


The essential skills for clinical study assistant jobs encompass mastery of:
These competencies are crucial for supporting research teams, ensuring compliance, enhancing collaboration, and ultimately contributing to the success of clinical trials through improved study outcomes and participant safety.
The landscape of clinical research is evolving rapidly, necessitating a robust skill set from professionals in clinical study assistant roles. Mastering essential competencies not only enhances individual performance but also significantly contributes to the integrity and success of medical studies. As the industry grapples with increasing complexity and regulatory scrutiny, aspiring clinical study assistants must prepare effectively for the challenges that lie ahead. This article delves into ten critical skills that are indispensable for success in this dynamic field, offering insights into how these abilities can be cultivated to drive innovation and improve patient outcomes.
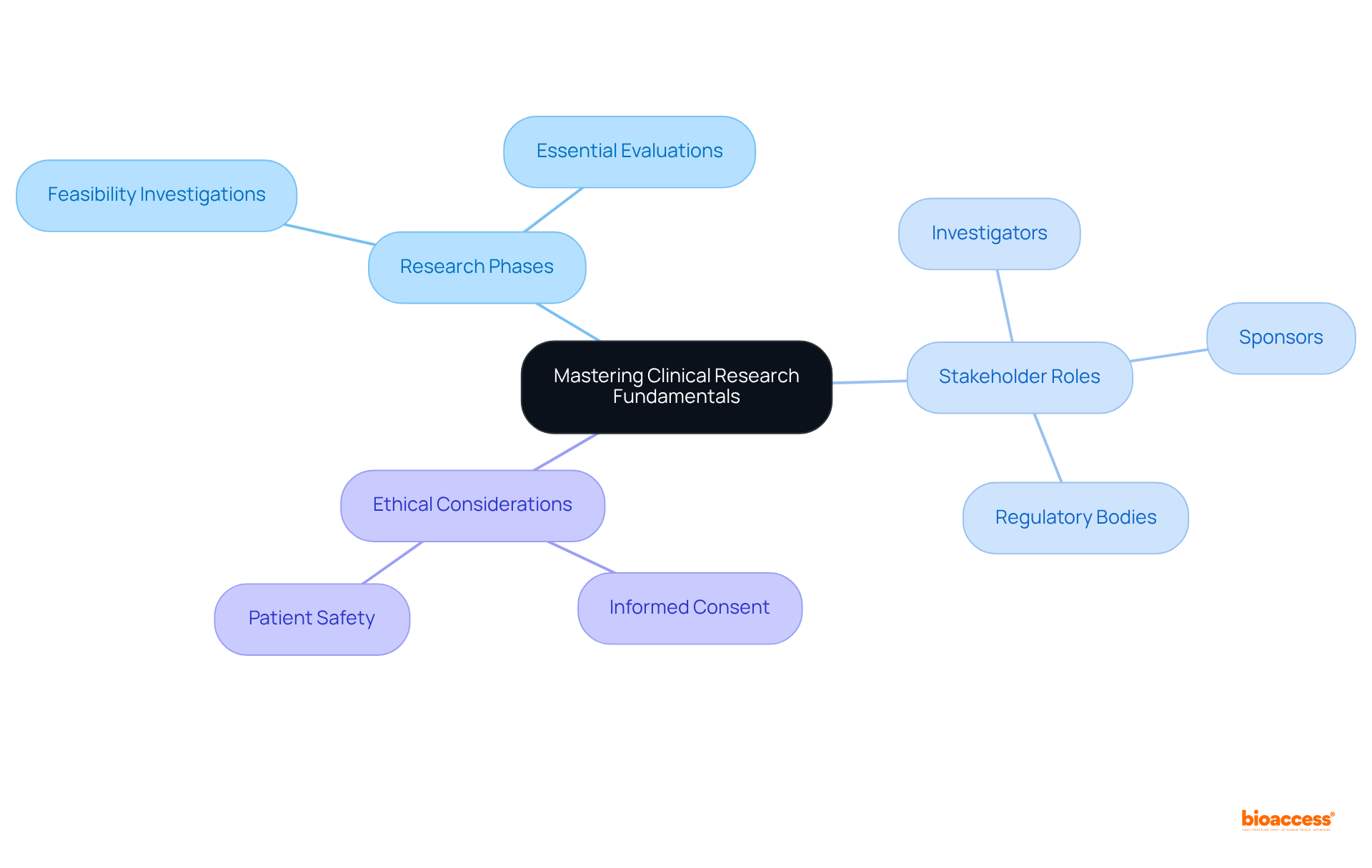
At bioaccess®, we emphasize the mastery of research fundamentals as a cornerstone for success in clinical study assistant jobs. A comprehensive understanding of the stages of medical studies is crucial, as each phase—from initial feasibility investigations to essential evaluations—serves a specific function in the drug development process. For example, knowledge of how to navigate these phases can significantly influence study outcomes; studies reveal that only 36 out of 70 negative trials (51.4%) possessed 80% power to detect a 50% difference in outcome rates.
