


Navigating the complex landscape of UK medical device regulations can be a daunting task for innovators, particularly in light of the recent legislative changes driven by Brexit. This article presents ten essential insights that clarify the current regulatory framework while highlighting strategic opportunities for medical device developers. As the industry grapples with the critical question of how to maintain compliance and ensure swift market entry, understanding these regulations is vital for success. What key strategies can innovators employ to thrive amidst these evolving requirements?
bioaccess® harnesses the compliance efficiency of Latin America, enabling medical device innovators to secure ethical approvals in an impressive 4-6 weeks. This expedited process is further bolstered by Colombia's competitive advantages, which include cost savings exceeding 30% compared to North America and Western Europe, alongside access to a diverse patient population of over 50 million, with 95% covered by universal healthcare. Moreover, the total IRB/EC and MoH (INVIMA) review in Colombia takes only 90-120 days, ensuring rapid site activation and regulatory compliance. These factors culminate in enrollment procedures that are 50% quicker than conventional systems.
In fact, nearly 90% of research studies globally fail to meet their enrollment goals, with 80% postponed due to challenges in finding sufficient participants. Yet, bioaccess® consistently achieves success by leveraging local expertise and robust recruitment strategies. The strong network of investigators in Latin America supports the execution of clinical trials, ensuring that these trials reflect diverse demographics. Inclusion in clinical trials is not merely a moral obligation; it is a scientific necessity for effective healthcare, addressing health disparities and enhancing treatment relevance.
By bridging these areas, bioaccess® presents a compelling value proposition for Medtech, Biopharma, and Radiopharma firms eager to accelerate their clinical research initiatives and capitalize on the burgeoning opportunities within the global medical equipment sector. Furthermore, the collaboration between bioaccess® and Caribbean Health Group, backed by Colombia's Minister of Health, aims to position Barranquilla as a premier destination for clinical trials in Latin America, thereby enriching the overall landscape for clinical research in the region.
The UK medical device regulations are shaped by the Medical Devices Regulations 2002, which have been updated to align with post-Brexit requirements. The UK medical device regulations, which encompass the UK MDR and the Medical Devices (Amendment) Regulations 2025, introduce crucial changes that impact compliance and market entry for innovators. Notably, producers are now required to assign a Unique Device Identification (UDI) to their products, which enhances traceability and post-market oversight. This requirement is part of a broader initiative aimed at improving patient safety and ensuring that devices meet stringent safety and efficacy standards before they can enter the market.
As the UK transitions to its new regulatory framework, innovators face several challenges related to the UK medical device regulations. For instance, maintaining essential EU regulations beyond the initial sunset date of May 2025 is vital to avoid regulatory gaps and ensure stability during this transition. Industry stakeholders have emphasized the importance of upholding current regulations to prevent delays in approvals, particularly for in vitro diagnostics (IVDs) and animal tissue-based products.
Experts in the field stress that engaging with oversight organizations early can expedite the approval process, while leveraging technological solutions can streamline compliance efforts. The introduction of the Early Access Service for innovative medical devices further supports creators by providing a pathway for quicker market entry.
Examples of companies successfully adapting to these changes highlight the importance of strategic planning in regulatory compliance. By prioritizing key sectors and collaborating with local distributors or consultants, innovators can gain a better understanding of specific industry needs and navigate the complexities of the UK medical device regulations framework. As the regulatory landscape continues to evolve, staying informed and proactive will be essential for achieving successful market entry.

To successfully introduce a medical product to the UK market, manufacturers must adhere to UK medical device regulations by registering their offerings with the Medicines and Healthcare products Regulatory Agency (MHRA) and obtaining either a UKCA or CE marking, depending on the product's classification. This process begins with appointing a UK Responsible Person (UKRP) who will manage compliance and communication with the MHRA. Extensive documentation is required to demonstrate compliance with the UK medical device regulations, which includes a technical file detailing the product's design, intended use, and risk assessment.
The average time for medical equipment registration in the UK can vary significantly, with many submissions facing delays due to initial administrative checks. For example, nearly 32 percent of FDA 510(k) submissions failed the acceptance for review check in the year ending September 2022. This statistic underscores the importance of thorough preparation to avoid similar pitfalls in the UK process. Innovators should also be aware that the MHRA conducts audits and inspections to verify compliance, which can further impact timelines.
