


The primary focus of the article titled "10 Essential Skills for Clinical Project Manager Jobs Remote" is to delineate the key competencies necessary for clinical project managers operating in remote environments. It underscores that skills such as:
are vital for effectively overseeing clinical trials and navigating the complexities of remote work. This ensures successful outcomes in medical research.
In the competitive landscape of clinical research, the role of a clinical project manager has evolved into a multifaceted position that demands a diverse skill set. As organizations strive to bring innovative medical solutions to market faster, the demand for professionals who can navigate complex regulatory environments, manage diverse teams, and utilize advanced technologies is at an all-time high. This article explores ten essential skills that remote clinical project managers must master to thrive in this dynamic field. How can these competencies not only enhance individual performance but also drive the success of entire research initiatives?
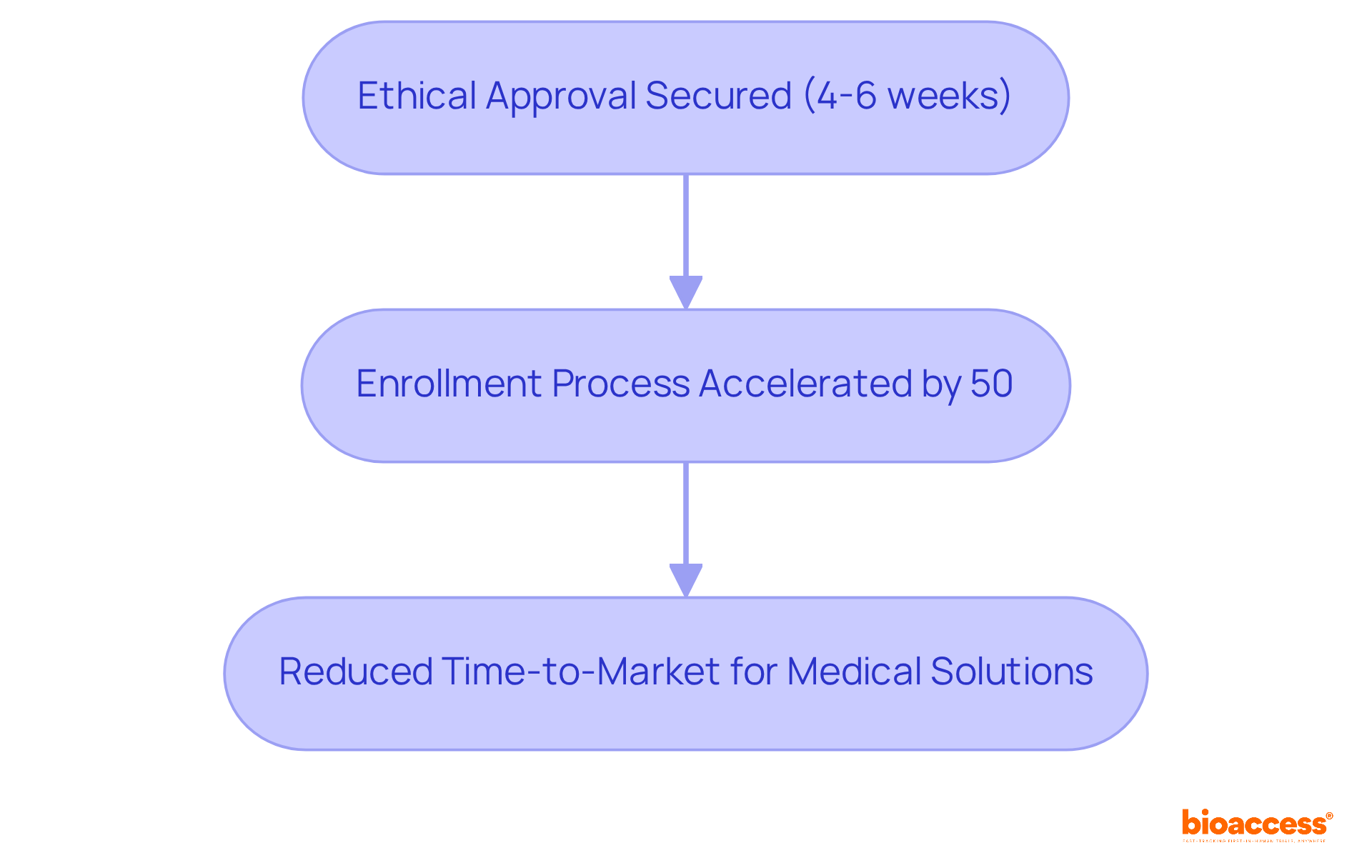
bioaccess® distinguishes itself in the research landscape through exceptional speed and efficiency. With ethical approvals secured in just 4-6 weeks and enrollment processes accelerated by 50% compared to conventional markets, trial supervisors can significantly reduce the time-to-market for innovative medical solutions. This agility is essential for Medtech, Biopharma, and Radiopharma companies, allowing them to adeptly navigate complex regulatory environments while ensuring compliance and upholding high-quality standards in their studies. The rapid pace of ethical approvals not only shortens the development timeline but also enhances the overall success rate of medical studies, positioning it as a vital component in the competitive arena of medical innovation.
