


The article titled "10 Essential Insights on European Union GMP Compliance" serves as a crucial examination of Good Manufacturing Practice (GMP) compliance within the European Union and its significant implications for public health and pharmaceutical quality. It asserts that adherence to EU GMP regulations is not merely a regulatory requirement but a fundamental necessity for ensuring the safety and efficacy of medicinal products. This assertion is backed by a comprehensive review of regulatory frameworks, real-world compliance challenges, and the critical role of ongoing training and inspections in maintaining high standards within the pharmaceutical industry.
The landscape of clinical research and pharmaceutical manufacturing is increasingly shaped by the stringent requirements of European Union Good Manufacturing Practice (GMP) compliance. As organizations strive to ensure the safety and efficacy of medicinal products, understanding the nuances of these regulations becomes essential. This article delves into ten critical insights that illuminate the complexities of EU GMP compliance, offering valuable guidance for navigating the regulatory landscape. However, with evolving guidelines and the rising stakes of public health, how can companies effectively adapt to ensure not only compliance but also excellence in their operations?
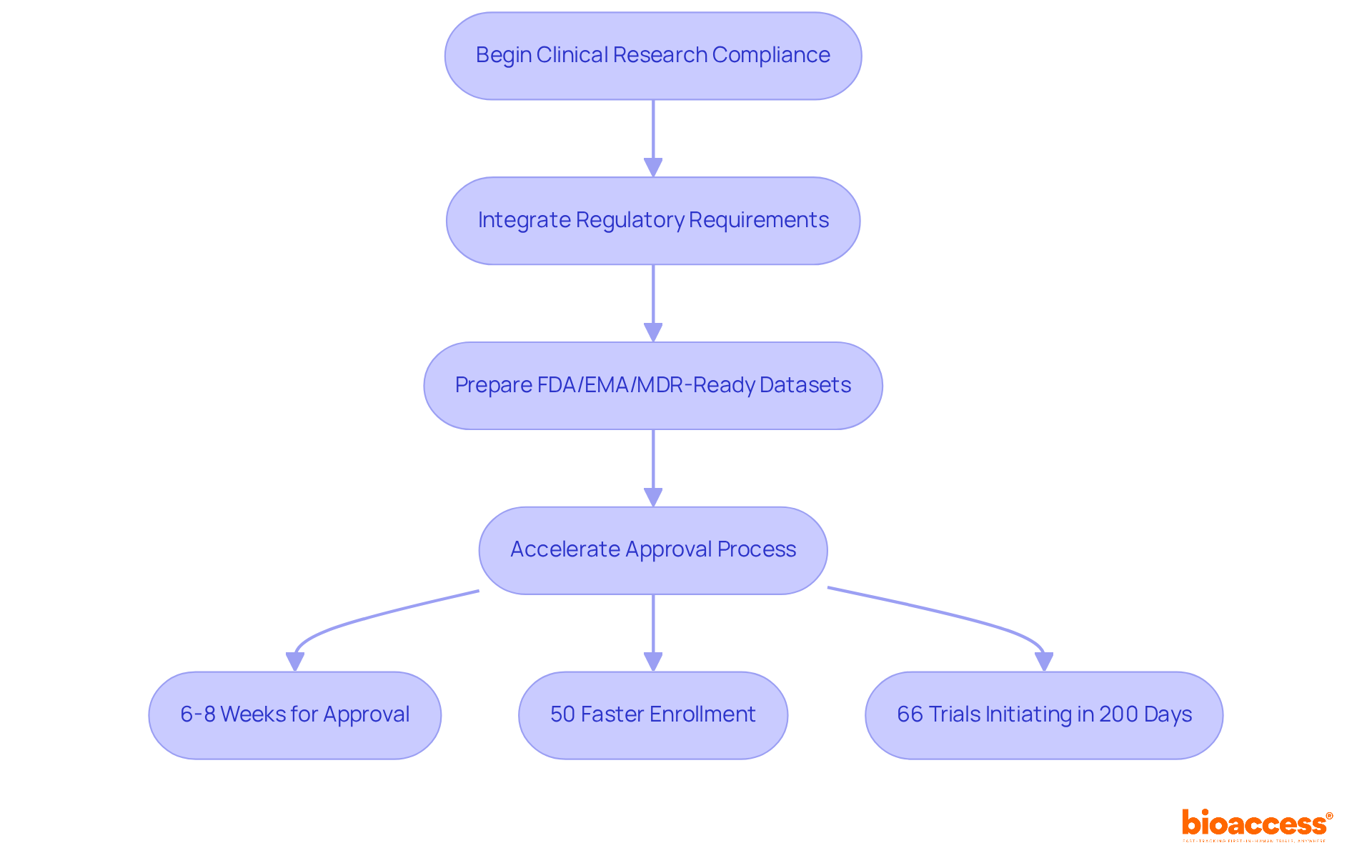
bioaccess® leverages its extensive clinical research experience to ensure that all studies comply with European Union GMP regulations. By integrating regulatory requirements into their operational framework, bioaccess® accelerates the approval process while upholding rigorous standards of excellence. This distinctive approach combines local regulatory expertise with global best practices, empowering clients to adeptly navigate the complexities of compliance.