Successful device registrations with the MHRA often involve proactive engagement with compliance experts who can navigate the complexities of the approval process. Industry feedback emphasizes that obtaining expert regulatory support early can significantly reduce delays and enhance the likelihood of a successful submission. Understanding the subtleties of UKCA and CE marking is essential, as these certifications not only guarantee adherence to UK medical device regulations but also enable market access in the UK and the European Economic Area, respectively.

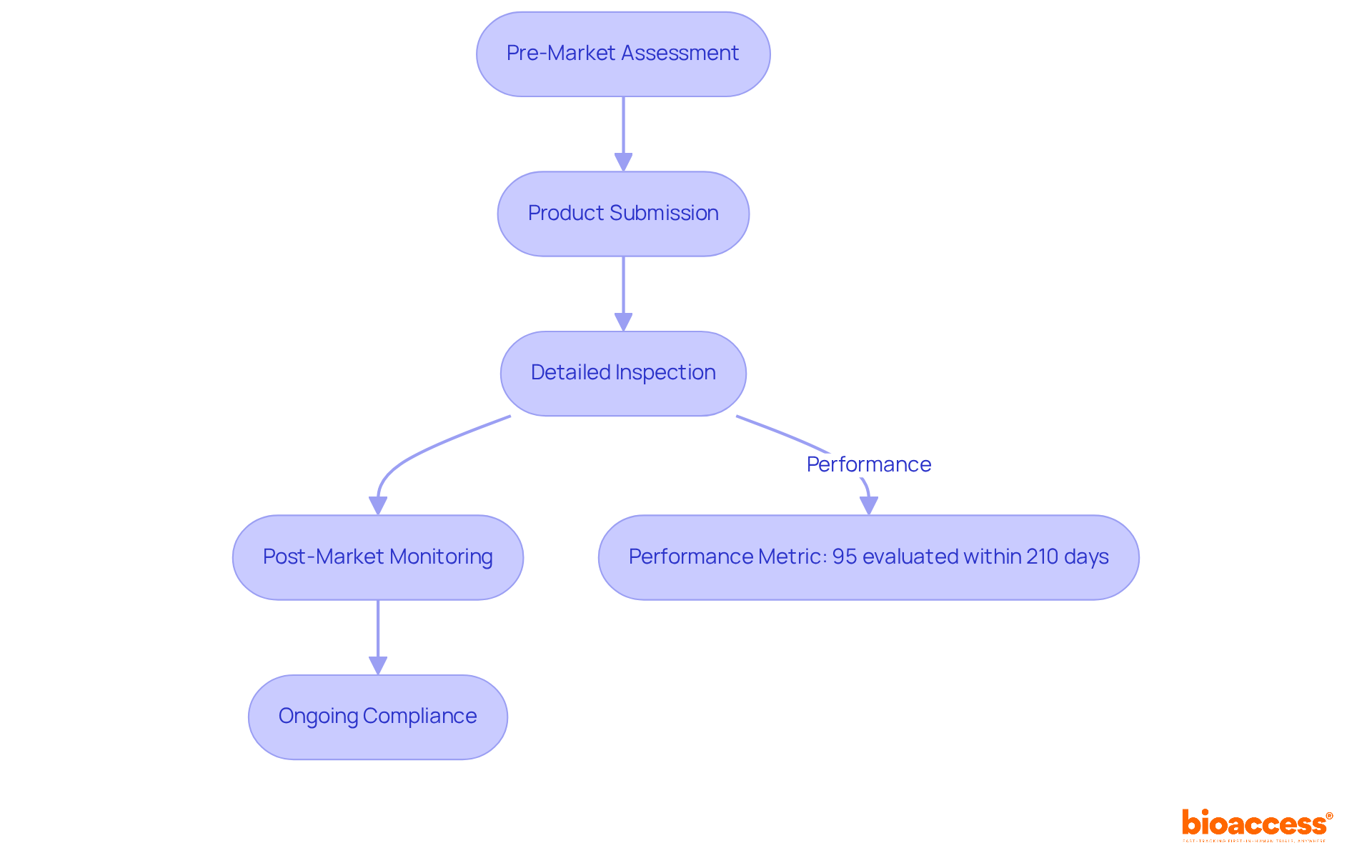
The Medicines and Healthcare products Regulatory Agency (MHRA) serves as the cornerstone of UK medical device regulations, ensuring the safety and efficacy of products through rigorous oversight. By assessing product submissions, conducting detailed inspections, and monitoring post-market performance, the agency upholds established regulations. Notably, recent data reveals that 95% of Clinical Trial Authorisation (CTA) and Clinical Investigation applications were evaluated within the statutory timeline of 210 days, underscoring the MHRA's commitment to timely assessments.
