


The article titled "10 Essential Statistics for Clinical Research Directors" focuses on crucial statistical insights essential for clinical research directors to effectively manage and analyze clinical trials. It is evident that the content will delve into key statistical methods and their applications within clinical research. Furthermore, it will explore how these statistics influence study design, patient enrollment, and regulatory compliance. By addressing these aspects, the article aims to enhance the overall effectiveness and reliability of clinical trials, underscoring the importance of data-driven decision-making in this field.
In the intricate world of clinical research, where innovation meets regulation, the role of statistics emerges as a cornerstone for success. For clinical research directors, mastering essential statistics not only enhances study design but also ensures compliance and accelerates the path to groundbreaking treatments. However, as the landscape evolves, how can these leaders effectively navigate the complex interplay of biostatistics, ethical approvals, and trial methodologies to optimize their research outcomes? This article delves into ten critical statistics that empower clinical research directors to elevate their studies and make informed decisions in an increasingly competitive environment.
bioaccess® expertly navigates the regulatory landscape across Latin America, the Balkans, and Australia, achieving ethical approvals in an impressive 4-6 weeks. This expedited procedure enables medical study leaders to initiate studies more quickly, thereby accelerating the path to market for innovative treatments. By optimizing the approval workflow, bioaccess® allows researchers to concentrate on advancing medical knowledge and enhancing patient care.
In contrast to traditional approval timelines that can extend for months, bioaccess® has consistently demonstrated its capability to secure ethical approvals rapidly. This efficiency not only simplifies medical studies but also significantly enhances their overall effectiveness. For instance, organizations collaborating with bioaccess® have reported substantial reductions in time to patient enrollment, with some achieving enrollment procedures that are 50% faster than conventional markets. Such advancements underscore the essential role of rapid ethical approvals in improving the operational effectiveness of medical studies.

Biostatistics plays a pivotal role in the planning of medical studies, ensuring that the statistics for clinical research are not only statistically sound but also compliant with regulatory standards. It encompasses critical tasks such as determining sample sizes, defining endpoints, and selecting appropriate statistical methods for data analysis, all of which involve statistics for clinical research. For research leaders, a robust understanding of biostatistics is vital for making informed decisions that significantly impact study outcomes.
In early-stage clinical studies, particularly for Medtech and Biopharma startups, overcoming patient recruitment challenges is paramount. Many potential patients may be hesitant, confronted with numerous options, or deemed ineligible, rendering swift recruitment nearly unfeasible. Within this framework, statistics for clinical research empower researchers to determine the minimum sample size needed to detect a treatment effect, optimizing resource allocation and ensuring that studies are adequately powered to yield valid results.
Phase I studies, typically involving 20 to 80 healthy participants and extending up to one year, benefit from biostatistics in evaluating safety profiles. Phase II studies, which include 100 to 300 patients, focus on both efficacy and safety, while Phase III studies, comprising at least 1,000 patients and lasting from one to four years, are designed to provide regulatory proof of a drug's safety and efficacy. By utilizing statistics for clinical research at each of these stages, research directors can enhance the reliability of their investigations and facilitate successful regulatory submissions.
Moreover, bioaccess offers comprehensive management services for research studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. This holistic approach not only aids in patient recruitment but also ensures that studies adhere to essential regulatory frameworks. Additionally, biostatistics is crucial in post-market surveillance, monitoring drugs post-approval to identify unexpected side effects or long-term effects. This continuous analysis is vital for maintaining compliance and safety in drug development. To effectively harness these insights, study leaders should prioritize the integration of biostatistical techniques and utilize statistics for clinical research in their designs, while also considering ongoing statistical assessments even after market approval.