bioaccess® provides FDA/EMA/MDR-ready datasets, enhancing the service's overall effectiveness. With a rapid regulatory approval process that delivers results in just 6-8 weeks, bioaccess® significantly shortens the time to market for innovative products. Notably, in cardiology and neurology cohorts, enrollment can occur 50% faster than at Western sites. Recent developments indicate that the European Union GMP is essential for progressing toward greater harmonization of regulatory requirements, which is a crucial factor for clinical research timelines. With the Clinical Trials Regulation (CTR) now fully enacted, it is anticipated that two-thirds (66%) of clinical trials will initiate patient recruitment within 200 calendar days of application submission, marking a substantial improvement over previous benchmarks.
This regulatory knowledge is essential, as it directly influences the speed and efficiency of clinical research, enabling bioaccess® to assist its clients in achieving timely market access for their innovative products. Moreover, the decline in the EEA's share of commercial trials—from 22% in 2013 to 12% in 2023—highlights the significance of bioaccess®'s expertise in navigating these challenges. This is exemplified through successful collaborations, such as Avantec Vascular's first-in-human clinical study in Latin America.
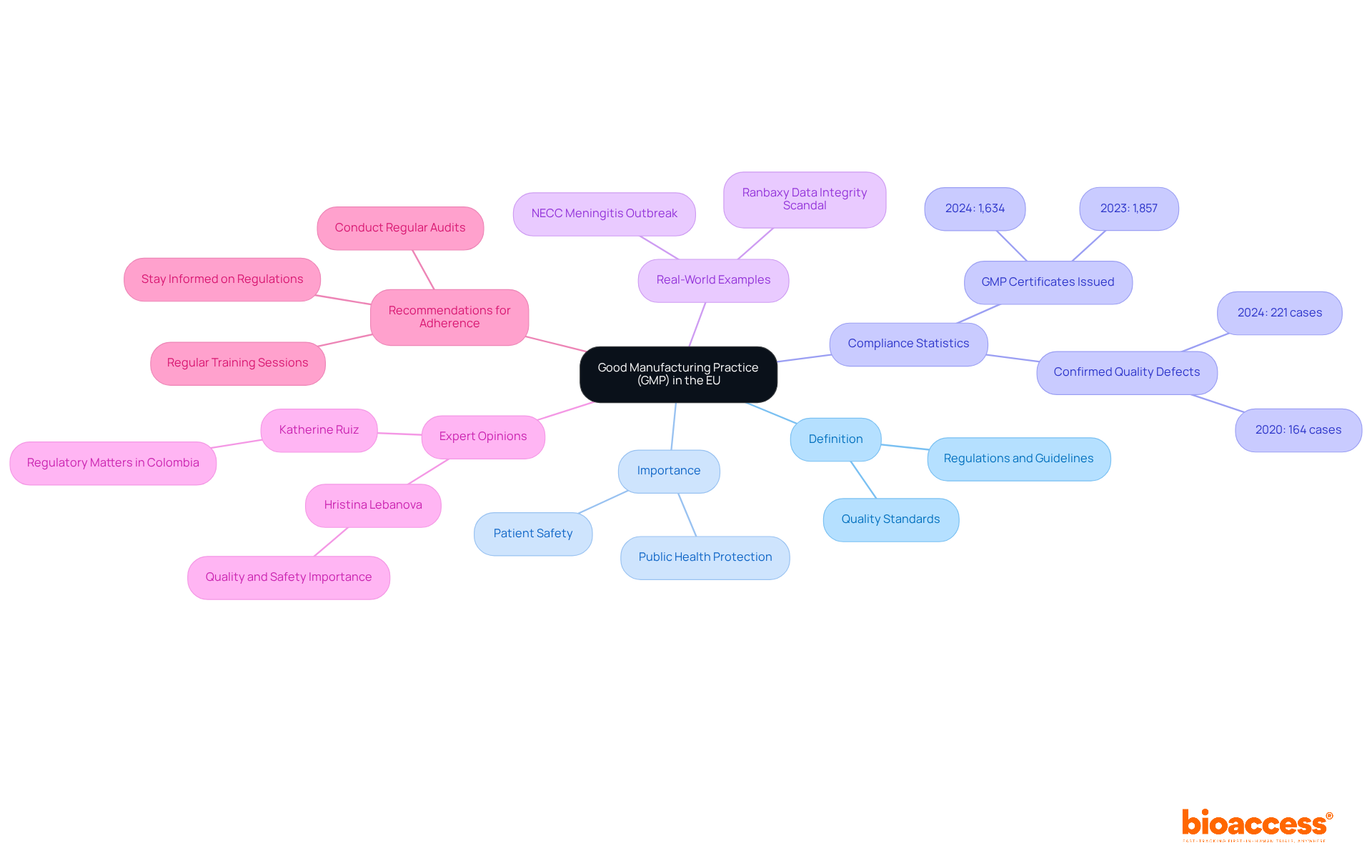
Good Manufacturing Practice (GMP) encompasses a series of regulations and guidelines designed to ensure that products are consistently produced and managed according to rigorous quality standards. Within the European Union, European Union GMP serves as a cornerstone of public health protection, ensuring that medicinal products are safe, effective, and of the highest quality. Compliance with GMP mandates strict documentation, control measures, and regular audits, which are vital for maintaining standards throughout the manufacturing process.