Innovators must actively engage with the MHRA throughout the product lifecycle, from pre-market assessments to ongoing post-market surveillance. This engagement is vital for maintaining regulatory compliance and safeguarding patient safety. For instance, the MHRA has achieved a performance rate of 96% in overseeing adverse reaction evaluations for combined medications and instruments, demonstrating its dedication to monitoring safety post-introduction.
Expert insights highlight the importance of adherence at every stage of the product lifecycle. Successful pre-market evaluations by the MHRA not only facilitate quicker market entry but also enhance the overall quality and safety of medical products. Furthermore, the agency's recent evaluations have reinforced the necessity for manufacturers to comply with UK medical device regulations, ensuring that only safe and effective products reach patients. By prioritizing adherence to regulations, innovators can effectively navigate the complexities of the regulatory landscape while contributing to improved healthcare outcomes.
CE marking signifies compliance with EU regulations, while UKCA marking is essential for medical products entering the Great Britain market after Brexit. Starting June 2025, all medical devices must display the UKCA mark to confirm adherence to UK regulations. Innovators face distinct requirements for each marking; non-compliance can result in significant delays or even denial of market access. For example, many manufacturers have adeptly transitioned from CE to UKCA marking, showcasing their adaptability in a changing regulatory environment. A notable case study, 'Transition from CE to UKCA Certification,' illustrates how manufacturers are managing this shift effectively.
However, compliance rates vary, with some studies indicating that only a fraction of equipment meets the new UKCA standards without additional support. As one source advises, "If you only sell in the UK - Begin collaborating with a UK Approved Body to ensure your products are UKCA-certified before the deadline." The implications of UKCA marking are profound for medical device innovators, as they now navigate a dual regulatory landscape shaped by UK medical device regulations that demand meticulous attention to documentation and conformity assessments, including the preparation of a UK Declaration of Conformity.
