


Navigating the complex landscape of biologics in Australia demands a thorough understanding of risk management plans (RMPs) and the essential templates that guide their submission. These templates not only ensure compliance with stringent regulatory standards but also significantly enhance the efficiency of the approval process, which has seen remarkable improvements in recent years.
With the rapid evolution of guidelines and the intricacies involved in RMP submissions, how can sponsors effectively leverage these tools to secure timely market access for their innovative products?
This article explores ten essential RMP submission templates that are pivotal for biologics in Australia, illuminating their benefits and the strategic approaches necessary for successful implementation.
The bioaccess® RMP submission templates for biologics in Australia are expertly crafted to streamline the submission of management plans (RMPs). This template not only integrates best practices but also aligns with the latest regulatory requirements, ensuring that all essential information is presented in a clear and concise manner. By leveraging this template, sponsors can anticipate a more efficient review process. This is particularly significant given that average approval times for biological products in Australia have improved dramatically, with many achieving market access within just 4 to 6 weeks in 2025.
The template features comprehensive sections dedicated to:
These sections are specifically tailored for the Australian regulatory landscape. This strategic approach enhances compliance and operational efficiency, empowering sponsors to navigate the complexities of RMP submission templates for biologics in Australia with greater confidence. Successful implementations of this template have proven its effectiveness in accelerating the approval process, thereby facilitating quicker access to innovative biologics for patients in need. As the Therapeutic Goods Administration (TGA) continues to refine its processes, the adoption of streamlined RMP submission templates becomes increasingly essential for Medtech innovators striving for timely market entry.

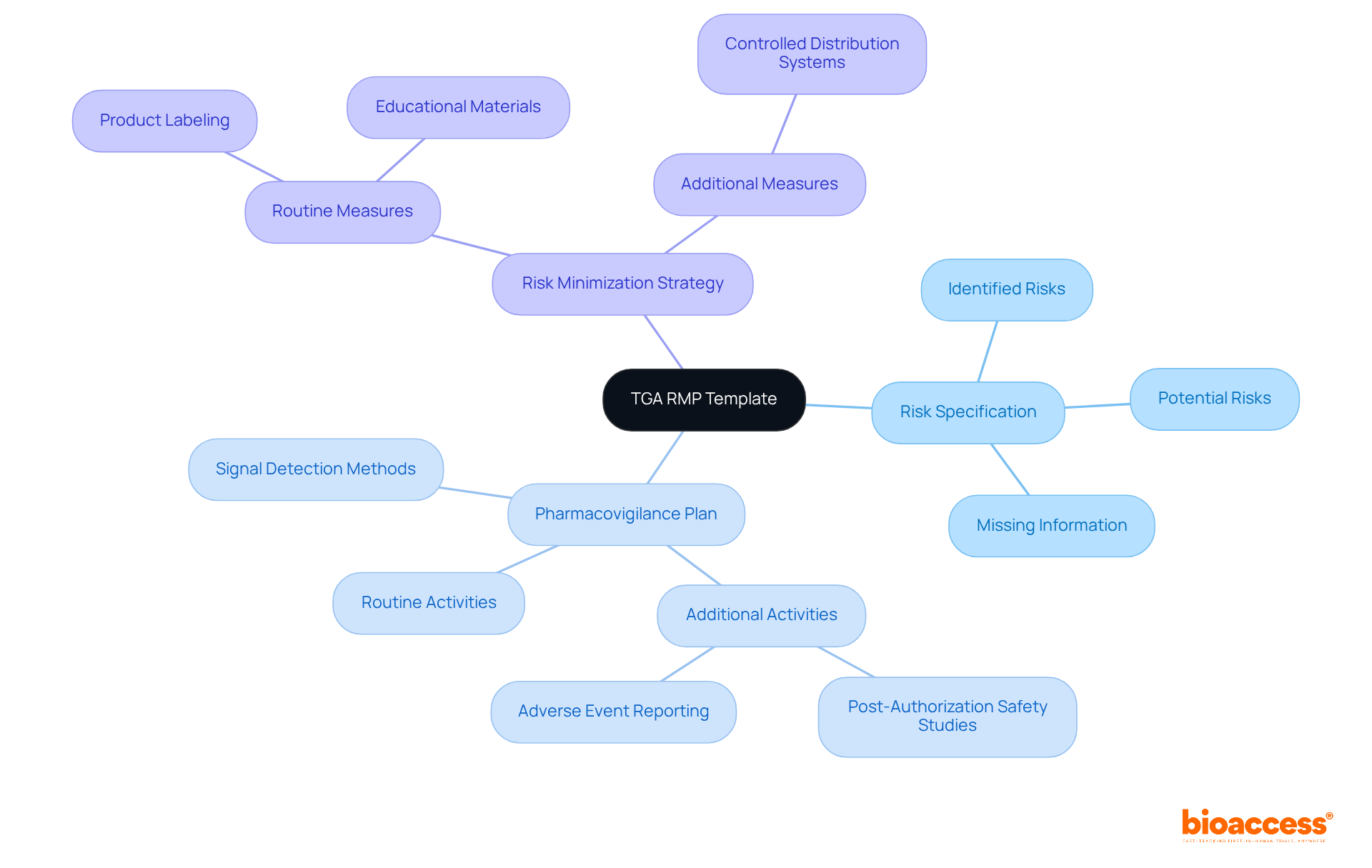
The TGA RMP Template serves as a crucial compliance framework that delineates the specific requirements for management plans in Australia. This template is vital for sponsors of prescription medicines and biologicals, as it serves as one of the RMP submission templates for biologics in Australia, ensuring that all essential components are incorporated into their submissions. Key elements of the TGA RMP Template encompass:
By adhering strictly to this template, sponsors not only demonstrate their commitment to patient safety but also enhance their regulatory compliance, significantly streamlining the approval process.
Notably, the average review time for RMP submission templates for biologics in Australia is around 4 to 6 weeks, highlighting the efficiency of the process when all requirements are met. Specialist insights reveal that a well-organized RMP can lead to successful submissions, as evidenced by numerous management plans positively assessed by the TGA. Furthermore, staying abreast of the latest TGA guidelines and integrating any necessary changes into the RMP is essential for maintaining compliance and ensuring the safety of biological products throughout their lifecycle.
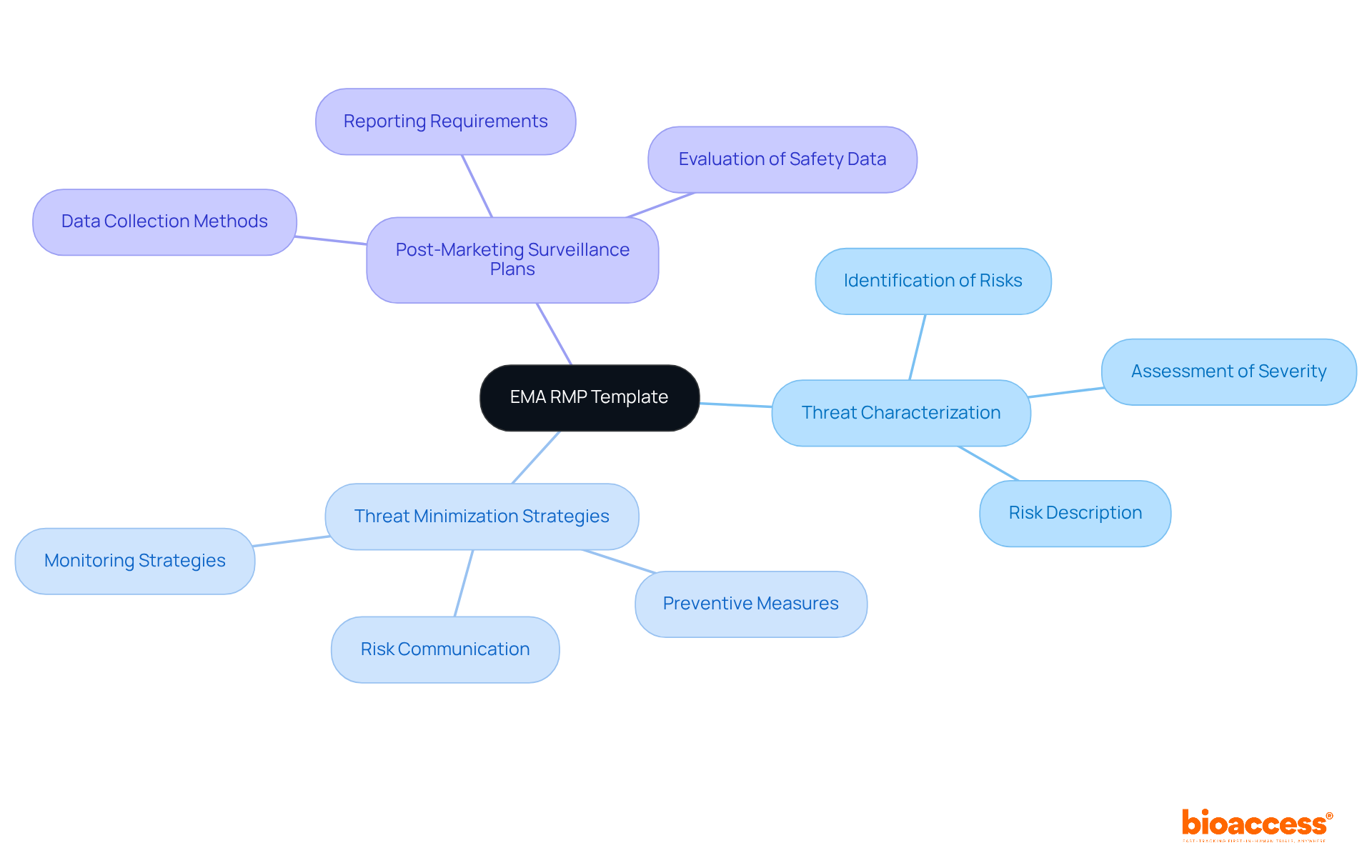
