, it is crucial for researchers to engage stakeholders early in the design phase. By gathering feedback from these stakeholders, researchers can implement iterative improvements to CRF drafts, ensuring that the forms meet both regulatory standards and user needs. Additionally, utilizing modular designs offers the required flexibility to adjust clinical research frameworks for diverse studies while maintaining vital core components.
Statistics indicate that engaging stakeholders in the development process significantly boosts involvement and adherence, ultimately leading to more efficient information gathering and improved results. For instance, the initial CRF library comprised 160 models, while the current library has expanded to 177 models totaling 1.5 gigabytes, illustrating the continuous efforts to enhance CRF design.
As Patricio Ledesma aptly noted, 'The design of a CRF is a vital element of research studies as it directly influences the precision and dependability of the information gathered.' By proactively addressing these challenges, researchers can develop clinical trial case report forms that not only facilitate precise information collection but also enhance the overall clinical trial procedure.
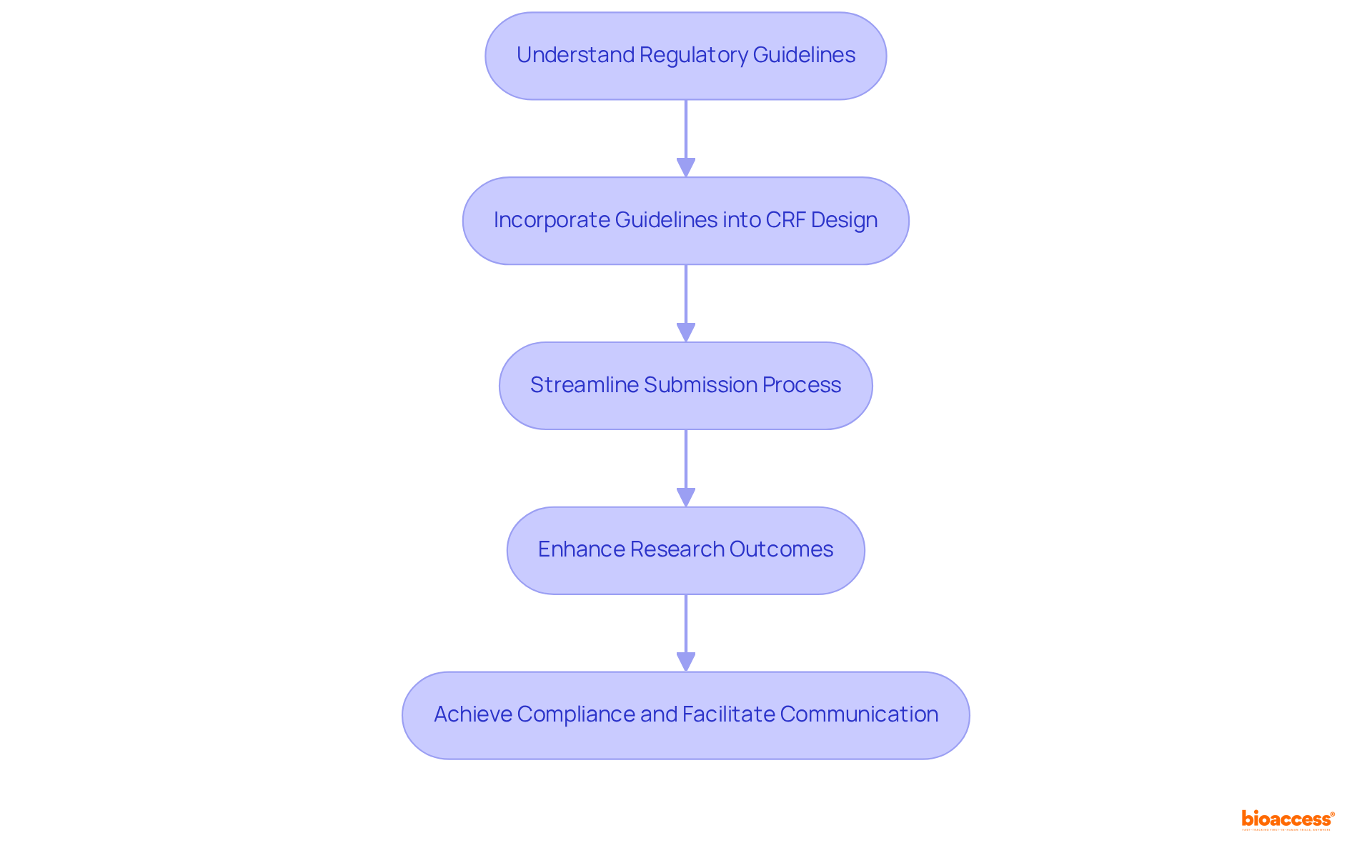
Regulatory compliance is paramount in the development of Case Report Forms (CRFs), necessitating adherence to the guidelines established by regulatory bodies such as the FDA and EMA. These guidelines stipulate that CRFs must thoroughly capture all requisite data for assessing safety and efficacy.