The challenges faced by producers post-Brexit necessitate distinct adherence strategies, particularly for Northern Ireland, which aligns with EU regulations. Staying informed about the latest compliance requirements for UKCA marking is crucial for ensuring successful product launches in this evolving environment.

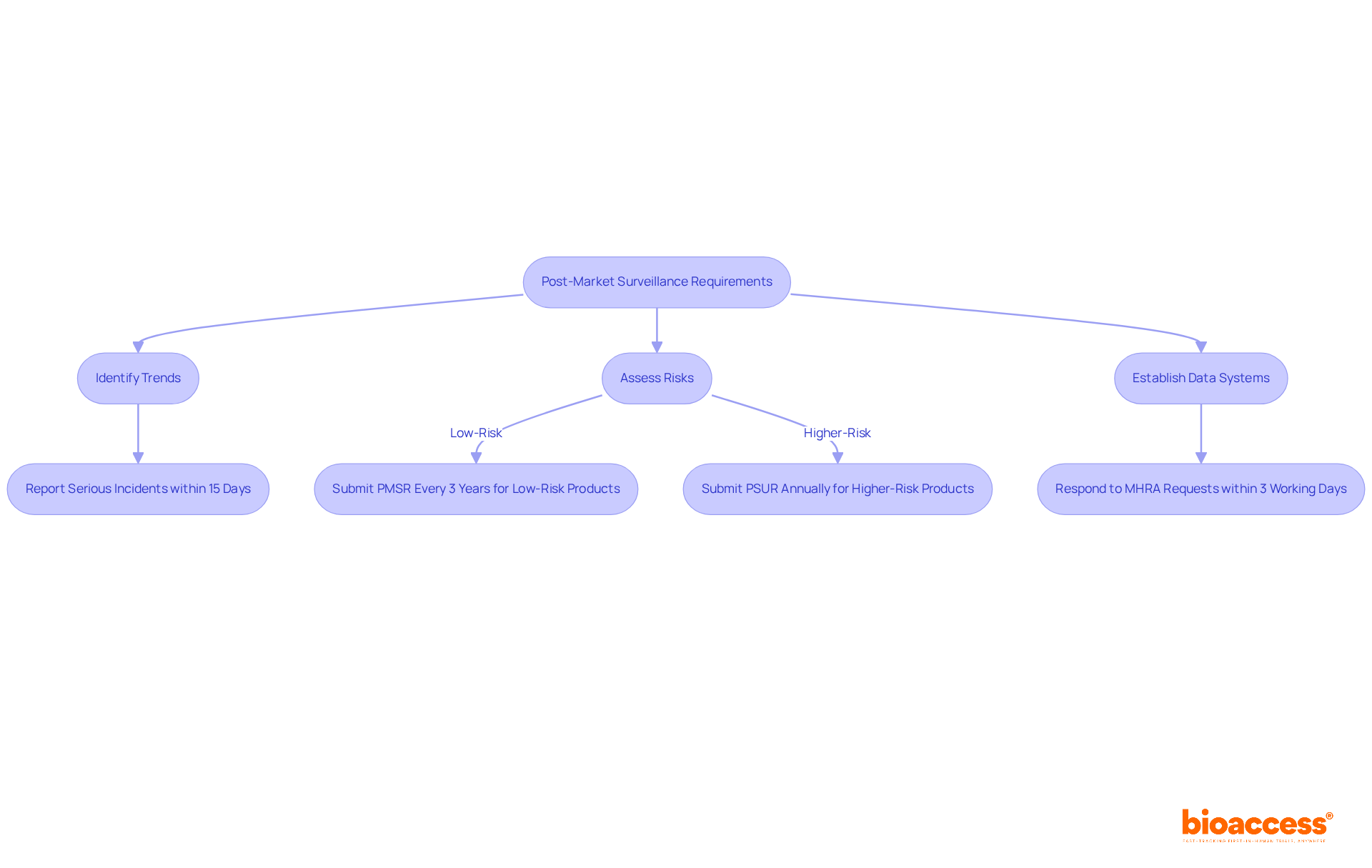
Post-market surveillance (PMS) plays a vital role in ensuring the safety and effectiveness of medical products once they hit the market. Starting June 2025, manufacturers will be mandated to implement comprehensive PMS plans that encompass regular performance assessments and prompt reporting of adverse incidents to the Medicines and Healthcare products Regulatory Agency (MHRA). This proactive strategy aims to swiftly identify and mitigate potential safety issues, ultimately safeguarding patient health.
The new regulations apply to all medical products, including in vitro diagnostics and active implantable medical instruments. Manufacturers are required to document their PMS strategies, which will involve:
For example, serious incidents must now be reported within 15 calendar days, down from the previous 30 days, significantly enhancing manufacturers' responsiveness to safety concerns. Additionally, low-risk products will require a Post-Market Surveillance Report (PMSR) at least every three years, while higher-risk items will need an annual Periodic Safety Update Report (PSUR) submitted to the UK Approved Body (UKAB).
Manufacturers must also respond to MHRA requests for PMSR or PSUR within three working days. The MHRA has noted that 96% of adverse reaction evaluations for combined medicines and equipment are reported, underscoring the importance of rigorous PMS practices. This shift not only enables quicker identification of safety issues but also promotes transparency in incident management, ultimately enhancing patient safety and fostering trust in medical technologies.
Medical device creators often face significant challenges related to the UK medical device regulations, including strict adherence requirements and evolving legislation. Staying informed about UK medical device regulations compliance updates is crucial for navigating these hurdles. Engaging with governing bodies early in the development process and seeking guidance from seasoned professionals can make a substantial difference in understanding UK medical device regulations.