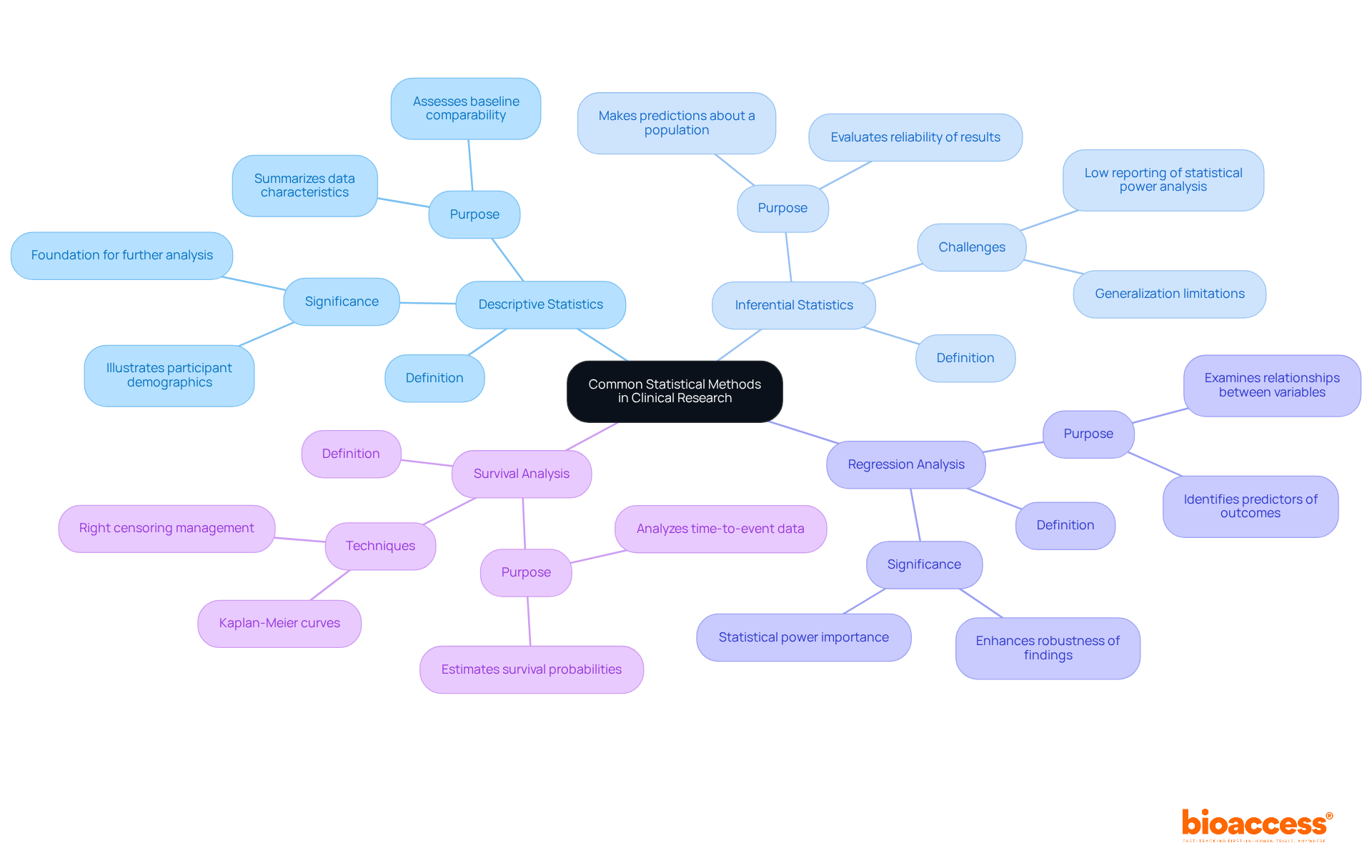
Common statistical techniques used in statistics for clinical research are crucial for obtaining valuable insights from study data. Key techniques include:
Descriptive Statistics: These methods summarize data characteristics, offering a clear picture of the sample population. They are essential for understanding participant demographics and treatment outcomes, with descriptive statistics often forming the foundation for further analysis. A case analysis on 'Descriptive Statistics for Single Variables' illustrates how these statistics are applied to assess baseline comparability in trials.
Inferential Statistics: This approach enables researchers to make predictions or inferences about a broader population based on sample data. It is vital for generalizing findings and evaluating the reliability of results, especially in research with limited sample sizes. Notably, only 3 out of 49 papers (6.1%) in British orthopedic journals reported a statistical power analysis, highlighting the challenges in statistical reporting.
Regression Analysis: This technique examines relationships between variables, identifying potential predictors of outcomes. It allows researchers to understand how different factors influence treatment effects, enhancing the robustness of clinical findings. As Howard Barkan observes, 'The likelihood of research producing a statistically significant outcome if its hypothesis is supported is referred to as its statistical power,' underscoring the significance of sufficient power in research design.
Survival Analysis: Concentrated on time-to-event data, survival analysis is vital for research involving patient survival rates. Techniques such as Kaplan-Meier curves help estimate the probability of survival over time, providing valuable insights into treatment efficacy. A case study on 'Survival Analysis and Right Censoring' discusses the importance of these techniques in accurately estimating time to events in studies with right-censored data.
Grasping these statistical techniques is essential for directors in the medical field, as they aid in the efficient analysis of results and bolster data-informed decision-making using statistics for clinical research. As Howard Barkan emphasizes, 'Statistical analysis is one of the foundations of evidence-based clinical practice,' reinforcing the importance of rigorous statistical methods in promoting clinical studies.