The importance of European Union GMP regulations is paramount. In 2024 alone, the European Economic Area (EEA) authorities issued 1,634 GMP certificates, underscoring a commitment to uphold these standards. Furthermore, there were a total of 8,365 notifications in 2024, with confirmed quality defects increasing from 164 in 2020 to 221 in 2024. This trend highlights the critical need for robust GMP compliance to mitigate risks associated with pharmaceutical products and safeguard public health.
Real-world examples underscore the impact of GMP implementation in pharmaceutical manufacturing. The Ranbaxy data integrity scandal and the NECC meningitis outbreak serve as stark reminders of the repercussions of overlooking GMP regulations, emphasizing the urgent necessity for stringent adherence to these standards. Such incidents reveal that maintaining high standards in GMP regulations is essential to avert detrimental effects on clinical research and public health.
Expert opinions further reinforce the significance of GMP in ensuring public health safety. Hristina Lebanova, a regulatory expert, asserts, "Ensuring the quality and safety of medicinal products is of paramount importance to the pharmaceutical industry." Regulatory agencies such as the FDA and EMA are tightening guidelines, making it increasingly crucial for researchers and manufacturers to understand and implement GMP compliance effectively. By prioritizing European Union GMP adherence, organizations can enhance patient safety, foster greater confidence in new treatments, and ultimately contribute to improved health outcomes throughout the EU.