The EMA RMP Template stands as the gold standard for risk management plans across Europe, serving as a crucial reference for international submissions. When companies apply for marketing authorization, they must submit a risk-management plan (RMP) to the European Medicines Agency (EMA), underscoring the necessity of utilizing the EMA RMP Template. This comprehensive template encompasses vital sections for:
All adhering to the modular format outlined in GVP Module V. By aligning their submissions with the EMA's RMP Template, sponsors not only fulfill European regulatory requirements but also bolster their credibility in the global market. This strategic alignment proves especially advantageous for companies aiming to penetrate European markets while ensuring compliance with Australian regulations, ultimately facilitating smoother international submissions and the effective use of RMP submission templates for biologics in Australia to enhance the overall success of their products.
Moreover, it is essential to recognize that RMPs are dynamic documents that require revisions as new safety information emerges, reflecting the evolving nature of risk management. As Safwan Azeem aptly noted, 'A well-crafted Risk Management Plan (RMP) is no longer a regulatory luxury - it’s a foundational requirement for any medicinal product seeking approval in today’s global market.' This statement reinforces the critical role of RMPs in navigating the complexities of regulatory landscapes.
The WHO RMP Template provides essential global guidelines for the development and approval of biologics. It underscores the importance of a systematic approach to managing uncertainties, which includes:
By following the WHO's recommendations, sponsors can ensure their products comply with local regulations and meet international quality standards. This compliance is crucial for building trust among healthcare professionals and patients, ultimately leading to improved health outcomes.