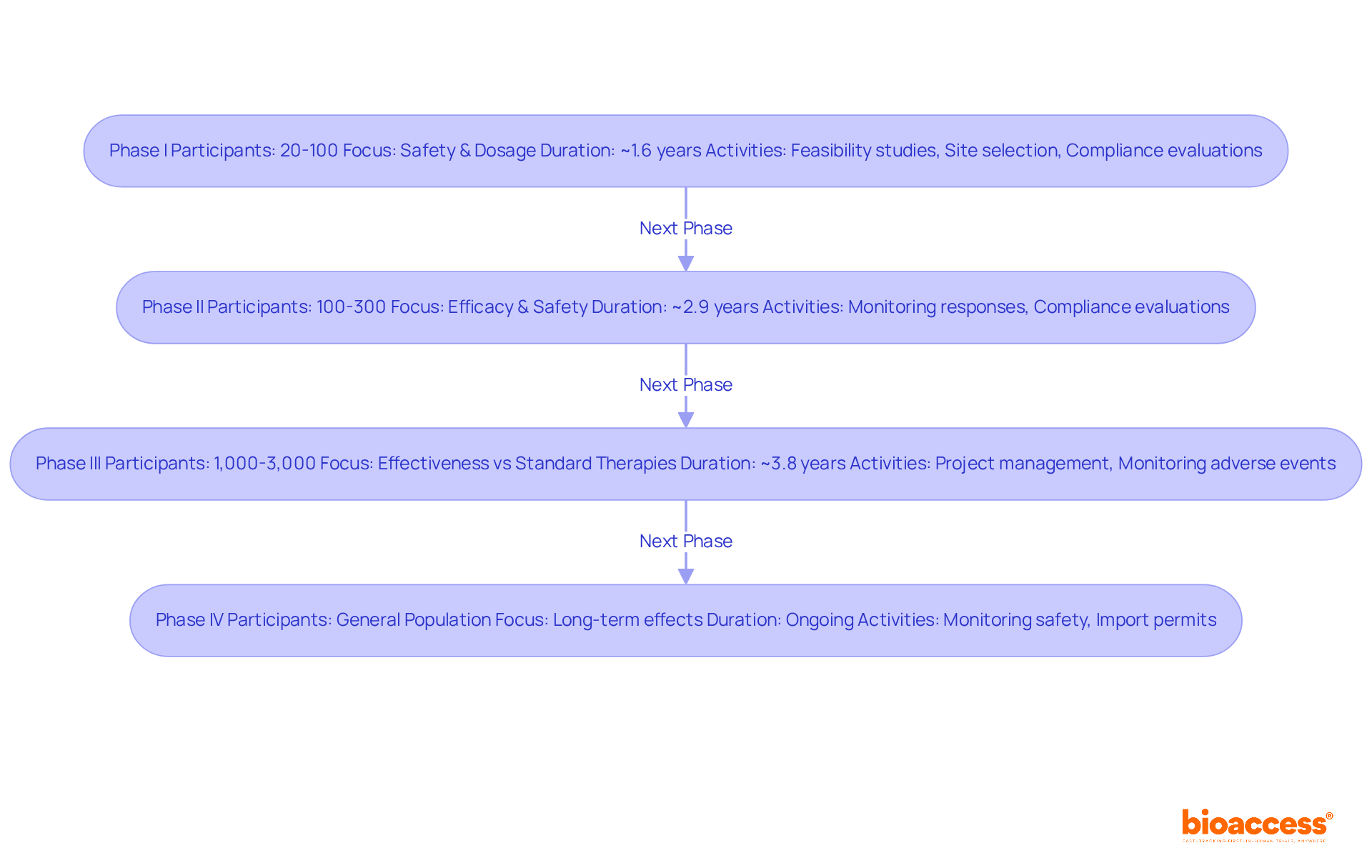
Clinical trials are systematically categorized into four distinct phases, each serving a critical role in the research lifecycle.
Phase I: This initial phase focuses on safety and dosage, typically involving 20 to 100 participants. It aims to assess the treatment's safety profile and determine the appropriate dosage. The typical length for Phase I studies is roughly 1.6 years. Comprehensive feasibility studies and site selection are essential at this stage to guarantee the appropriate principal investigator (PI) is selected, greatly influencing the success of the research. Furthermore, experiment setup and compliance evaluations are crucial to satisfy regulatory requirements.
Phase II: In this phase, the treatment's efficacy is tested on a larger group, usually ranging from 100 to 300 participants. This phase further evaluates safety and side effects, with an average duration of about 2.9 years. Insights from clinical research directors indicate that managing this phase requires careful monitoring of participant responses to ensure accurate data collection. Compliance evaluations and input on study documents are crucial to enable seamless setup and guarantee conformity with national requirements.