Keeping abreast of evolving regulations is crucial; researchers must incorporate these requirements into CRF design from the outset. This proactive strategy not only streamlines the regulatory submission process but also bolsters the reliability of research outcomes.
Notably, CRFs that align with FDA and EMA standards have been shown to significantly improve trial success rates, underscoring the importance of meticulous documentation.
Regulatory experts assert that comprehending and complying with these legal requirements is essential for ensuring adherence and facilitating effective communication among stakeholders involved in the clinical trial case report form.
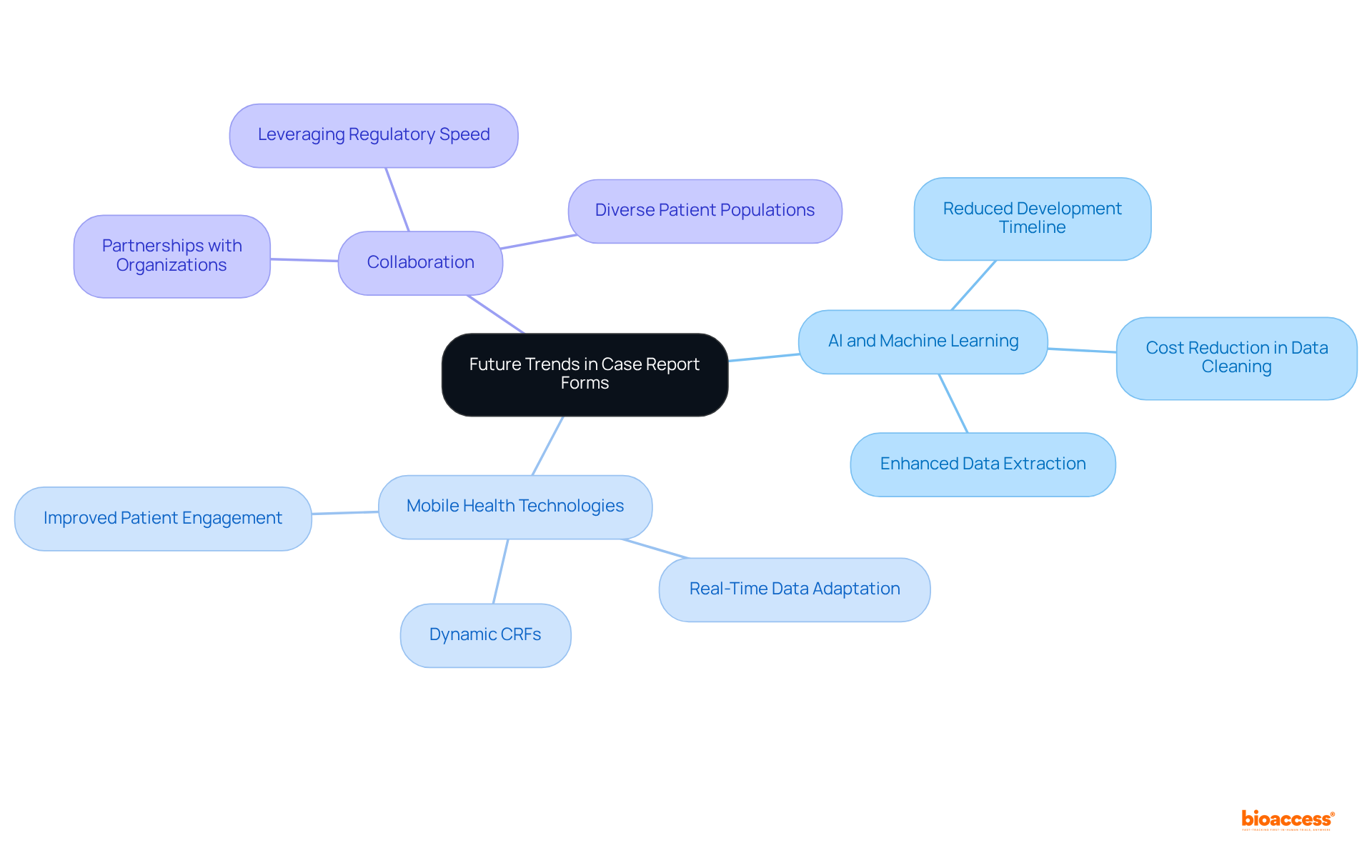
The future of the clinical trial case report form is poised for a transformative shift, driven by technological advancements and analytical insights. Innovations such as artificial intelligence (AI) and machine learning are revolutionizing the processes of information gathering and analysis, enabling researchers to derive more nuanced insights into patient outcomes. Notably, AI-driven methods can significantly reduce the eCRF development timeline from 70 days to an impressive 5-7 days, presenting a competitive edge in research studies.