To prioritize GMP adherence in your practices, consider implementing regular training sessions for your team and staying informed about the latest regulatory changes. Engaging in workshops and professional networks can also provide valuable insights into best practices for upholding regulations.
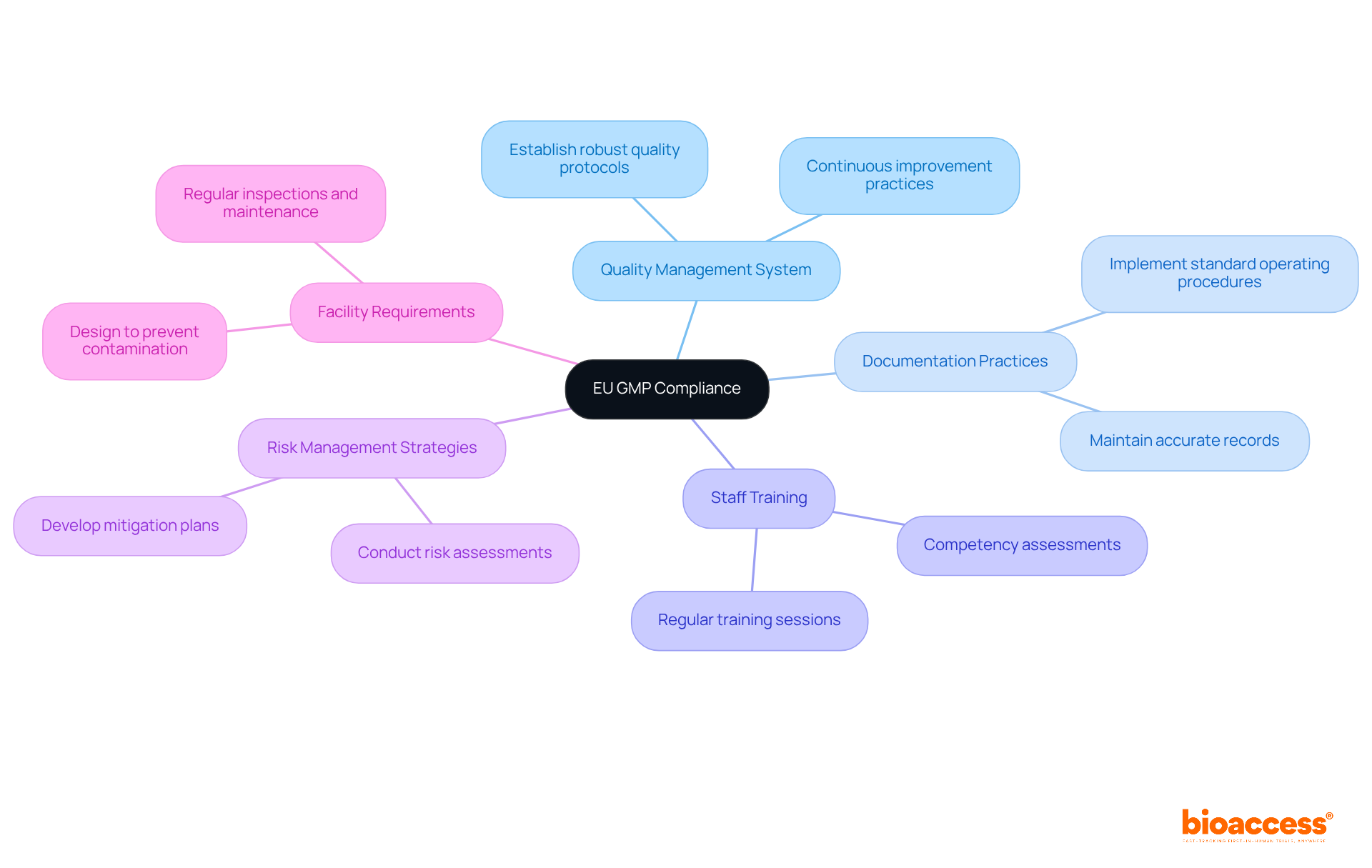
Key regulatory requirements for European Union GMP adherence are critical in maintaining the integrity of health products. These include:
Additionally, companies must ensure that their facilities meet specific design and operational criteria to avert contamination and uphold product integrity. Regular inspections by regulatory authorities, such as INVIMA in Colombia—recognized as a Level 4 health authority by PAHO/WHO—are indispensable to compliance. Experts like Ana Criado, with extensive knowledge in regulatory matters and biomedical engineering, underscore the necessity of adhering to European Union GMP guidelines to ensure the safety and efficacy of health products.