Phase III: This phase involves a larger population, often 1,000 to 3,000 participants, and compares the new treatment against standard therapies to confirm its effectiveness. The typical length for Phase III studies is about 3.8 years, with a completion rate of roughly 84.9%. Successful examples include oncology studies, which, despite having a low overall success rate of 3.4%, have led to significant advancements in cancer treatment. Comprehensive project management and monitoring during this phase are vital to track study status and manage adverse events effectively, including reporting serious and non-serious adverse events.
Phase IV: Conducted post-approval, Phase IV studies monitor long-term effects and effectiveness in the general population. These experiments are essential for comprehending the treatment's effect over time and ensuring continuous safety. The completion rate for Phase IV studies is approximately 87.2%. The capability to obtain import permits and nationalize investigational devices can improve the effectiveness of these studies, aiding healthcare advancement and economic development in local communities.
Grasping these stages equips study directors with the insight to handle the intricacies of management in statistics for clinical research, guaranteeing adherence to regulatory requirements and enhancing the likelihood of favorable results. Furthermore, it is crucial to highlight that the typical duration for creating a new medication is projected to be between 10 to 15 years. As of May 2023, 31% of registered research studies are carried out solely within the US, while 53% take place outside the US. Moreover, 56% of sites indicated that trials are more intricate than they were three years prior, emphasizing the evolving challenges in medical studies.
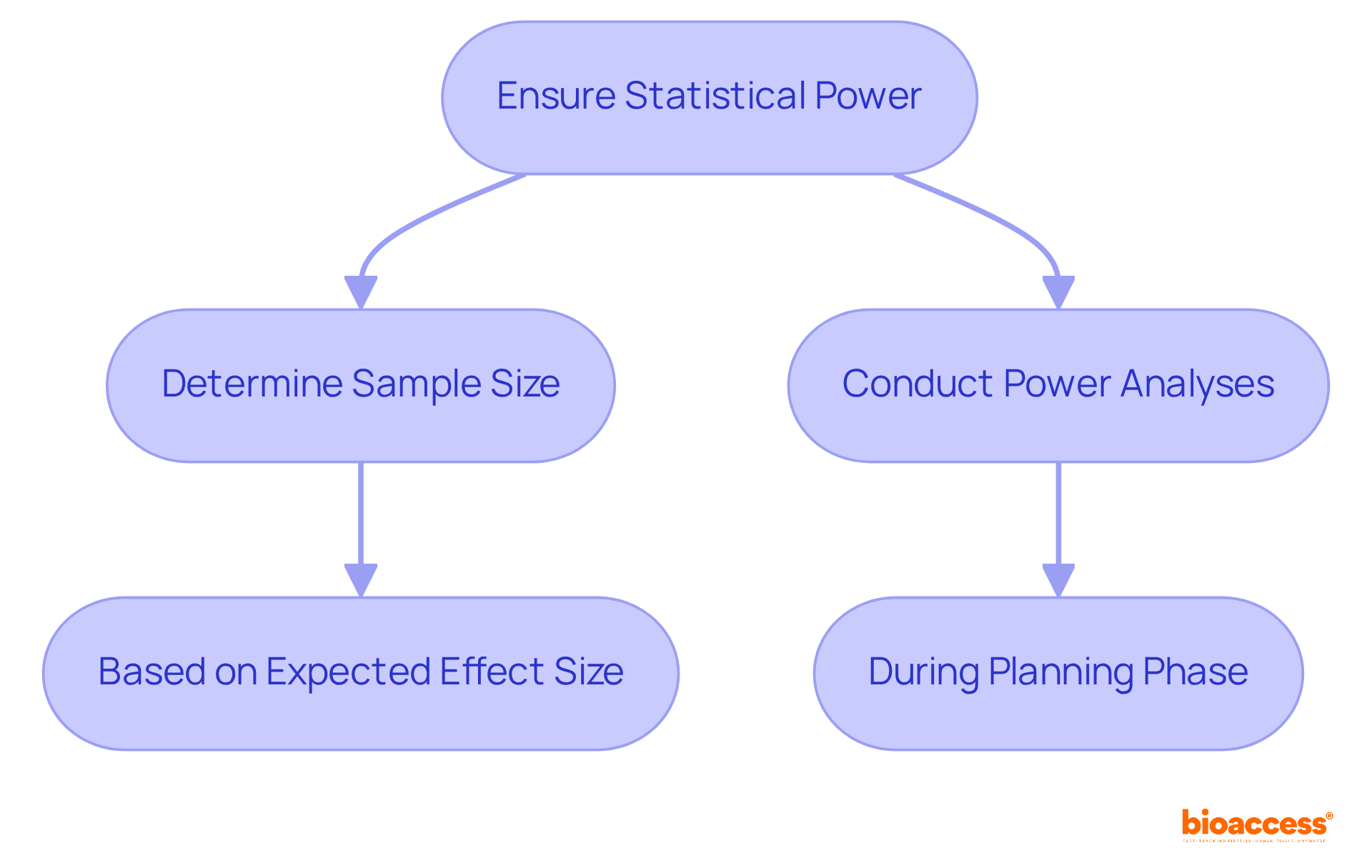
Statistical power represents the likelihood that a research effort will accurately dismiss a null hypothesis when it is indeed untrue. A higher power significantly reduces the risk of Type II errors, which occur when an existing effect goes undetected. To ensure adequate power, clinical research directors must take decisive action:
For instance, consider a study with low power; it may fail to identify a significant treatment effect, ultimately leading to incorrect conclusions and wasted resources. This highlights the critical importance of understanding and applying statistics for clinical research in order to ensure statistical power.