Furthermore, the integration of mobile health technologies and wearables is leading to the emergence of dynamic clinical trial case report forms that can adapt in real-time to patient data, thereby enhancing the relevance and accuracy of the information collected. As the sector transitions towards more patient-centered designs, embracing these technological trends will be vital for researchers aiming to improve the efficiency and effectiveness of their studies.
Collaboration with organizations like bioaccess® exemplifies how leveraging regulatory speed and diverse patient populations can further optimize the CRF process, ultimately resulting in enhanced study outcomes. Significantly, bioaccess® can expedite patient enrollment by 50%, underscoring the critical role of collaborations in overcoming challenges in research studies.
To ensure the effectiveness of Case Report Forms in research studies, it is essential for researchers to adhere to the following best practices:
By following these practices, researchers can significantly improve the quality and efficiency of their clinical trials.
The design of clinical trial case report forms (CRFs) stands as a pivotal factor influencing the success of medical research. By prioritizing clarity, standardization, and technological integration, researchers can significantly enhance the quality and efficiency of data collection, ultimately yielding more reliable study outcomes. The insights presented underscore the necessity of meticulous CRF design, which not only ensures compliance with regulatory standards but also optimizes the overall clinical trial process.
Key arguments discussed encompass:
In conclusion, embracing these principles and innovations in clinical trial case report form design is essential for advancing research efficiency and effectiveness. As the landscape of clinical trials evolves, researchers are urged to leverage technology and stakeholder feedback to create CRFs that not only meet current demands but also pave the way for future advancements in medical research.
What is bioaccess® and how does it enhance clinical trial case report forms (CRFs)?
bioaccess® leverages its extensive experience in early-stage research to improve the design and execution of clinical trial case report forms (CRFs) by integrating regulatory efficiency with diverse patient populations, ensuring compliance and tailoring CRFs to the unique demands of Medtech, Biopharma, and Radiopharma innovators.
What is the purpose of a clinical trial case report form (CRF)?
A clinical trial case report form (CRF) is designed to collect systematic and consistent information from each participating patient to facilitate accurate data analysis and regulatory submissions, documenting patient outcomes, adverse events, and other critical details necessary for evaluating medical interventions.
Why are clinical trial case report forms important in medical research?
CRFs are vital for ensuring data accuracy and efficiency in medical trials, safeguarding patient safety, ensuring regulatory compliance, and optimizing cost efficiency. The quality of data collected through CRFs directly impacts the integrity of the database and the validity of study outcomes.
What are the benefits of using electronic Case Report Forms (eCRFs) over paper-based forms (pCRFs)?
eCRFs offer numerous advantages, including real-time data entry, automated validation checks, reduced entry errors (with no mistakes reported in eCRF conditions compared to a 5% error rate for pCRFs), and time savings for patients and researchers. eCRFs also enhance collaboration through cloud-based platforms.
How does the time taken to complete eCRFs compare to pCRFs?
Completing an eCRF averages 8.29 minutes, while completing a pCRF takes about 10.54 minutes, indicating a significant efficiency gain.
What role do innovations like cloud-based platforms play in the use of eCRFs?
Cloud-based platforms enable seamless collaboration among research teams, facilitating faster data access and analysis, which contributes to the overall efficiency of clinical trials.
What impact do eCRFs have on the timeline for clinical trials?
The use of eCRFs simplifies the regulatory submission process and accelerates the overall timeline for trials, ultimately leading to quicker delivery of new therapies to patients.