Furthermore, recognizing the roles of various stakeholders, including investigators, sponsors, and regulatory bodies, is vital for effective collaboration and adherence to protocols and regulations. Ethical considerations, such as informed consent and patient safety, must remain at the forefront of any research endeavor in healthcare.
Effective training programs for clinical study assistant jobs should encompass these fundamentals, equipping them with the skills necessary to support research teams efficiently. By cultivating a robust foundation in research principles, bioaccess® ensures that its team members are well-prepared to contribute to the advancement of medical innovations.
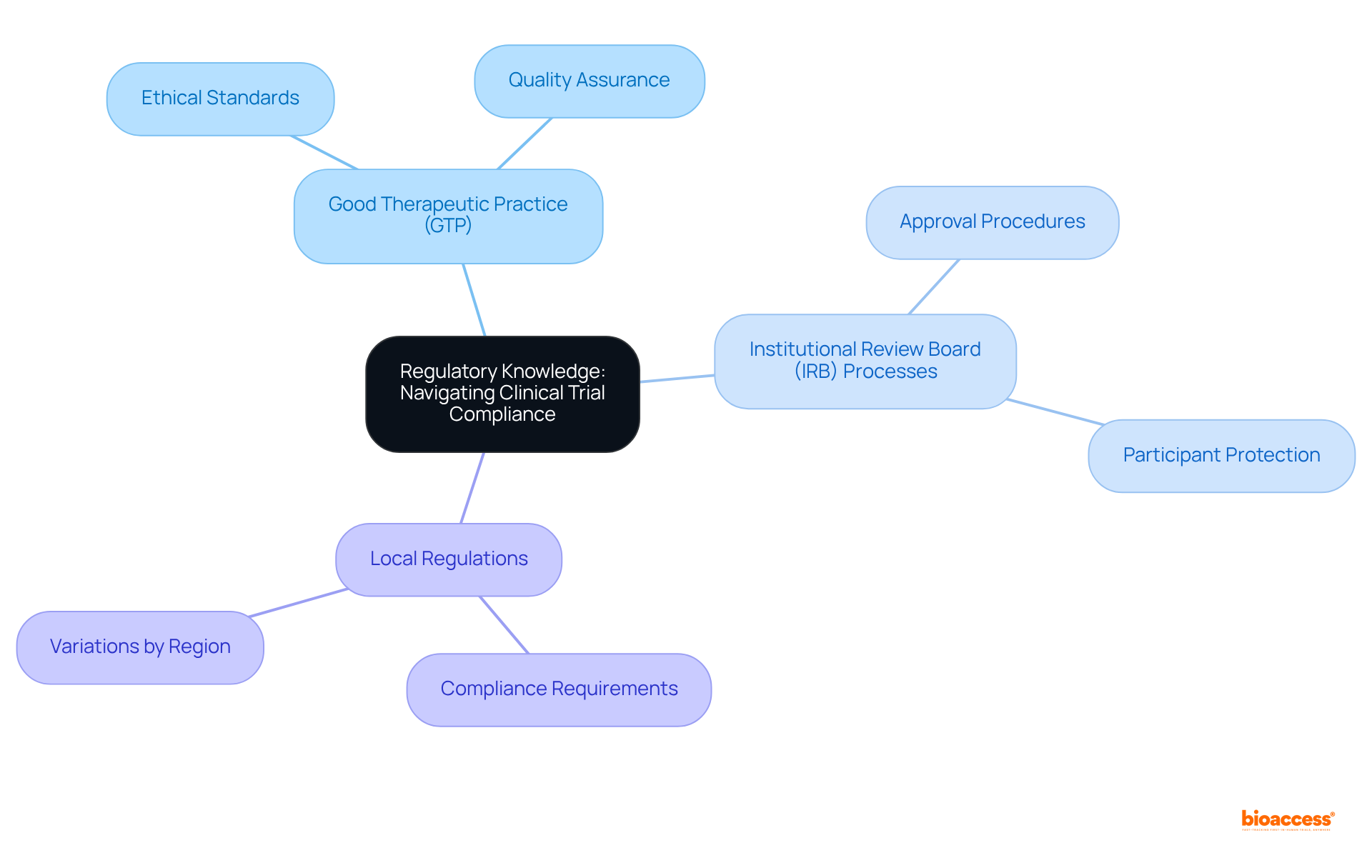
Research Assistants must possess a comprehensive understanding of the regulatory requirements governing clinical trials. This includes a thorough knowledge of:
Familiarity with these regulations is crucial, as it ensures that studies are conducted ethically and legally, thereby protecting both participants and the integrity of the research. By adhering to these standards, Research Assistants not only uphold the principles of ethical research but also contribute to the overall credibility and reliability of clinical studies.
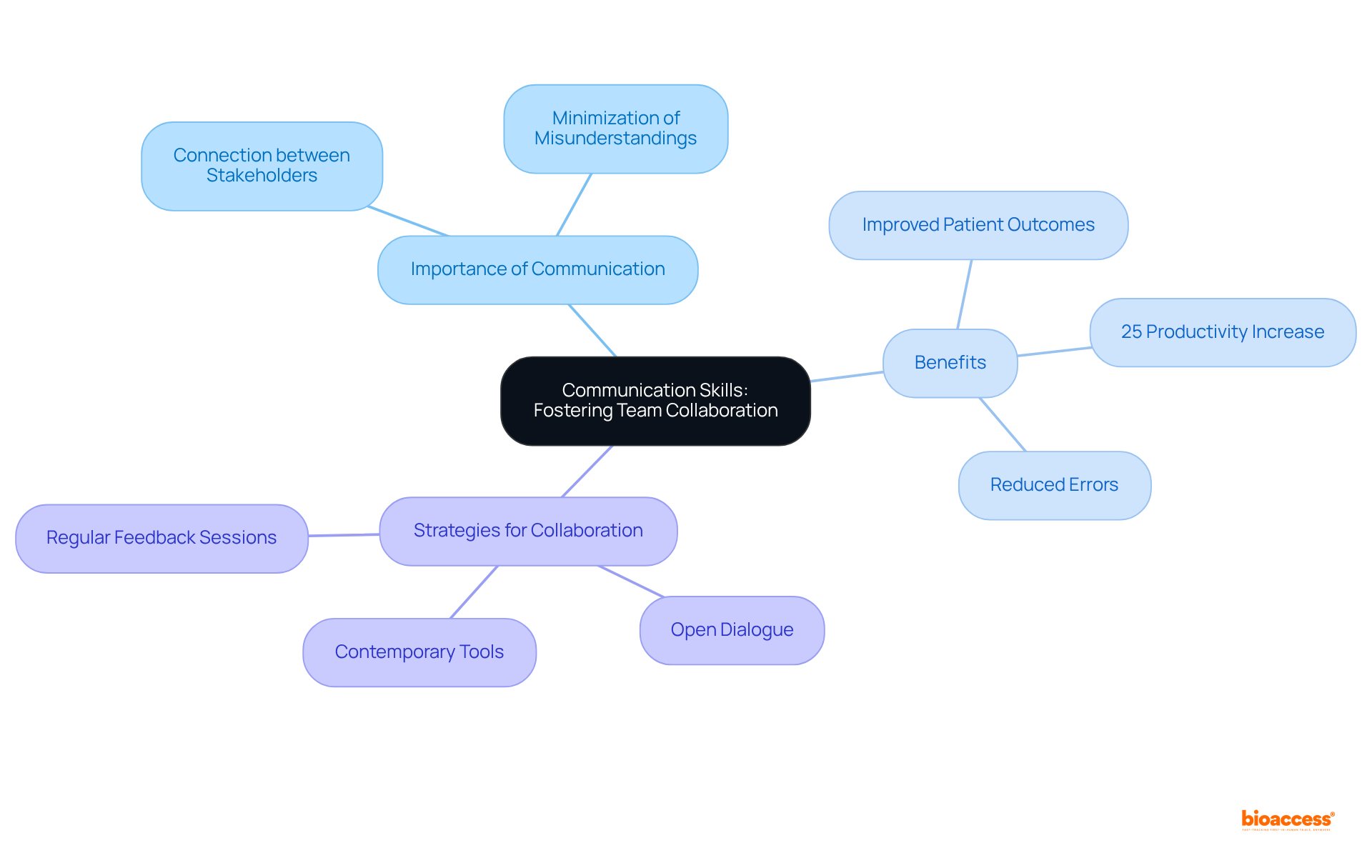