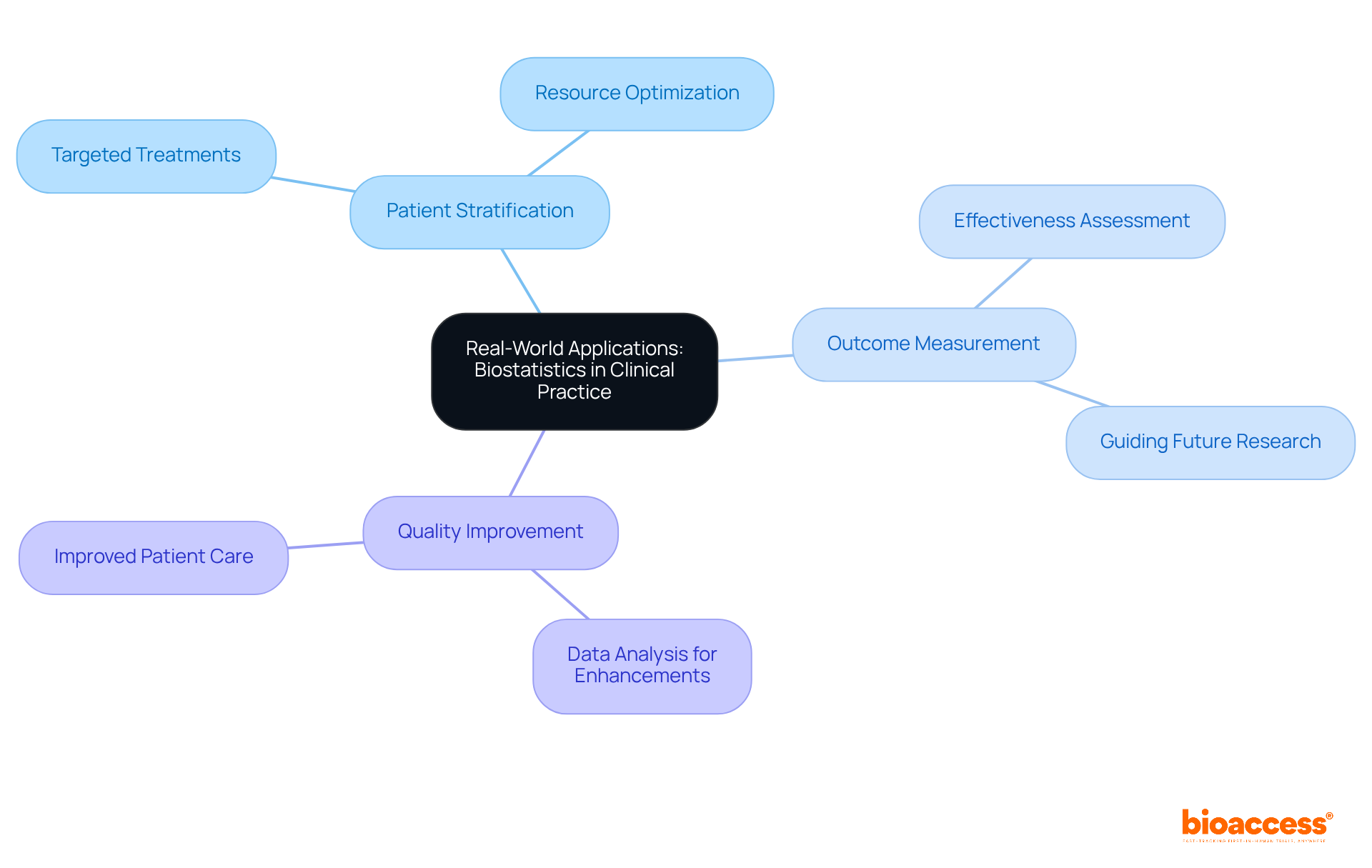
Statistics for clinical research serves as a vital link between investigation and medical practice, providing practical applications that enhance patient care. Key areas include:
The demand for skilled data experts is escalating at a rate of 36%, highlighting the increasing importance of statistics for clinical research in health studies. Furthermore, companies are incurring losses of approximately $5.2 million in revenue due to untapped data, highlighting the financial ramifications of ineffective data utilization in medical research. Understanding these applications allows healthcare leaders to align their initiatives more closely with real-world needs, ultimately resulting in better patient outcomes and more effective healthcare solutions.