Inspections and certifications are paramount for upholding the European Union GMP guidelines. Regulatory authorities, such as INVIMA in Colombia, conduct routine inspections to assess compliance with GMP regulations. Recognized as a Level 4 health authority by PAHO/WHO, INVIMA is instrumental in overseeing the marketing and manufacturing of health products, ensuring strict adherence to established guidelines.
These inspections evaluate:
Successful inspections culminate in certifications that affirm a company's dedication to quality and safety, which is essential for market access, consumer trust, and adherence to European Union GMP regulations. Experts like Ana Criado, Director of Regulatory Affairs, leverage her extensive experience in biomedical engineering and health economics to significantly influence this regulatory landscape, ensuring that companies meet the stringent standards required for certification.

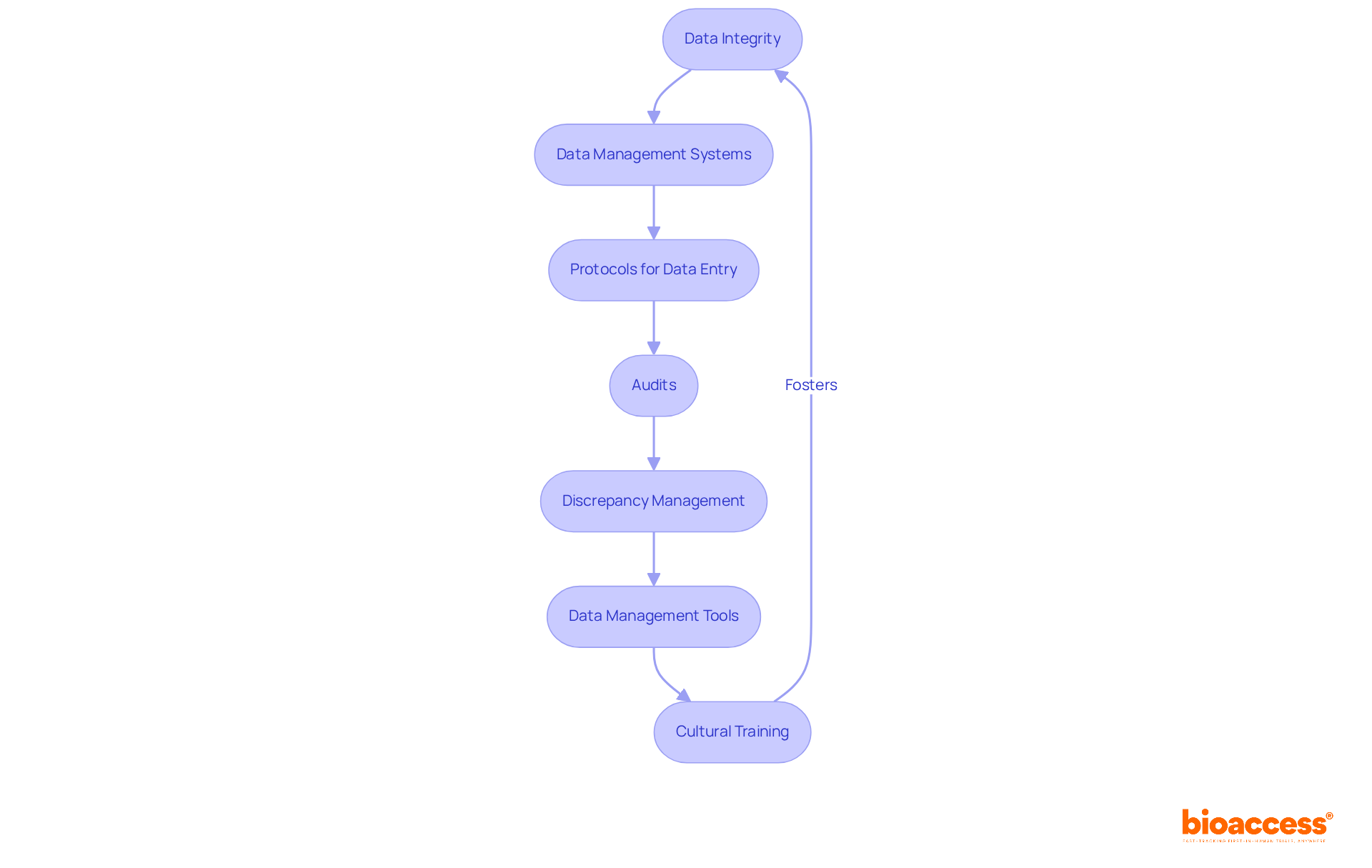
Ensuring data integrity is paramount for compliance with European Union GMP. Organizations must implement comprehensive data management systems that guarantee the accuracy, consistency, and reliability of data throughout the research lifecycle. This necessitates the establishment of clear protocols for data entry, storage, and retrieval, coupled with regular audits to identify and rectify discrepancies.
Discrepancy management stands as the most critical activity in the Clinical Data Management (CDM) process. Moreover, utilizing sophisticated data management tools, such as electronic data capture systems, can automate processes and enhance data integrity, significantly reducing the risk of errors. In fact, most CDM systems comply with 21 CFR Part 11 regulations, further validating their role in maintaining data integrity.
Additionally, fostering a culture of data integrity among clinical trial personnel is essential, supported by comprehensive training initiatives that empower teams to emphasize excellence at every stage of data management and adherence to international data guidelines like CDISC. By prioritizing data integrity, organizations not only meet regulatory requirements but also enhance the credibility of their research findings, ultimately leading to more reliable outcomes and improved patient safety.