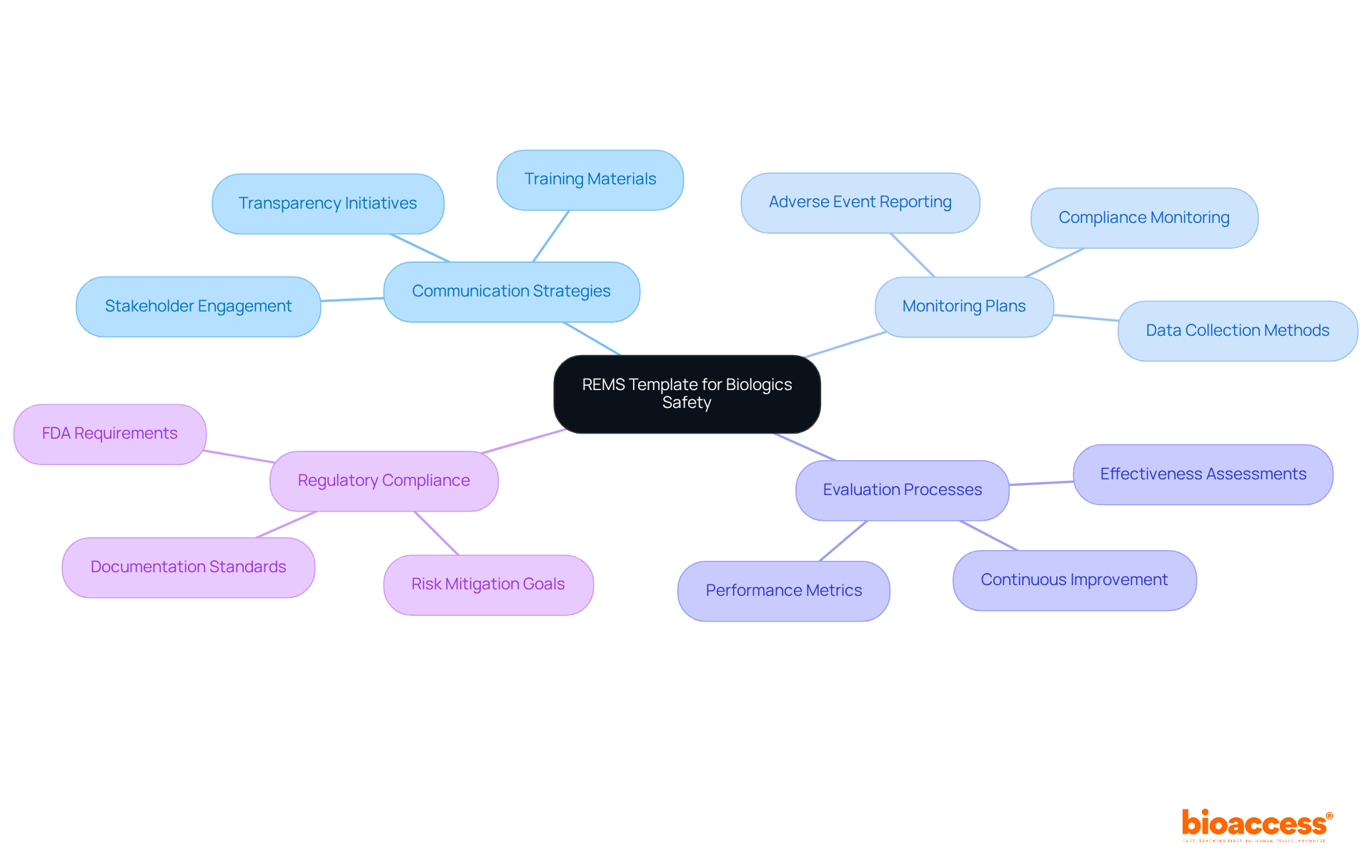
The REMS Template serves as a crucial framework for sponsors of high-risk biologics, delineating essential strategies for assessing and mitigating associated hazards. This comprehensive template encompasses organized plans for communication, monitoring, and evaluation, ensuring that all stakeholders remain well-informed and actively engaged in the protection process. By implementing a robust REMS, sponsors not only bolster patient safety but also ensure compliance with regulatory requirements, thereby fostering trust in their products.
