


The article underscores ten essential statistics pivotal for achieving success in clinical research, asserting the critical role of methodologies such as descriptive statistics, inferential statistics, and ethical considerations. These statistics not only enhance study design but also ensure diverse representation and uphold integrity. Ultimately, they lead to reliable outcomes and informed medical decision-making, reinforcing the necessity of a robust statistical foundation in the clinical research landscape.
In the intricate realm of clinical research, statistics are the cornerstone of successful outcomes, intricately woven into the fabric of medical advancements. By leveraging critical statistical insights, organizations like bioaccess® not only streamline processes but also guarantee that studies produce reliable data essential for patient care.
As the demand for diverse and representative research escalates, researchers face the pressing question: how can they effectively navigate the challenges of bias and data integrity while maximizing the impact of their findings?
This article explores ten pivotal statistics vital for achieving clinical research success, providing valuable perspectives on methodologies that can transform trials into credible sources of medical knowledge.
bioaccess® stands at the forefront of medical research, leveraging essential statistics clinical research to streamline processes and enhance outcomes. By incorporating advanced statistical techniques, bioaccess® accelerates the approval and enrollment stages in statistics clinical research, ensuring that studies are not only efficient but also yield trustworthy data that propels medical progress. Their unique approach merges regulatory speed with diverse patient pools, establishing them as a leader in the Medtech, Biopharma, and Radiopharma sectors.
On March 29, 2019, bioaccess® forged a collaboration with Caribbean Health Group to position Barranquilla as a premier site for medical studies in Latin America, a strategic decision endorsed by Colombia's Minister of Health. This partnership, alongside GlobalCare Clinical Trials, has led to over a 50% reduction in recruitment time and impressive retention rates of 95%. Insights from Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, further underscore the positive impact of bioaccess® during its initial human testing in Colombia, showcasing the effectiveness of their statistics clinical research methodologies in real-world applications.
Statistics clinical research are pivotal in summarizing essential characteristics of the patient population, such as age, gender, and health status. This information is vital for contextualizing medical trials and ensuring that the sample accurately reflects the broader population. For instance, from 2014 to 2021, women represented an average of 51% of participants in FDA-approved medications, and at least 50% of participants in studies funded by the National Institute of Child Health and Human Development were women throughout all reporting years. This underscores the importance of gender representation in research. By analyzing these demographics, researchers can pinpoint potential biases and refine their methodologies, ultimately leading to more accurate and generalizable results.
As we look ahead to 2025, the evolution of medical research will place a premium on descriptive statistics. Current trends reveal that:
This highlights the necessity for targeted research that addresses specific health conditions. Furthermore, the exclusion of certain demographics, such as expectant individuals, from research studies can compromise the relevance of findings. Notably, 68% of NIH-funded actively recruiting investigations specifically excluded pregnant women.
By employing statistics clinical research, researchers can enhance trial design, ensuring that diverse patient populations are adequately represented. This approach not only bolsters the validity of findings but also addresses health inequalities, which are projected to impose over $5 trillion in diabetes-related costs on society by 2050. Ultimately, the effective application of statistics clinical research is essential for advancing medical research and achieving equitable health outcomes.

Inferential statistics empower researchers to make predictions and generalizations about a population based on sample data, a critical aspect in the realm of experiments overseen by bioaccess®. Techniques such as hypothesis testing and confidence intervals play a pivotal role in determining the likelihood that observed effects arise from chance. By employing these methodologies, clinical researchers can rigorously evaluate the effectiveness of new treatments through statistics in clinical research, particularly in initiatives like:
This statistical rigor not only supports informed decision-making regarding the potential impact of new medical devices on patient care but also ensures that trial outcomes are both reliable and actionable.
Statistical power is the probability of correctly rejecting the null hypothesis when it is false. In the realm of clinical research, a well-structured study should strive for a power of at least 80% according to statistics clinical research. Achieving this level of power typically requires careful consideration of sample size.
By conducting power analyses, researchers can ascertain the minimum number of participants necessary to detect a meaningful effect. This approach not only enhances the reliability of their findings in statistics clinical research but also minimizes the risk of Type II errors, thereby reinforcing the integrity of the research process.
P-values serve as a critical measure of the probability that observed results occurred by chance under the null hypothesis. A p-value below 0.05 is widely regarded as statistically significant, indicating that the results are unlikely to stem from random variation.
However, it is crucial to interpret p-values within the context of the research design and other statistics clinical research measures. They do not convey information regarding the magnitude or practical significance of the findings, which are essential for comprehensive understanding in statistics clinical research.