Effective communication abilities are crucial for Research Assistants, who serve as vital connections between researchers, participants, and regulatory agencies. Clear and concise communication minimizes misunderstandings and fosters collaboration within teams. This encompasses both verbal and written communication, alongside the ability to tailor communication styles to suit diverse audiences.
Studies indicate that groups employing effective communication methods can enhance productivity by as much as 25%, significantly influencing success rates in medical studies. Furthermore, effective communication correlates with improved patient outcomes and reduced errors; for instance, 80% of serious medical errors stemmed from miscommunication during patient handovers.
To cultivate a collaborative atmosphere, Research Assistants should prioritize:
By doing so, they can ensure that all stakeholders are aligned, ultimately resulting in more efficient and successful studies.
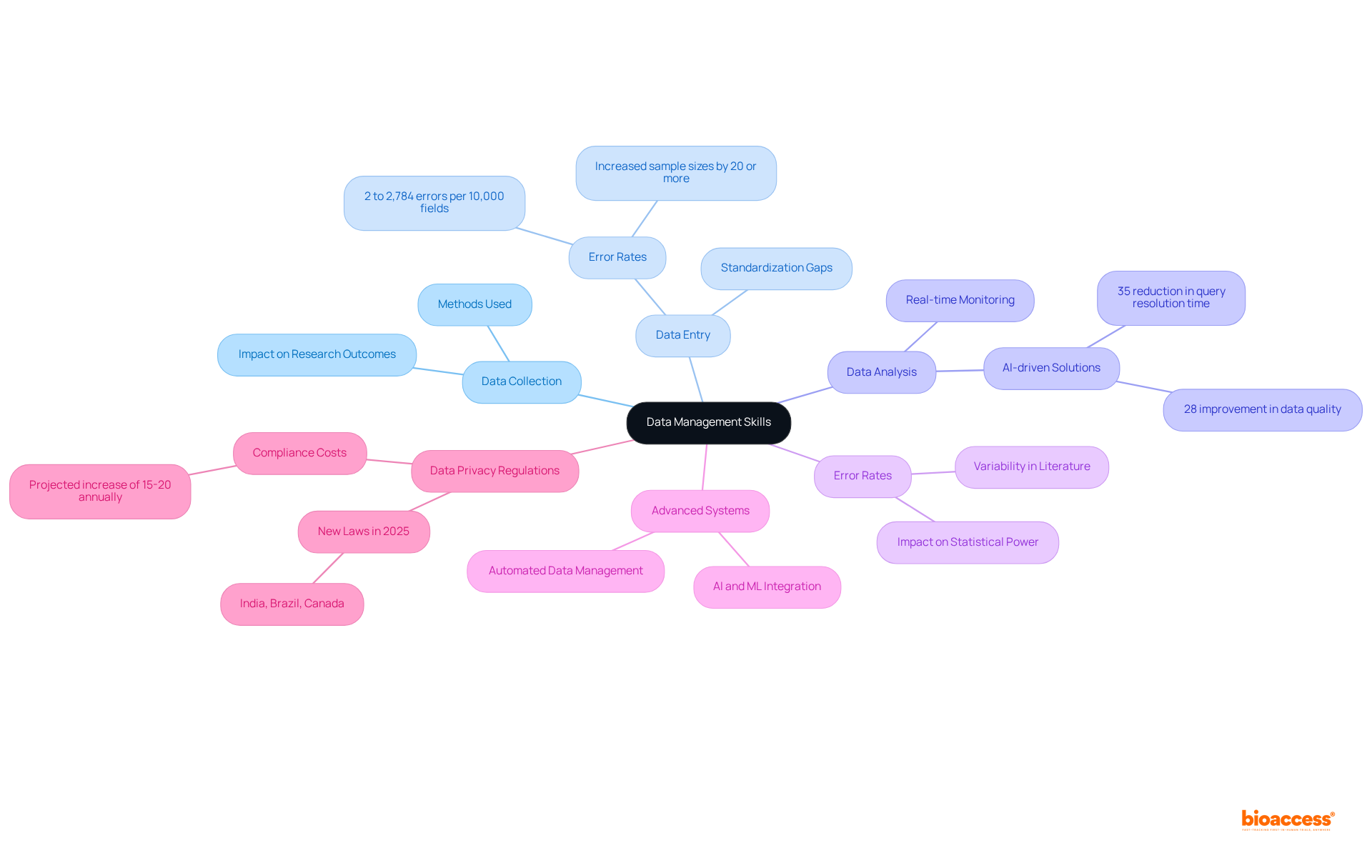
Those pursuing clinical study assistant jobs must excel in data management, which encompasses data collection, entry, and analysis. The precision and integrity of information are essential, as they greatly impact research outcomes. High error rates in data processing can necessitate increases in sample sizes by 20% or more to maintain statistical power, underscoring the need for rigorous data management practices.