Effective communication strategies within the REMS framework are vital, as they promote transparency and instill confidence among healthcare providers and patients alike. The timeline for implementing REMS for high-risk biological products can vary; however, early planning during the clinical trial phases is recommended to streamline the process and avert delays. Recent updates in hazard assessment and mitigation strategies for biologics underscore the evolving landscape of regulatory expectations, highlighting the necessity for continuous improvement in protection protocols.
The RMP submission templates for biologics in Australia serve a crucial purpose for sponsors conducting clinical trials. It outlines the necessary management plans that must be submitted as part of the Clinical Trials Notification process. Essential elements include:
By utilizing the RMP submission templates for biologics in Australia, sponsors can ensure their clinical trials comply with Australian regulations, which facilitates smoother approvals and enhances the well-being of trial participants.

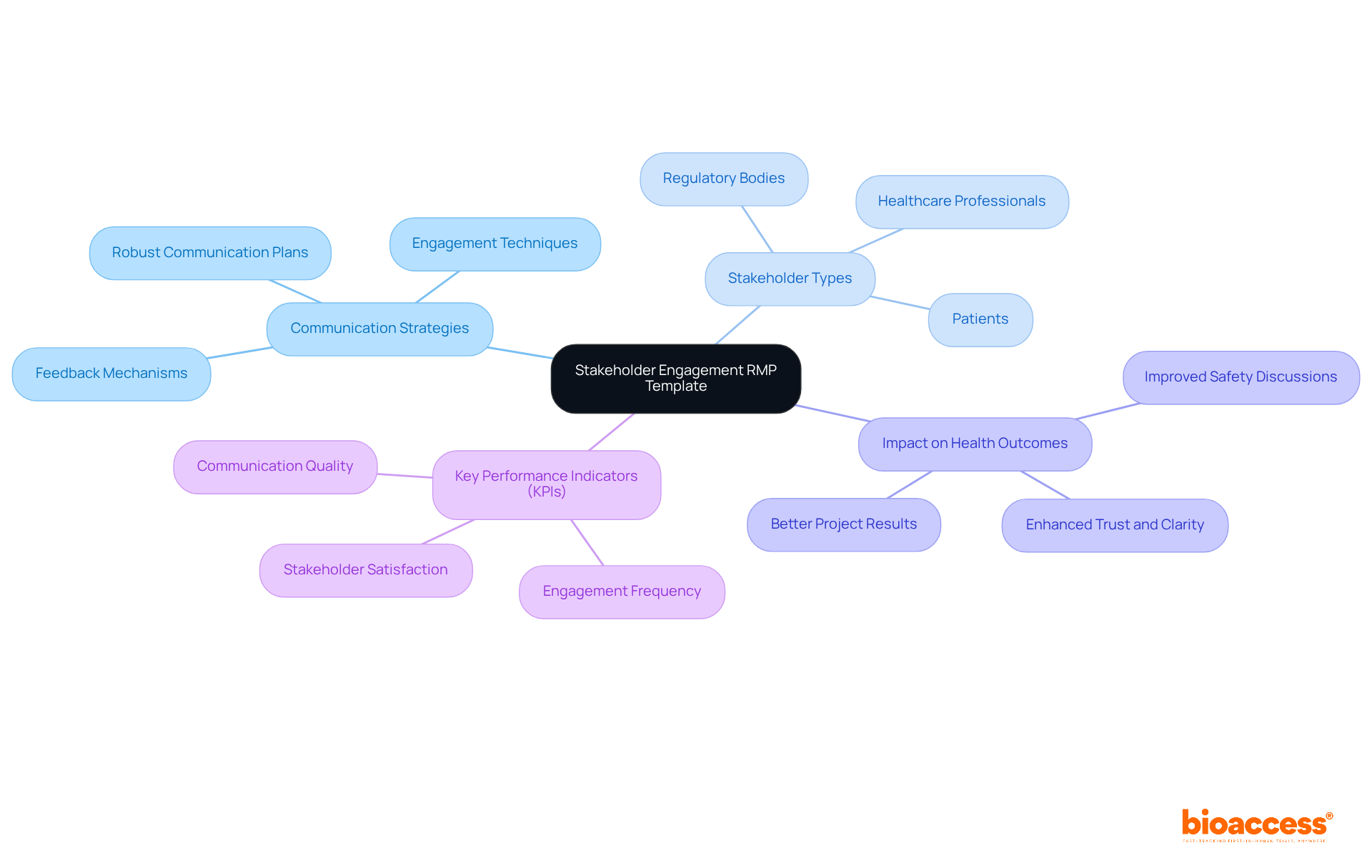
The Stakeholder Engagement RMP Submission Templates for biologics in Australia highlight the critical role of communication in managing biologics. This template delineates strategies for engaging healthcare professionals, regulatory bodies, and patients, ensuring that all stakeholders remain well-informed and actively participate in safety discussions. Effective communication fosters clarity and trust-elements essential for successfully navigating uncertainties. By adopting robust communication strategies, sponsors can significantly boost the effectiveness of their Risk Management Plans (RMPs), ultimately leading to improved health outcomes.