A thorough understanding of regulatory requirements is essential for clinical leaders, particularly in the evolving environments of Latin America, the Balkans, and Australia. By staying informed about local and international regulations, supervisors can prepare accurate documentation, facilitate audits, and ensure that all activities adhere to ethical standards. This diligence not only safeguards the integrity of the research process but also enhances credibility with stakeholders.
In 2025, updates to regulatory requirements will necessitate that individuals overseeing initiatives adapt to evolving compliance landscapes, especially in regions where regulatory frameworks can be complex and varied. For instance, navigating the regulatory environment in Latin America often involves addressing unique challenges such as varying approval timelines and diverse patient populations, while Australia offers streamlined pathways that can expedite the approval process.
By utilizing their knowledge in feasibility assessments, site selection, and compliance evaluations, research coordinators can successfully navigate regulatory obstacles, ensuring favorable study results and building trust among regulatory authorities and participants alike.
Furthermore, the thorough procedure for advancing medical device evaluations—encompassing the acquisition of IRB/EC approval, INVIMA authorization, and MinCIT import permits—is crucial for upholding regulatory compliance and enabling efficient management.

Project managers in the healthcare sector are pivotal to the successful implementation of research studies, necessitating a robust foundation in project management skills. Their key responsibilities encompass:
The application of methodologies such as Agile and Waterfall can substantially enhance operational efficiency. For instance, Agile practices facilitate rapid iteration and flexibility, crucial in the evolving landscape of medical research. Conversely, the Waterfall methodology provides a structured approach, ensuring that each phase of the process is meticulously planned and executed.
Data indicates that approximately 52.2% of research studies are funded by the industry, underscoring the importance of efficient management in navigating the complexities of these investigations. A notable example is the collaboration between Welwaze Medical Inc. and bioaccess™ for the launch of the Celbrea® medical device in Colombia, where bioaccess™ offered essential regulatory and market access consulting. This partnership exemplifies how comprehensive research management services—including:
can streamline the process and enhance study success.
Moreover, a significant challenge in clinical studies is patient recruitment, with 45.4% of discontinuations attributed to this issue. Consequently, managers must not only oversee logistics for the initiative but also implement strategies to bolster recruitment and retention. Notably, industry-sponsored studies exhibited a 70% lower likelihood of discontinuation due to slow recruitment compared to nonindustry research, emphasizing the impact of effective management on study outcomes.
Insights from industry leaders further highlight the importance of these methodologies. For example, Peter Drucker noted that 'time is the most limited resource and unless it is handled nothing else can be handled,' stressing the necessity of efficient time management in research studies. Additionally, embracing an Agile mindset can cultivate a culture of continuous improvement, enabling teams to swiftly address challenges and feedback.
Ultimately, the ability to manage research studies effectively hinges on a coordinator's skill in merging strategic planning with adaptable approaches. To enhance study results, coordinators should focus on integrating targeted research management services and persistently refining their strategies to meet the evolving demands of medical research.
Effective communication stands as a cornerstone for project managers tasked with coordinating diverse teams, which include researchers, sponsors, and regulatory bodies. This pivotal role necessitates not only the capacity to convey information with clarity but also the skill to actively listen to team members’ concerns and feedback.
Regular meetings and updates, complemented by collaborative tools, significantly enhance communication, ensuring that all parties remain informed and engaged throughout the process. Furthermore, efficient communication is critical for facilitating the review and feedback on research documents, as well as for providing updates on study status—both of which are essential components of research management.
As the landscape of medical research evolves, the integration of effective communication methods will be indispensable for navigating the complexities of contemporary studies.
Clinical program supervisors must possess strong data evaluation skills to accurately interpret trial outcomes. This requires a solid understanding of statistical methods and the ability to analyze trends within complex datasets. As Brad Schneider aptly puts it, "Wrangling data is like interrogating a prisoner. Just because you wrangled a confession doesn’t mean you wrangled the answer," highlighting the intricacies involved in data interpretation.