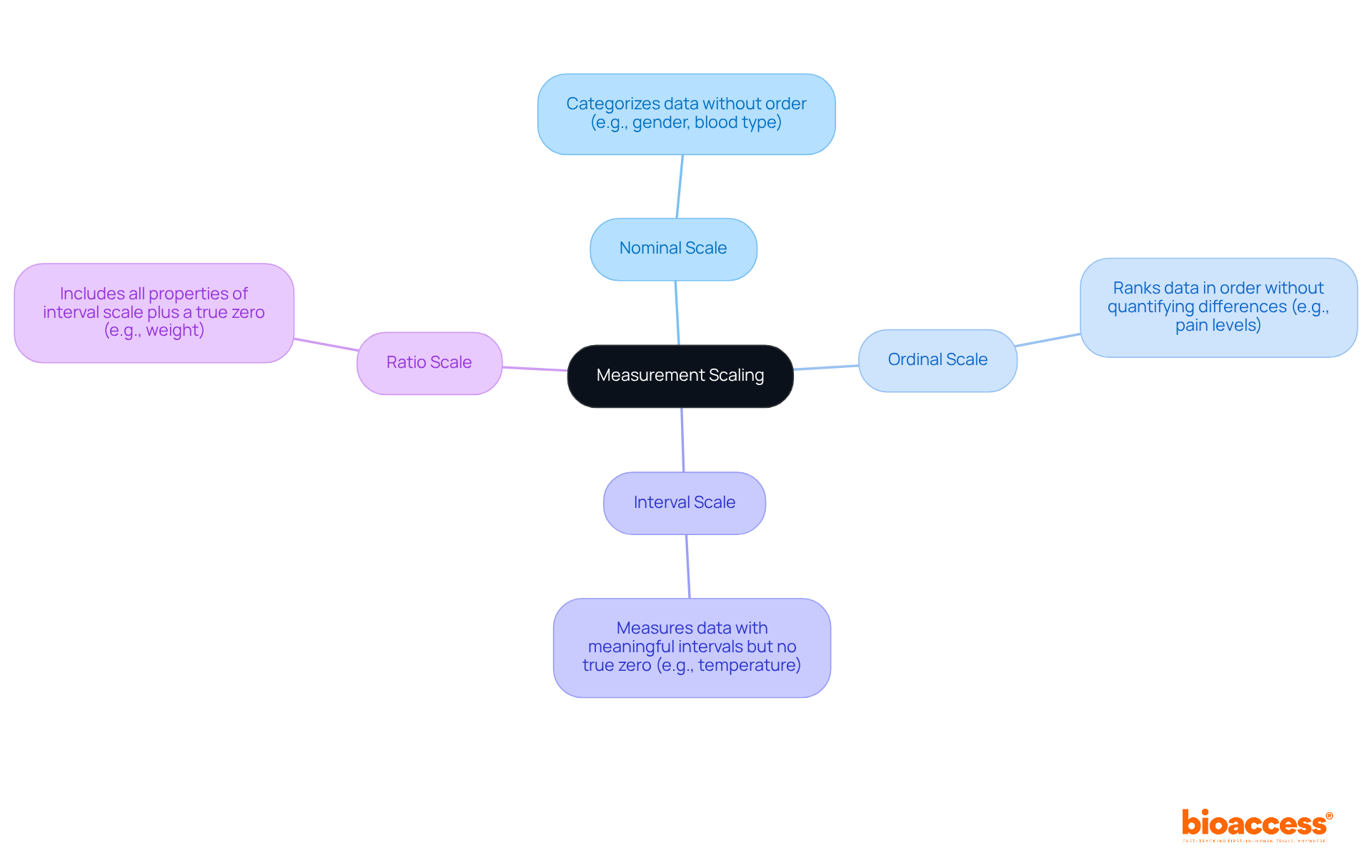
Measurement scaling is a fundamental aspect of data analysis, as it involves categorizing data into distinct levels. Understanding these levels is essential for clinical research, as it ensures that statistics for clinical research are analyzed accurately and effectively. The primary types of measurement scales include:
Grasping these measurement scales empowers clinical study directors to select appropriate statistical techniques for statistics for clinical research, thereby ensuring the precise interpretation of results.