Research shows that companies with strong stakeholder engagement are 30% more likely to succeed with new products. A well-structured communication strategy not only keeps stakeholders updated on RMP progress but also encourages their involvement by utilizing RMP submission templates for biologics in Australia, enhancing project results and mitigating challenges associated with biological products. Furthermore, effective stakeholder engagement necessitates clear, measurable Key Performance Indicators (KPIs) to assess the success of communication strategies. Understanding the regulatory landscape, including the oversight from authorities like INVIMA in Colombia-recognized as a Level 4 health authority by PAHO/WHO-can further amplify the effectiveness of these engagement strategies within the Australian context.
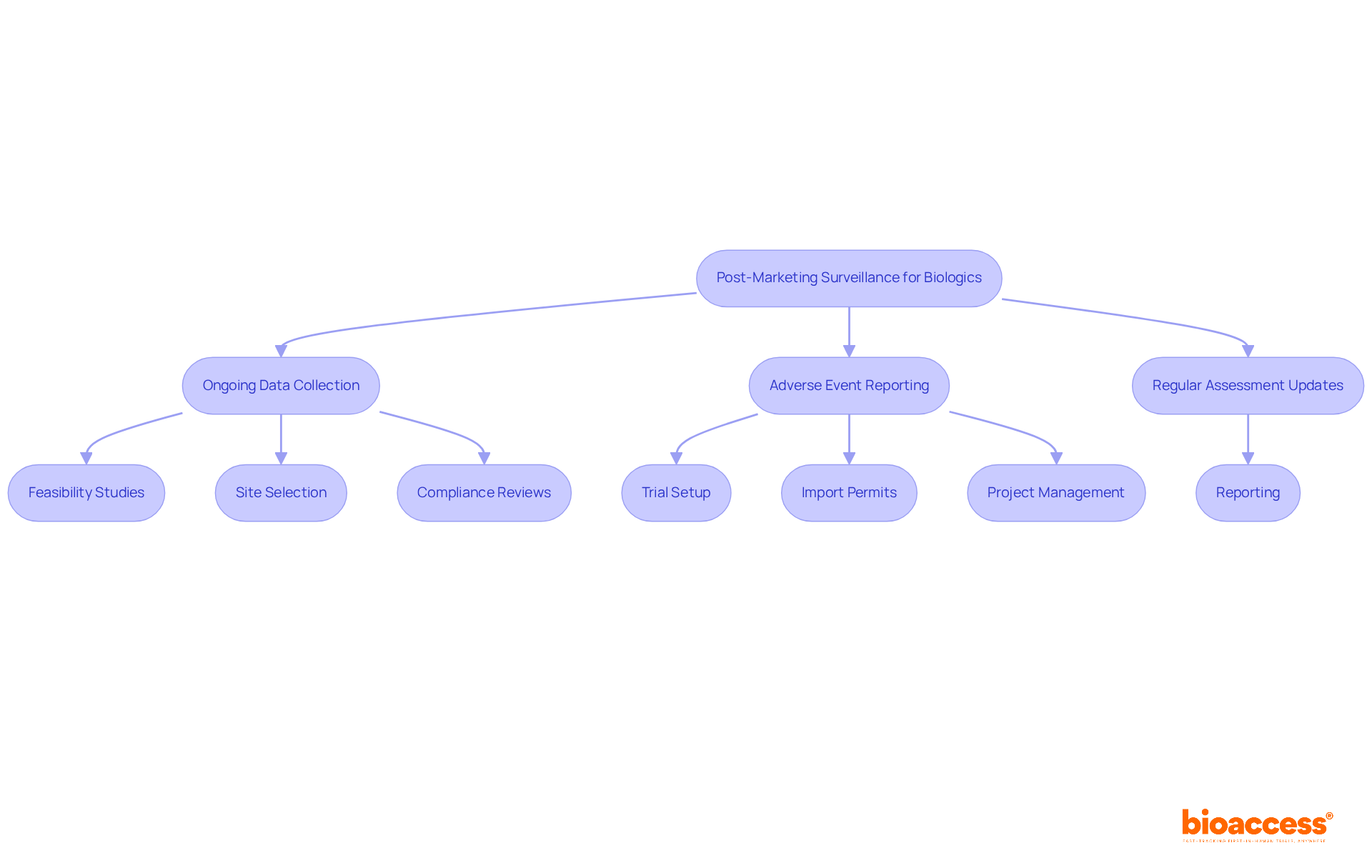
The RMP submission templates for biologics in Australia are essential for tracking the safety and efficacy of biologics after approval. This template, which is one of the RMP submission templates for biologics in Australia, outlines strategies for ongoing data collection, prompt adverse event reporting, and regular assessment updates. Given that the average time for adverse event reporting in Brazil is critical for patient well-being, a robust post-marketing surveillance strategy enables sponsors to swiftly identify potential issues and implement necessary risk mitigation measures.