Familiarity with advanced data management systems and software, particularly AI-driven solutions, is essential; these tools can enhance overall data quality by up to 28%. Understanding data privacy regulations is vital for maintaining compliance and safeguarding participant information, especially with new laws in 2025 in India, Brazil, and Canada introducing unique data protection requirements.
As the intricacy of research studies continues to rise, effective data integrity practices will be a key differentiator in achieving successful outcomes.
Problem-solving abilities are essential for those in clinical study assistant jobs, as they frequently encounter immediate challenges requiring swift resolution. These challenges encompass:
Notably, 80% of medical studies face delays or terminations due to recruitment issues, costing sponsors approximately 40% of the overall budget. This statistic underscores the financial implications of such obstacles. Developing a proactive approach not only mitigates these issues but also enhances the overall efficiency of clinical research operations, particularly in clinical study assistant jobs.
Successful recruitment strategies, including multimodal approaches that integrate in-person recruitment with social media outreach, have demonstrated effectiveness in achieving enrollment targets, with a remarkable completion rate of 92.3% attributed to social media efforts. As Dr. Man Hung noted, 'A significant enabler of recruitment success was the creation of a multimodal recruitment strategy, strategically planned prior to the study commencing.'
By fostering teamwork and interaction among group members, Research Assistants can significantly improve participant involvement and retention, ultimately leading to more successful research outcomes. Moreover, addressing logistical challenges, such as providing assistance to participants living far from study sites, can further enhance recruitment rates and the overall success of the study.
Research Assistants must exhibit an exceptional level of attention to detail, a critical factor in minimizing errors within investigations. This responsibility encompasses:
A meticulous approach not only upholds the integrity of the study but also ensures compliance with all regulatory requirements. Ultimately, these efforts are vital to the successful execution of clinical trials.
Time management abilities are essential for those in clinical study assistant jobs, who navigate a complex array of responsibilities. These responsibilities for clinical study assistant jobs encompass:
Effective prioritization strategies are crucial; research indicates that implementing time management techniques could potentially recover 20% of work hours. Utilizing organizational tools, such as Trial Management Systems (CTMS), can streamline processes and enhance productivity. For instance, a 2017 Veeva study revealed that data analysis through CTMS improved patient recruitment cycles by 48%, underscoring the significant impact of effective time management on study timelines.