Clinical trials are categorized into distinct types, each with unique methodologies that cater to specific research objectives.
Comprehending these trial types is vital for directors of studies, particularly as bioaccess® focuses on overseeing a variety of medical device trials, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). By identifying the specific methodologies and services provided by bioaccess®, study directors can make informed choices about the most appropriate design for their inquiries, ultimately resulting in more effective and dependable outcomes.

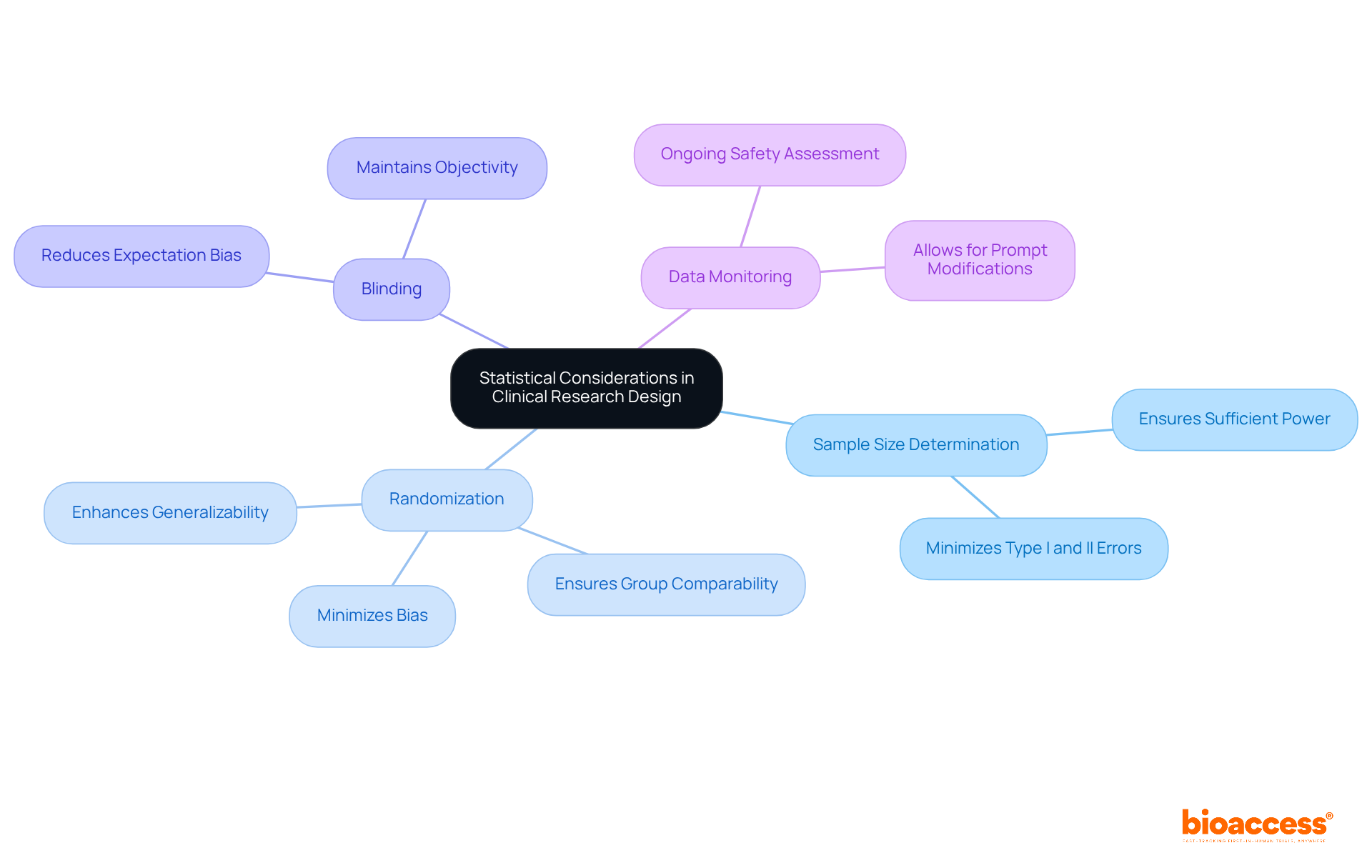
In clinical research design, several statistical considerations are paramount for ensuring robust and reliable outcomes:
Sample Size Determination: It is essential to calculate an adequate sample size to ensure the study is sufficiently powered to detect meaningful effects. A well-defined sample size minimizes the risk of Type I and Type II errors, thereby enhancing the credibility of the findings.