Continuous oversight is vital for ensuring the long-term safety of biological products. It allows for the adjustment of strategies based on real-world data, reinforcing the importance of effective monitoring. Not only do these strategies enhance product safety, but they also foster trust with regulatory bodies, ultimately leading to improved patient outcomes. Furthermore, bioaccess provides comprehensive clinical trial management services that are crucial in supporting these post-marketing surveillance efforts. These services encompass:
Ensuring meticulous management of all aspects of the clinical trial process.
The RMP submission templates for biologics in Australia, including the Risk Identification and Assessment template, serve as a vital resource for sponsors involved in the development of biological products. This template not only facilitates a systematic approach to identifying potential threats, such as security concerns and efficacy issues, but also outlines effective strategies for evaluating these uncertainties. By utilizing RMP submission templates for biologics in Australia, sponsors are equipped to proactively address the common challenges encountered during the biological product development process, ultimately enhancing the security and effectiveness of their biologics.
Moreover, continuous updates to security information and efficacy assessments are essential. These revisions guide harm reduction strategies and align with evolving regulatory standards. This structured approach not only ensures compliance but also reinforces a commitment to patient safety and therapeutic effectiveness. In a landscape where collaboration is key, utilizing the RMP submission templates for biologics in Australia positions sponsors to navigate the complexities of clinical research with confidence.