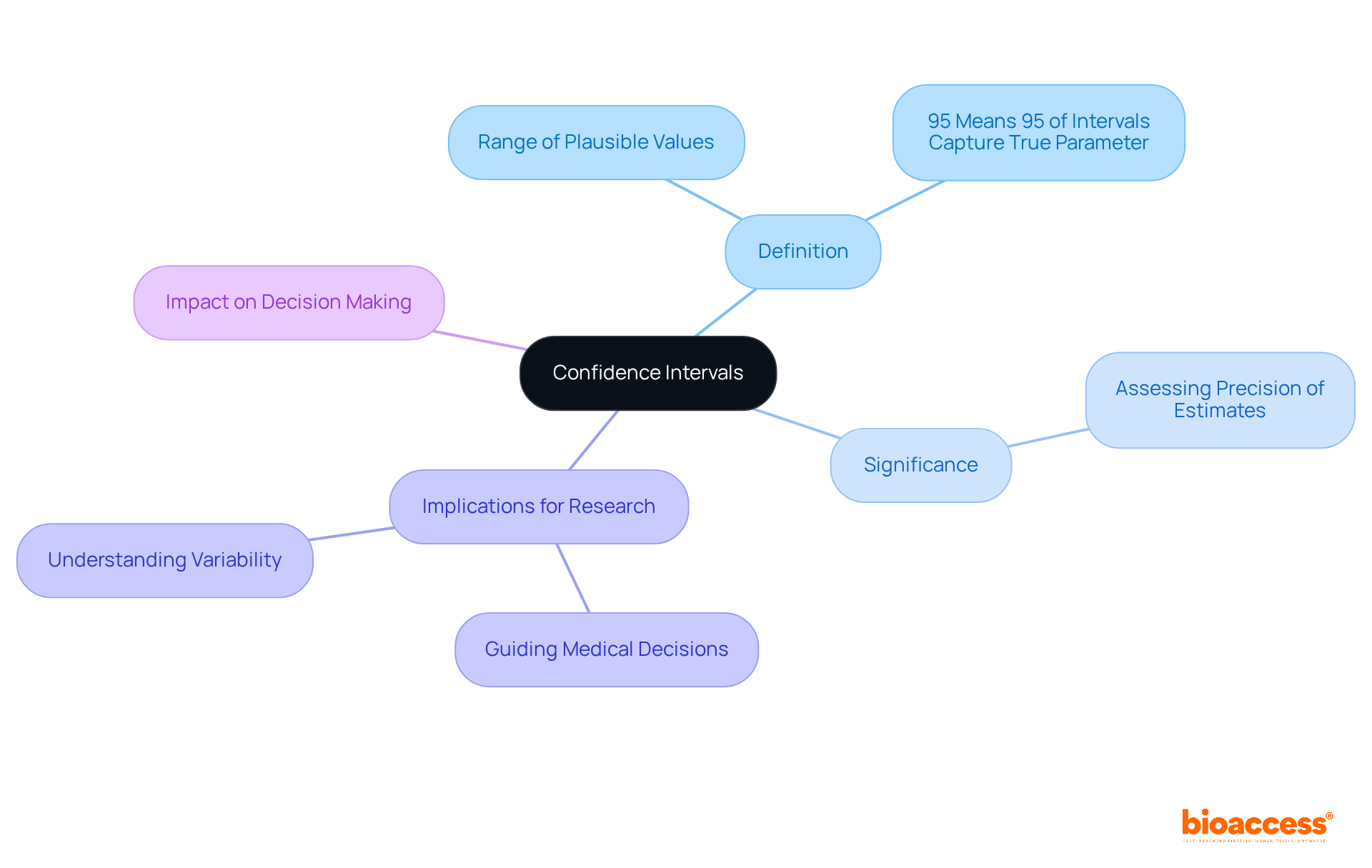
Confidence intervals (CIs) are essential in clinical research, offering a range of plausible values for a population parameter and providing critical insight into the precision of an estimate. For instance, a 95% CI indicates that if the study were to be repeated numerous times, 95% of the calculated intervals would encompass the true parameter. This statistical tool is crucial for interpreting the reliability of results in statistics clinical research and understanding the potential variability in treatment effects, significantly guiding medical decision-making. By leveraging CIs, researchers can better navigate the complexities of clinical data, ensuring informed choices that enhance patient outcomes.