Recent updates to the European Union GMP guidelines highlight the dynamic landscape of pharmaceutical manufacturing and clinical research, especially considering technological advancements. These modifications aim to enhance compliance with European Union GMP by addressing novel manufacturing processes and refining control measures. For example, the introduction of Annex 22 specifically governs the use of artificial intelligence in manufacturing, underscoring the necessity for rigorous selection, training, and validation of AI models. This reflects a broader trend where companies are increasingly mandated to integrate advanced technologies into their operations while adhering to stringent regulatory standards.
Organizations must remain vigilant concerning these updates to ensure alignment with the latest regulatory expectations. The European Commission's revisions aim to minimize variability in performance results and enhance the pharmaceutical standards system in line with European Union GMP, which has been in effect since January 31, 2013. Consequently, companies are encouraged to implement regular training and updates for their personnel to effectively adapt to these changes. The proactive identification of manufacturing risks is now emphasized, with the goal of preventing shortages and mitigating supply chain vulnerabilities.
Examples of businesses successfully adjusting to these shifts underscore the importance of compliance in maintaining product standards and availability. By embracing the revised guidelines, organizations can not only fulfill regulatory requirements but also harness new technologies to improve their operational efficiency and product integrity. Staying informed and prepared for these evolving regulations is essential for success in the competitive realm of pharmaceutical manufacturing.