The Continuous Improvement RMP Submission Templates for biologics in Australia emphasize the critical need for ongoing assessment and enhancement of management strategies for biological products. This template delineates mechanisms for the regular review and updating of assessments, ensuring that new data and emerging information are seamlessly integrated. As W. Edwards Deming aptly noted, "Learning is not compulsory; it’s voluntary. Improvement is not compulsory; it’s voluntary. But to survive, we must learn." By fostering a culture of continuous enhancement, sponsors can adapt their strategies to meet evolving challenges, thereby bolstering the reliability and efficacy of their biologics, which is essential for adhering to RMP submission templates for biologics in Australia.
Frequent updates to threat evaluations are not just beneficial; they are essential. Such updates facilitate swift responses to new insights and regulatory demands, ultimately promoting a proactive management strategy. For example, the case study on the "Human Side of Change Management" illustrates how organizations that prioritize continuous improvement can adeptly navigate challenges and refine their processes. Furthermore, it is advisable that risk assessments, particularly those involving RMP submission templates for biologics in Australia, be updated at least biannually to ensure alignment with the latest regulatory expectations and safety data.

The significance of effective RMP submission templates for biologics in Australia is paramount. These templates not only ensure compliance with regulatory standards but also streamline the submission process, ultimately granting patients quicker access to innovative treatments. By implementing structured frameworks like the bioaccess® RMP submission template and the TGA RMP Template, sponsors can navigate the complexities of regulatory requirements with enhanced ease and confidence.
This article has presented key insights into various RMP templates, each designed to address specific regulatory needs and challenges. From the comprehensive guidelines provided by the WHO and EMA to the localized compliance frameworks essential for Australian clinical trials, these templates are indispensable tools for sponsors. They encompass critical elements such as:
All vital for ensuring the safety and efficacy of biologics.
As the biologics landscape evolves, embracing these RMP submission templates is crucial for upholding high standards of patient safety and regulatory compliance. Companies must prioritize the integration of these templates into their processes, fostering a culture of continuous improvement and proactive risk management. By doing so, they not only enhance their chances of successful market entry but also contribute to the overall advancement of healthcare innovation in Australia and beyond.
What is the purpose of the bioaccess® RMP submission template for biologics in Australia?
The bioaccess® RMP submission template is designed to streamline the submission of risk management plans (RMPs) for biologics in Australia, integrating best practices and aligning with the latest regulatory requirements to enhance the efficiency of the review process.
What sections are included in the bioaccess® RMP submission template?
The template includes comprehensive sections dedicated to threat identification, evaluation, and mitigation strategies, specifically tailored for the Australian regulatory landscape.
How has the approval time for biological products in Australia changed recently?
Average approval times for biological products in Australia have improved significantly, with many achieving market access within just 4 to 6 weeks in 2025.
What is the significance of the TGA RMP Template?
The TGA RMP Template serves as a compliance framework that outlines the specific requirements for management plans in Australia, ensuring all essential components are included in submissions for prescription medicines and biologicals.
What are the key elements of the TGA RMP Template?
Key elements of the TGA RMP Template include a thorough risk specification, a robust pharmacovigilance plan, and a comprehensive risk minimization strategy.
What is the average review time for RMP submission templates for biologics in Australia?
The average review time for RMP submission templates for biologics in Australia is around 4 to 6 weeks.
How does the EMA RMP Template relate to international submissions?
The EMA RMP Template is considered the gold standard for risk management plans in Europe and is essential for companies applying for marketing authorization, ensuring compliance with European regulatory requirements.
What sections are included in the EMA RMP Template?
The EMA RMP Template includes sections for threat characterization, threat minimization strategies, and post-marketing surveillance plans, adhering to the modular format outlined in GVP Module V.
Why is it important for RMPs to be dynamic documents?
RMPs are dynamic documents that require revisions as new safety information emerges, reflecting the evolving nature of risk management and ensuring ongoing compliance and safety for biological products.
What is the foundational requirement for any medicinal product seeking approval in today's global market?
A well-crafted Risk Management Plan (RMP) is considered a foundational requirement for any medicinal product seeking approval in today's global market.