By employing advanced data visualization tools, team leaders can effectively showcase findings to stakeholders, enhancing clarity and facilitating informed decision-making. Current trends underscore the significance of utilizing visual aids, such as Kaplan-Meier plots and forest plots, which are frequently applied in trials—evident from their presence in 32 articles examined. These tools convey survival probabilities and treatment effects across diverse patient subgroups, making them invaluable for effective communication.
By mastering these techniques and understanding statistical uncertainty, research coordinators can ensure that insights derived from trial data are actionable, ultimately guiding strategic planning for subsequent research phases.
In a remote work environment, healthcare team leaders must exhibit strong leadership skills to effectively motivate and direct their groups. This entails:
By nurturing a cooperative and inclusive atmosphere, leaders can significantly boost team morale and promote success, even when operating from diverse locations.
The comprehensive management services for studies offered by bioaccess—including:
play a crucial role in supporting these leadership efforts. By ensuring that all aspects of the examination are meticulously managed, including the import permit and nationalization of investigational devices, supervisors can focus on inspiring their teams and achieving successful outcomes. This ultimately contributes to the enhancement of global health through innovative medtech solutions.

Healthcare program leaders must possess robust problem-solving capabilities to effectively tackle the myriad challenges that arise during research studies. This entails not only identifying potential risks but also formulating comprehensive contingency plans and executing solutions with agility. A proactive approach to problem-solving is vital; it significantly reduces disruptions and preserves the integrity of the research process.
As of 2025, the landscape of medical studies has grown increasingly intricate, with 56% of locations indicating that studies are more complex than three years prior. This complexity necessitates that managers remain vigilant regarding potential issues, leveraging historical performance data for site selection and implementing strategic enrollment planning.
At bioaccess, our extensive research management services encompass:
All crucial for effective risk management. Industry leaders underscore that the integration of technology and patient-centric designs can enhance recruitment and retention outcomes. For instance, decentralized trials have demonstrated potential in overcoming participant engagement challenges, facilitating faster enrollment and fostering greater diversity among trial participants.
By embracing these strategies, healthcare program leaders can adeptly navigate the evolving challenges of medical research and contribute to the advancement of healthcare innovation, ultimately enhancing global health through international collaboration.
In the rapidly evolving realm of medical research, flexibility emerges as a vital competency for leaders. They must be prepared to adjust plans and strategies in response to new data, regulatory changes, or unforeseen challenges. Adopting a flexible mindset not only boosts individual performance but also cultivates a culture of resilience within teams. This adaptability is crucial for navigating the complexities of research studies, where the ability to pivot can significantly impact outcomes.
For example, bioaccess® excels in managing a range of studies, including:
Their accelerated regulatory approval process enables authorizations in just 6-8 weeks, compared to the typical 6-12 months in the US/EU, empowering team leaders to respond swiftly to changing circumstances. Effective adjustments to research plans often necessitate real-time data analysis and stakeholder engagement, ensuring that teams remain coordinated and agile.
As the demand for research trial activities continues to grow, evidenced by a notable 12.2% increase in recent years, the ability to thrive in dynamic environments will distinguish effective supervisors. Industry experts emphasize that strong decision-making skills are critical for making rapid, informed choices in these high-pressure scenarios, further highlighting the necessity of adaptability.

Clinical program managers play a vital role in stakeholder management, which is essential for fostering strong relationships with sponsors, regulatory bodies, and research sites. Meaningful involvement encompasses consistent communication, prompt updates, and organized feedback sessions, promoting collaboration and alignment with objectives. In 2025, sustaining these connections has become increasingly critical due to evolving regulatory environments and the growing complexity of research studies. Leaders at bioaccess leverage extensive research management services, including:
to enhance transparency and streamline interactions, ensuring that all stakeholders are informed and engaged throughout the research process.
Proactive outreach to sponsors may include sharing progress reports and addressing concerns promptly, which not only builds trust but also facilitates smoother approvals. Similarly, establishing open lines of communication with regulatory agencies aids in navigating compliance challenges more effectively. By emphasizing stakeholder involvement, healthcare program leaders can enhance the overall effectiveness of studies and contribute to achieving successful results.
Moreover, understanding the barriers and facilitators for the broader use of patient-reported outcomes (PROs) in real-world evidence (RWE) generation is crucial. Addressing operational challenges and promoting collaborative efforts among stakeholders can significantly enhance the execution of PROs, ultimately benefiting outcome results. Engaging stakeholders early in the process not only enhances credibility but also ensures that the systematic review findings are relevant and actionable. By incorporating these insights into their stakeholder management approaches, healthcare leaders can navigate the intricacies of medical studies more efficiently.