Training and education are pivotal for achieving adherence to European Union GMP within the pharmaceutical sector. Comprehensive training on European Union GMP principles, regulatory requirements, and specific operational procedures is essential for all personnel involved in manufacturing and research processes. Typically, it takes 1.5 to 2 years for new employees to become proficient in their roles, highlighting the necessity for structured training programs that foster competency and retention.
Regular refresher courses and updates are vital for keeping staff informed about evolving practices and regulations, particularly those related to European Union GMP. A well-trained workforce not only ensures adherence to European Union GMP standards but also enhances product quality and safety. Implementing a blended approach to GMP training—combining classroom instruction, online modules, and practical workshops—can significantly boost engagement and knowledge retention. This method allows for the introduction of fundamental concepts through online learning, followed by hands-on applications in real-world scenarios.
Current trends in European Union GMP training highlight the importance of continuous education and professional development. Organizations are encouraged to conduct regular internal audits to pinpoint areas for improvement in adherence to European Union GMP and to adjust training materials to reflect the latest industry standards. Effective education strategies incorporate real-life examples and case studies into training sessions, aiding employees in grasping the practical applications of GMP principles.
Best practices for GMP training include establishing a feedback loop to capture insights from employees, ensuring that training remains relevant and effective. Furthermore, maintaining comprehensive training records is crucial for adherence to European Union GMP regulations, as inspectors frequently request these during audits. By fostering an environment of ongoing enhancement and promoting innovation in training techniques, organizations can bolster their GMP adherence efforts and ultimately safeguard patient safety.
In the European Union GMP, compliance for Active Pharmaceutical Ingredients (APIs) is governed by stringent regulations that dictate manufacturing processes to ensure product safety and efficacy. Companies must create APIs in regulated settings, applying strict control measures throughout the production cycle. This includes comprehensive testing and validation of raw materials, alongside meticulous documentation of all production processes. For instance, humidity levels in pharmaceutical cleanrooms must be monitored with tolerances as precise as one percent, ensuring optimal conditions for manufacturing.
Additionally, adherence to the ALCOA+ principles—where data must be Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, and Available—is crucial for maintaining data integrity. Compliance with these regulations, including European Union GMP, not only safeguards the quality of medicinal products but also mitigates risks associated with contamination and recalls, reinforcing the importance of quality control in the API manufacturing landscape. Moreover, the Europe active pharmaceutical ingredients (API) market is expected to expand at a CAGR of 8.02% from 2025 to 2032, emphasizing the rising importance of adherence in a growing market.
The historical context of GMP regulations, enacted in 1963 following the thalidomide incident, underscores the critical nature of these regulations in ensuring product safety. Adherence to the European Union GMP guidelines must also be validated in writing by the exporting nation's competent authority since July 2, 2013, highlighting the strictness of the regulatory framework. Furthermore, maintaining equipment used in production is essential, as it must be calibrated, cleaned, and verified according to GMP recommendations. The European Medicines Agency (EMA) plays an essential role in organizing inspections to confirm adherence to these guidelines, further strengthening the regulatory framework in line with European Union GMP.
Biopharmaceuticals encounter distinct challenges in meeting European Union GMP standards, largely due to intricate manufacturing processes and stringent regulatory requirements. Key issues encompass maintaining data integrity, ensuring consistent product quality, and navigating supply chain complexities. To effectively tackle these challenges, companies can implement several robust solutions:
Furthermore, the case study of Chi Pharmaceuticals Limited illustrates the financial advantages of investing in enhancement interventions, achieving a benefit-cost ratio of 5.3. By proactively addressing these challenges and implementing these solutions, biopharmaceutical companies can significantly enhance their compliance with European Union GMP, ultimately ensuring the safety and efficacy of their products.