Moreover, inadequate time management can result in stress and burnout, highlighting the necessity for effective practices. By embracing these strategies, Research Assistants can maintain high-quality standards while adhering to timelines, ultimately contributing to the success of research projects.
Collaboration is essential in medical research, as Research Assistants engage with a diverse array of stakeholders, including scientists, sponsors, and regulatory bodies. By cultivating strong relationships and fostering a collaborative environment, communication can be enhanced, and processes streamlined. The significance of collaboration cannot be overstated; it ensures that all group members are aligned in their objectives and responsibilities. This unity ultimately contributes to the project's success, underscoring the necessity for a cohesive approach in clinical research.
Flexibility is a critical competency for clinical study assistant jobs, enabling professionals to adeptly navigate the complexities of research studies. In an environment where study protocols, participant needs, and regulatory requirements can shift unexpectedly, cultivating a flexible mindset is essential.
Adaptive frameworks in medical studies, such as adaptive enrichment approaches and seamless designs, allow for modifications based on interim data. These methodologies facilitate the rapid identification of ineffective therapies and optimize resource allocation, ultimately enhancing the likelihood of FDA approval, which hovers around a mere 10% for medications entering testing phases.
By embracing adaptability, we foster an environment where challenges are met with innovative solutions, exemplified by bioaccess® in early-phase studies, propelling the advancement of medical technologies and therapies.
Upholding ethical considerations is paramount for Clinical Study Assistants, who are instrumental in ensuring that studies are conducted with integrity. This responsibility encompasses:
A robust ethical foundation not only fosters trust among participants and stakeholders but also significantly enhances the credibility and success of clinical trials.
Mastering the essential skills required for clinical study assistant jobs is paramount for success in the dynamic field of clinical research. From regulatory knowledge to effective communication, each competency significantly contributes to the efficient and ethical conduct of studies. By honing these skills, individuals not only enhance their career prospects but also make meaningful contributions to the advancement of medical research.
This article highlights ten fundamental skills that are indispensable for clinical study assistants. Key areas such as:
are emphasized, demonstrating their direct impact on the integrity and outcomes of clinical trials. Moreover, the importance of teamwork and ethical considerations cannot be overstated, as they underpin the collaborative nature of research and the trust placed in study protocols.
As the landscape of clinical research continues to evolve, embracing these essential skills is not merely beneficial but necessary. By investing in training and development in these areas, aspiring clinical study assistants can position themselves as invaluable assets to research teams, ultimately driving innovation and improving patient outcomes in the healthcare sector.
What is the importance of mastering clinical research fundamentals at bioaccess®?
Mastering clinical research fundamentals is crucial for success in clinical study assistant jobs, as it provides a comprehensive understanding of the stages of medical studies, which significantly influences study outcomes.
What are the stages of medical studies that are emphasized at bioaccess®?
The stages of medical studies include initial feasibility investigations and essential evaluations, each serving a specific function in the drug development process.
Why is it important to recognize the roles of various stakeholders in clinical research?
Recognizing the roles of stakeholders, such as investigators, sponsors, and regulatory bodies, is vital for effective collaboration and adherence to protocols and regulations.
What ethical considerations are highlighted in clinical research?
Ethical considerations include informed consent and patient safety, which must remain a priority in any healthcare research endeavor.
What should effective training programs for clinical study assistants include?
Effective training programs should encompass the fundamentals of clinical research, equipping assistants with the necessary skills to support research teams efficiently.
What regulatory knowledge is essential for Research Assistants in clinical trials?
Research Assistants must understand Good Therapeutic Practice (GTP), Institutional Review Board (IRB) processes, and local regulations to ensure ethical and legal conduct in studies.
How do effective communication skills impact Research Assistants?
Effective communication skills are crucial for Research Assistants as they connect researchers, participants, and regulatory agencies, minimizing misunderstandings and fostering collaboration.
What are some benefits of effective communication in clinical research?
Effective communication can enhance productivity by up to 25%, improve patient outcomes, and reduce errors, as a significant portion of serious medical errors arises from miscommunication.
What practices should Research Assistants prioritize to cultivate a collaborative atmosphere?
Research Assistants should prioritize open dialogue, utilize contemporary communication tools, and engage in regular feedback sessions to ensure alignment among all stakeholders.