Clinical program managers must demonstrate a high level of technical expertise in trial management software to enhance processes and bolster data integrity. Familiarity with electronic data capture (EDC) systems, management tools, and statistical analysis software is not merely beneficial; it is essential. These technologies streamline workflows, significantly reduce errors, and improve communication among team members. The adoption of cloud-based EDC systems has proven to enhance real-time data access and collaboration, critical in today's fast-paced research environment.
As the Clinical Trial Data Management Services sector is projected to grow at a CAGR of 12.8% from 2024 to 2031, integrating advanced tools will be vital for managers to navigate increasing complexities. Furthermore, bioaccess® offers comprehensive clinical study management services, including:
All crucial for successful execution. By leveraging innovative technologies, such as AI-powered tools for predictive analysis, bioaccess® can facilitate more efficient study management, ultimately accelerating the development of new therapies and improving patient outcomes. Experts assert that these advancements in data collection and safety can further streamline drug development, enhancing data integrity and regulatory compliance while empowering researchers to conduct trials with both efficiency and precision.

The landscape of clinical project management is evolving rapidly, necessitating a diverse array of skills for success in remote roles. From regulatory knowledge to effective communication, the ability to navigate complex environments while maintaining compliance and fostering collaboration is paramount. The emphasis on adaptability and problem-solving skills further highlights the need for leaders who can manage challenges dynamically and drive teams toward successful outcomes.
Key insights from the article underscore the importance of:
Each skill contributes to a comprehensive approach that enhances the efficiency and effectiveness of clinical trials. As the demand for innovative medical solutions continues to rise, the significance of these competencies in clinical project management cannot be overstated.
In a world where the pace of medical research is accelerating, embracing these essential skills is not just beneficial but critical. Professionals in the field must commit to continuous learning and adaptation to thrive in this dynamic environment. By prioritizing these competencies, clinical project managers can significantly impact the advancement of healthcare, ultimately contributing to improved patient outcomes and the successful delivery of groundbreaking therapies.
What is bioaccess and how does it benefit clinical research?
bioaccess® accelerates clinical research by achieving ethical approvals in just 4-6 weeks and speeding up enrollment processes by 50% compared to conventional markets. This agility helps Medtech, Biopharma, and Radiopharma companies reduce time-to-market for medical solutions while ensuring compliance and maintaining high-quality standards.
Why is regulatory knowledge important for clinical leaders?
Regulatory knowledge is essential for clinical leaders to navigate compliance in clinical trials. Understanding local and international regulations allows supervisors to prepare accurate documentation, facilitate audits, and ensure ethical standards are met, which safeguards the integrity of the research and enhances credibility with stakeholders.
What challenges exist in navigating regulatory environments in different regions?
In regions like Latin America, challenges include varying approval timelines and diverse patient populations. Conversely, Australia offers streamlined pathways that can expedite the approval process. Staying informed about these regional differences is crucial for effective compliance management.
What are the key responsibilities of project managers in healthcare research?
Project managers in healthcare research are responsible for developing detailed timelines, managing budgets, and coordinating resources effectively. They apply methodologies like Agile and Waterfall to enhance operational efficiency in clinical trials.
How do Agile and Waterfall methodologies impact clinical research?
Agile methodologies promote rapid iteration and flexibility, which are crucial in the evolving landscape of medical research. Waterfall provides a structured approach, ensuring meticulous planning and execution of each phase of the research process.
What percentage of research studies are funded by the industry, and why is this significant?
Approximately 52.2% of research studies are funded by the industry. Efficient management is vital in navigating the complexities of these investigations, which can improve study outcomes.
What is a notable example of successful collaboration in clinical research?
A notable example is the partnership between Welwaze Medical Inc. and bioaccess™ for the launch of the Celbrea® medical device in Colombia, where bioaccess™ provided essential regulatory and market access consulting.
What are common challenges in patient recruitment for clinical studies?
Patient recruitment poses a significant challenge, with 45.4% of study discontinuations attributed to this issue. Effective management and recruitment strategies are crucial for improving retention and study success.
How do industry-sponsored studies compare to nonindustry research regarding recruitment?
Industry-sponsored studies have a 70% lower likelihood of discontinuation due to slow recruitment compared to nonindustry research, highlighting the importance of effective management in clinical studies.
What should coordinators focus on to enhance study results?
Coordinators should focus on integrating targeted research management services and continuously refining their strategies to adapt to the evolving demands of medical research, ensuring effective management and improved study outcomes.