In the Medtech landscape, establishing strong connections with the MHRA is vital for understanding UK medical device regulations. Utilizing resources from entities like bioaccess™, which offers comprehensive clinical trial management services - including feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, and reporting - can significantly enhance the chances of successful market entry. For instance, bioaccess™ played a pivotal role in assisting Avantec Vascular with their first-in-human clinical study in Latin America, demonstrating their expertise in facilitating access and ensuring compliance throughout the trial process.
Collaboration is key in overcoming the complexities of clinical research. By leveraging the right partnerships and resources, medical device creators can navigate the UK medical device regulations more effectively and achieve their goals.
The UK medical device regulations, influenced by the EU Medical Devices Regulation (MDR), play a crucial role in shaping the oversight environment for devices marketed in both regions. UK innovators face the challenge of navigating the differences between the UK medical device regulations and EU MDR, particularly regarding adherence timelines and requirements. While the UK has developed its own regulatory framework, many manufacturers still need to comply with EU regulations alongside the UK medical device regulations to access the European market, making a dual adherence strategy essential.
For instance, the transitional phase for Unique Identification of Medical Equipment (UDI) compliance is three years for general medical products and five years for in vitro diagnostic (IVD) products under the EU MDR. This stands in contrast to the UK's evolving regulations, which are still being finalized. The UKCA marking requirement will be phased out once UDI is fully operational; however, until that point, manufacturers must remain vigilant about both regulatory sets.
The impact of the EU MDR on UK medical device regulations for manufacturers is profound, as the increased requirements and costs associated with compliance can strain resources. Many manufacturers are grappling with challenges stemming from the backlog at Notified Bodies, complicating the certification process. As industry specialists emphasize, "Manufacturers need to recognize the significance of clinical data for demonstrating the adherence of their products," highlighting the necessity for comprehensive clinical data collection both before and after approval.
Key differences between the UK medical device regulations and EU MDR include the approval pathways and classification systems for devices. The UK is adopting a more risk-proportionate approach, particularly for Class B IVDs, which may permit UKCA self-assessment and Quality Management System (QMS) certification from accredited bodies in CPTPP countries. This flexibility is vital for manufacturers aiming to streamline their regulatory processes.