The significance of Good Manufacturing Practices (GMP) in ensuring the safety and efficacy of medicinal products is paramount. Adhering to GMP regulations is crucial for safeguarding public health, as it mandates that products are produced to the utmost excellence. This rigorous adherence not only enhances patient safety but also fosters trust in the pharmaceutical industry. For instance, Dr. N. Tamilselvan notes that "harmonizing practices could not only ensure higher product standards but also foster global regulatory cooperation and trust."
Furthermore, public trust in pharmaceutical products is closely linked to GMP adherence; a survey revealed that over 80% of consumers feel more confident in medications produced by companies that follow GMP guidelines, as highlighted by Catarina P Reis. By committing to these standards, organizations contribute to the advancement of medical knowledge and play a crucial role in developing effective treatments that ultimately benefit patients.
The historical context of GMP is also significant, with the first draft GMP document adopted by the World Health Assembly in 1969, marking the beginning of a global commitment to pharmaceutical quality.

The insights provided on European Union GMP compliance underscore the critical importance of adhering to Good Manufacturing Practices in ensuring the safety and efficacy of medicinal products. By emphasizing the integration of regulatory requirements into operational frameworks, organizations can streamline their clinical research processes and enhance public trust in the pharmaceutical industry. The commitment to GMP safeguards public health and fosters a culture of excellence and accountability within the sector.
Key arguments throughout the article highlight the necessity of:
The discussion also points to the evolving landscape of GMP regulations, especially with the advent of new technologies and the emphasis on data integrity. Organizations like bioaccess® exemplify how a proactive approach to compliance can significantly accelerate clinical research timelines and improve market access for innovative products.
In light of these insights, it is crucial for stakeholders in the pharmaceutical industry to prioritize GMP adherence and stay informed about the latest regulatory changes. Engaging in continuous education, fostering a culture of compliance, and implementing effective quality management practices are essential steps toward safeguarding public health and enhancing the integrity of medicinal products. As the landscape of GMP compliance continues to evolve, embracing these principles will contribute to better health outcomes and reinforce the industry's commitment to excellence and safety.
What is bioaccess® and how does it contribute to clinical research compliance?
bioaccess® is a clinical research organization that ensures studies comply with European Union GMP regulations. It integrates regulatory requirements into its operational framework, accelerating the approval process while maintaining high standards of excellence.
How does bioaccess® enhance the effectiveness of clinical research?
bioaccess® provides FDA/EMA/MDR-ready datasets and has a rapid regulatory approval process that delivers results in just 6-8 weeks, significantly shortening the time to market for innovative products.
What is the impact of bioaccess® on patient enrollment in clinical trials?
In cardiology and neurology cohorts, patient enrollment can occur 50% faster at bioaccess® compared to Western sites.
Why are European Union GMP regulations important for clinical research?
EU GMP regulations are essential for ensuring that medicinal products are safe, effective, and of the highest quality. They help harmonize regulatory requirements and improve clinical research timelines.
What are the key statistics regarding clinical trials and EU GMP?
It is anticipated that two-thirds (66%) of clinical trials will initiate patient recruitment within 200 calendar days of application submission under the Clinical Trials Regulation (CTR). Additionally, the share of commercial trials in the EEA declined from 22% in 2013 to 12% in 2023.
What are Good Manufacturing Practices (GMP)?
GMP encompasses regulations and guidelines that ensure products are consistently produced and managed according to rigorous quality standards, safeguarding public health by ensuring the safety and efficacy of medicinal products.
What are the consequences of non-compliance with GMP regulations?
Non-compliance with GMP can lead to significant public health risks, as exemplified by the Ranbaxy data integrity scandal and the NECC meningitis outbreak, highlighting the necessity for stringent adherence to these standards.
What are the key regulatory requirements for EU GMP compliance?
Key requirements include establishing a robust quality management system, implementing proper documentation practices, conducting regular staff training, and adopting effective risk management strategies.
How can organizations prioritize GMP adherence?
Organizations can prioritize GMP adherence by implementing regular training sessions for their teams, staying informed about regulatory changes, and engaging in workshops and professional networks to gain insights into best practices.