Randomization: Randomly assigning participants to treatment groups is crucial for minimizing bias. Effective randomization helps ensure that the groups are comparable, which is vital for the integrity of the study results. Successful instances of randomization can be observed in studies where varied populations were included, resulting in more generalizable outcomes.
Blinding: Implementing blinding—where participants and researchers are unaware of group assignments—further reduces bias. This practice is essential in maintaining objectivity and ensuring that the outcomes are not influenced by expectations. Significant clinical studies have shown the effectiveness of blinding in obtaining impartial results.
Data Monitoring: Conducting interim analyses allows for ongoing assessment of safety and efficacy throughout the trial. This proactive strategy can result in prompt modifications, ensuring participant safety and enhancing research design.
By carefully addressing these statistical factors and applying statistics for clinical research, trial directors can significantly enhance the validity and reliability of their studies, paving the way for impactful medical advancements.
To remain competitive in the rapidly evolving field of clinical research, directors must leverage a variety of resources.
By engaging with these resources, clinical research directors can significantly enhance their knowledge and skills, ensuring they remain at the forefront of the field.

Understanding the essential statistics for clinical research directors is crucial for navigating the complexities of medical studies. This article highlights the significance of rapid ethical approvals, the role of biostatistics in study design, and the importance of statistical methods in ensuring valid and reliable outcomes. By integrating these elements, research directors can enhance their operational efficiency and improve patient care.
Key points discussed include the expedited ethical approval process facilitated by bioaccess®, which significantly shortens the timeline for initiating studies. The article underscores the critical application of biostatistics throughout various phases of clinical trials, from sample size determination to post-market surveillance. Furthermore, it emphasizes the necessity of employing robust statistical techniques, such as regression and survival analysis, to draw meaningful conclusions from study data.
Ultimately, the insights presented serve as a call to action for clinical research directors to embrace continuous learning and stay updated on the latest trends and methodologies in the field. By prioritizing ethical considerations, biostatistical rigor, and effective research design, directors can not only advance medical knowledge but also contribute to better healthcare outcomes. Engaging with professional organizations, online courses, and industry publications will further empower these leaders to navigate the evolving landscape of clinical research effectively.
What is bioaccess and what role does it play in clinical research?
Bioaccess is a service that navigates the regulatory landscape across Latin America, the Balkans, and Australia, achieving ethical approvals for medical studies in 4-6 weeks. This expedited process allows study leaders to initiate research more quickly, accelerating the path to market for innovative treatments.
How does bioaccess improve the efficiency of medical studies?
Bioaccess optimizes the approval workflow, enabling researchers to focus on advancing medical knowledge and enhancing patient care. Organizations working with bioaccess have reported significant reductions in patient enrollment time, with some achieving processes that are 50% faster than conventional markets.
Why is biostatistics important for clinical trials?
Biostatistics is crucial for planning medical studies as it ensures that the statistical methods used are sound and compliant with regulatory standards. It involves determining sample sizes, defining endpoints, and selecting appropriate statistical methods, all of which are essential for making informed decisions that affect study outcomes.
What challenges do early-stage clinical studies face regarding patient recruitment?
Early-stage clinical studies often encounter challenges such as potential patients being hesitant, overwhelmed with options, or deemed ineligible, which can impede swift recruitment. Biostatistics helps researchers determine the minimum sample size needed to detect treatment effects, optimizing resource allocation.
What are the different phases of clinical studies and how does biostatistics apply to them?
Phase I studies involve 20 to 80 healthy participants and focus on safety profiles. Phase II studies include 100 to 300 patients, concentrating on both efficacy and safety. Phase III studies involve at least 1,000 patients and provide regulatory proof of a drug's safety and efficacy. Biostatistics enhances the reliability of findings at each phase and facilitates successful regulatory submissions.
What comprehensive management services does bioaccess provide for research studies?
Bioaccess offers services including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, which aid in patient recruitment and ensure adherence to regulatory frameworks.
What statistical methods are commonly used in clinical research?
Common statistical methods include: Descriptive Statistics: Summarizes data characteristics to understand participant demographics and treatment outcomes. Inferential Statistics: Allows predictions about a broader population based on sample data. Regression Analysis: Examines relationships between variables to identify predictors of outcomes. Survival Analysis: Focuses on time-to-event data, crucial for estimating patient survival rates.
Why is understanding statistical techniques essential for medical directors?
Grasping statistical techniques is vital for efficient analysis of results and informed decision-making in clinical research. Rigorous statistical methods support evidence-based clinical practice, enhancing the credibility and reliability of study outcomes.