In conclusion, UK innovators must implement dual compliance strategies to effectively navigate the complexities of both the UK medical device regulations and the EU regulatory landscapes. This approach not only facilitates access to the European market but also ensures that producers remain competitive in an increasingly regulated environment.
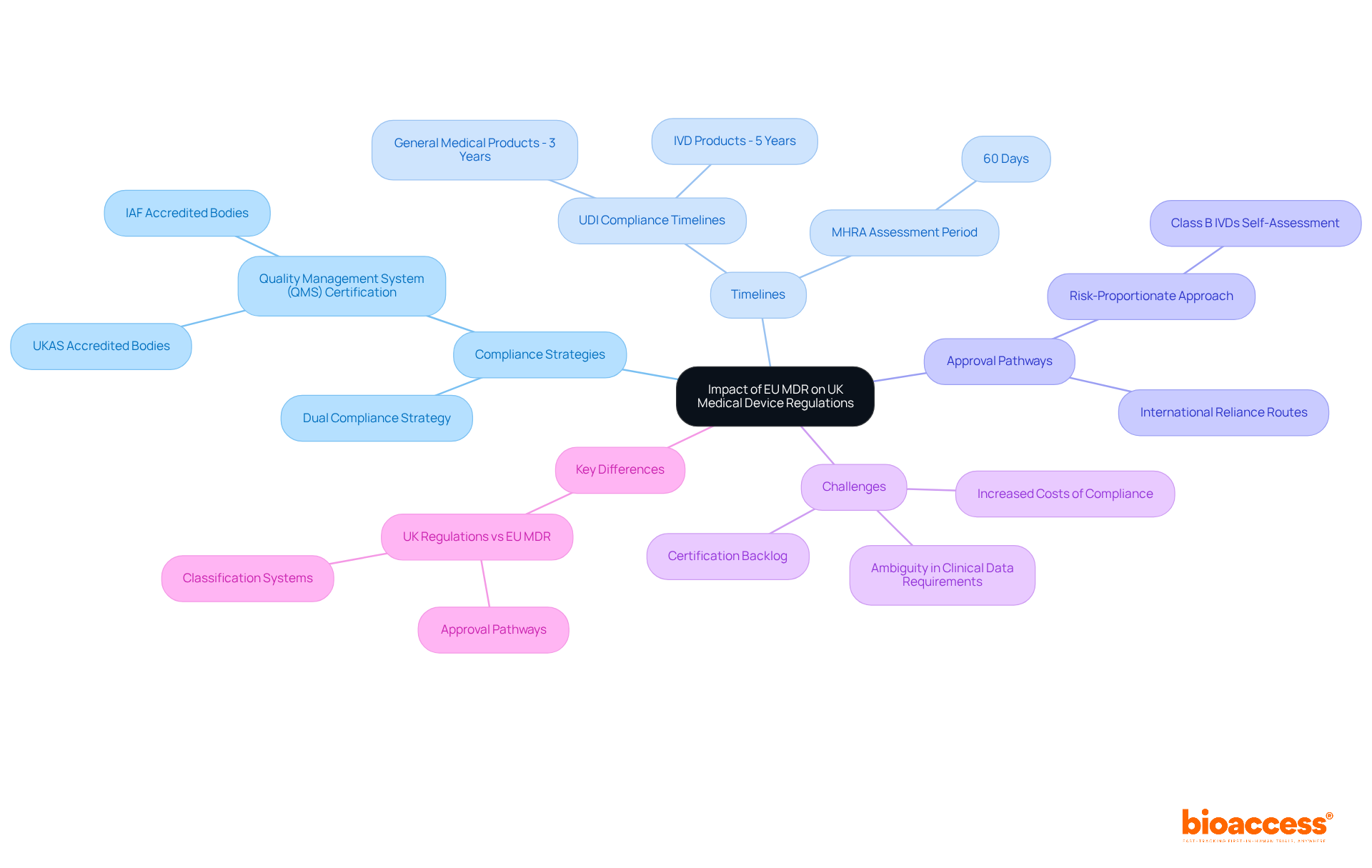
Medical instruments intended for the Northern Ireland market must comply with EU regulations, specifically CE marking. According to the UK medical device regulations established under the Medical Products (Northern Ireland Protocol) Regulations 2021, manufacturers are mandated to register their products with the Medicines and Healthcare products Regulatory Agency (MHRA). This registration procedure is vital, as it ensures compliance with the UK medical device regulations, guaranteeing that all items meet the required safety and performance standards before being sold in Northern Ireland.
Significantly, the MHRA has affirmed that CE-marked products can persist in being offered in Northern Ireland, indicating the area's conformity with EU standards. Moreover, producers must recognize that any Class I and general in vitro diagnostic products not meant for self-testing must be reported to the MHRA when introduced in Northern Ireland, in compliance with UK medical device regulations. The typical duration for medical equipment registration under the Northern Ireland Protocol is about 28 days, enabling a fairly quick entry into the industry.
Recent updates suggest that the MHRA is actively engaged in improving the oversight framework in accordance with the UK medical device regulations, with discussions scheduled for 2024 concentrating on clinical investigations and unique product identification (UDI). This will further clarify requirements for manufacturers. Specialists such as Ana Criado, with her vast experience in oversight matters and biomedical engineering, and Katherine Ruiz, who focuses on UK medical device regulations, emphasize the significance of grasping these requirements for successful market entry.
Ethical approvals are not just a formality; they are essential for conducting clinical trials involving medical devices, safeguarding the rights and welfare of participants. Innovators must submit their trial protocols to ethics committees, which play a pivotal role in reviewing and approving these protocols. This process guarantees adherence to regulatory standards and promotes public trust in clinical research. Recent data reveals that ethical approval is granted in 90.9% of studies, underscoring the effectiveness of ethics committees in maintaining high standards of research integrity.
The MHRA provides comprehensive guidance on the ethical considerations necessary for conducting clinical investigations, which must be strictly adhered to throughout the trial process. Expert insights highlight that ethics committees are instrumental in trial protocol reviews, ensuring that studies are designed with participant safety and ethical considerations at the forefront. Successful submissions to ethics committees often reflect a well-structured approach to addressing potential ethical concerns, significantly enhancing the likelihood of approval.
Moreover, participant trust in clinical trials is closely linked to ethical compliance. Studies indicate that when participants are informed about their rights and the ethical standards governing the research, their willingness to engage in trials increases. This trust is vital for recruitment, especially in first-in-human studies where diverse participant selection is essential for external validity. By prioritizing ethical considerations, innovators can not only meet regulatory requirements but also contribute to the overall success and credibility of their clinical investigations.
Navigating the complex landscape of UK medical device regulations is crucial for innovators seeking successful market entry. Grasping the nuances of compliance-ranging from the assignment of Unique Device Identifications to understanding the differences between CE and UKCA markings-is vital for ensuring that medical devices adhere to essential safety and efficacy standards. The evolving regulatory framework, shaped by both local and EU directives, presents unique challenges and opportunities for manufacturers.
This article has highlighted the significance of early engagement with regulatory bodies like the MHRA, the necessity of meticulous documentation, and the importance of post-market surveillance in maintaining compliance and ensuring patient safety. By leveraging local expertise and resources, such as those provided by bioaccess®, innovators can effectively navigate the complexities of ethical approvals and clinical trials, streamlining their paths to market.
Ultimately, staying informed and proactive about the regulatory landscape not only facilitates smoother market entry but also enhances the overall quality and safety of medical devices. As the industry continues to evolve, embracing collaboration and strategic planning will be essential for success in an increasingly competitive and regulated environment. Innovators must prioritize compliance and ethical considerations, ensuring their contributions lead to improved healthcare outcomes and greater trust in medical technologies.
What is bioaccess® and what advantages does it offer for clinical research in Latin America?
bioaccess® accelerates clinical research for medical devices in Latin America by enabling innovators to secure ethical approvals in 4-6 weeks. It offers cost savings exceeding 30% compared to North America and Western Europe and provides access to a diverse patient population of over 50 million, with 95% covered by universal healthcare.
How long does the review process take for clinical trials in Colombia?
The total review process by the IRB/EC and Ministry of Health (INVIMA) in Colombia takes only 90-120 days, facilitating rapid site activation and regulatory compliance.
What challenges do global research studies face regarding participant enrollment?
Nearly 90% of research studies globally fail to meet their enrollment goals, with 80% postponed due to difficulties in finding sufficient participants.
How does bioaccess® ensure successful enrollment in clinical trials?
bioaccess® achieves successful enrollment by leveraging local expertise and robust recruitment strategies, supported by a strong network of investigators in Latin America.
Why is inclusion in clinical trials important?
Inclusion in clinical trials is crucial not only as a moral obligation but also as a scientific necessity to address health disparities and enhance treatment relevance.
What is the collaboration between bioaccess® and Caribbean Health Group aimed at?
The collaboration aims to position Barranquilla as a premier destination for clinical trials in Latin America, enriching the overall landscape for clinical research in the region.
What are the key regulations governing medical devices in the UK?
The UK medical device regulations are shaped by the Medical Devices Regulations 2002, updated to align with post-Brexit requirements, including the UK MDR and the Medical Devices (Amendment) Regulations 2025.
What is the Unique Device Identification (UDI) requirement?
Producers are required to assign a UDI to their products to enhance traceability and post-market oversight, which is part of a broader initiative to improve patient safety.
What challenges do innovators face with the UK medical device regulations?
Innovators must maintain essential EU regulations beyond the initial sunset date of May 2025 to avoid regulatory gaps and ensure stability, especially for in vitro diagnostics and animal tissue-based products.
How can innovators expedite the approval process under the new UK regulations?
Engaging with oversight organizations early and leveraging technological solutions can streamline compliance efforts and expedite the approval process.
What is the process for placing medical devices on the UK market?
Manufacturers must register their products with the Medicines and Healthcare products Regulatory Agency (MHRA) and obtain either a UKCA or CE marking, which requires appointing a UK Responsible Person (UKRP) and preparing extensive documentation.
What factors can affect the registration timeline for medical devices in the UK?
The registration timeline can vary significantly, with delays often occurring due to initial administrative checks and the MHRA conducting audits and inspections to verify compliance.
How can manufacturers enhance their chances of successful device registration?
Engaging with compliance experts early in the process can significantly reduce delays and enhance the likelihood of a successful submission, as well as understanding the requirements for UKCA and CE marking.