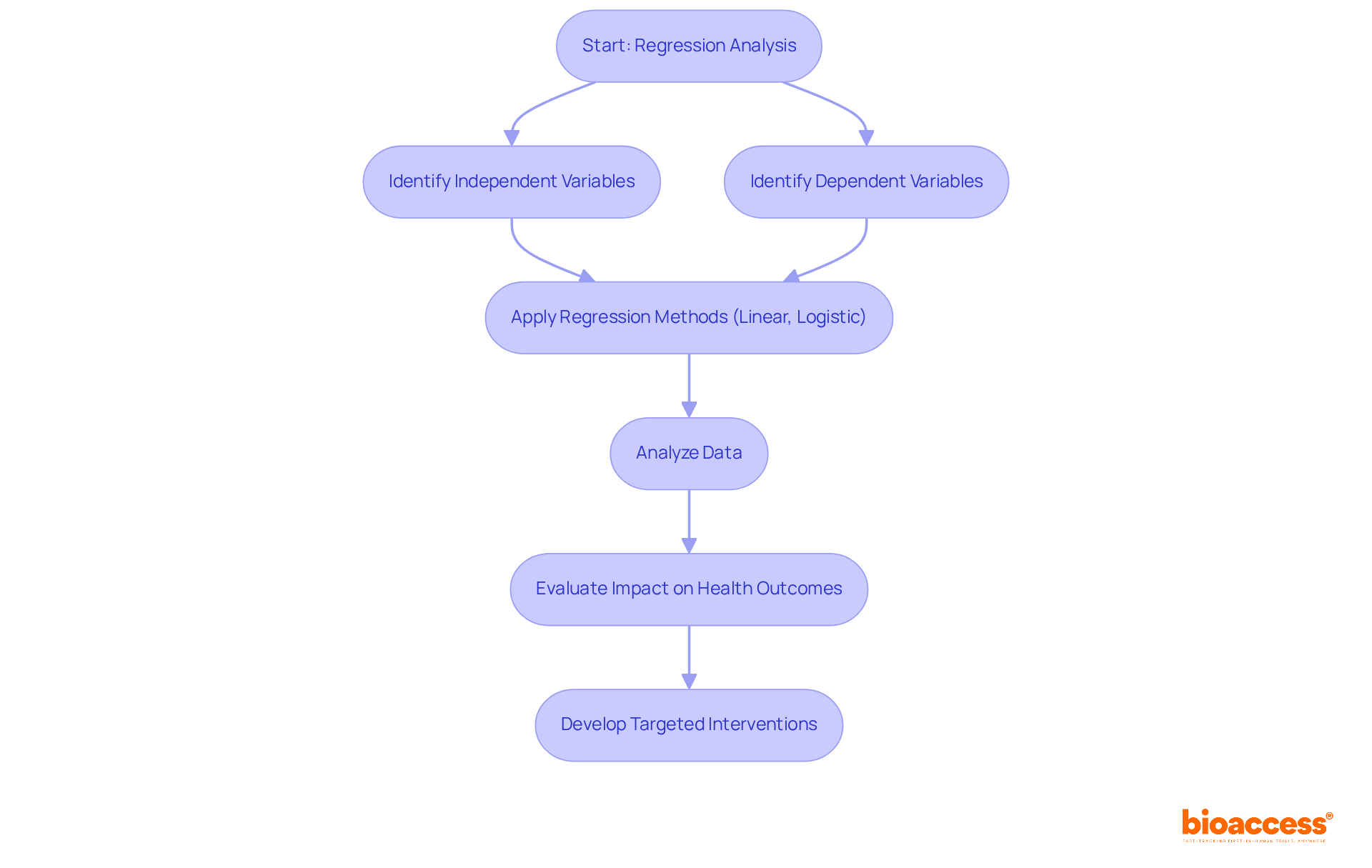
Regression analysis serves as a pivotal tool for researchers in statistics clinical research aiming to explore the intricate connections between dependent and independent variables, thereby identifying the factors that significantly influence health outcomes. By effectively modeling these relationships, researchers in statistics clinical research can evaluate the impact of various predictors on patient health, which in turn facilitates the development of more targeted interventions and personalized treatment strategies.
Within the scope of bioaccess's management services for studies, regression analysis assumes a critical role in investigations such as:
Here, a comprehensive understanding of these relationships is essential for guiding decision-making and refining study designs. Methods such as linear regression and logistic regression are routinely employed in statistics clinical research to analyze data from medical studies, yielding valuable insights that contribute to successful outcomes.
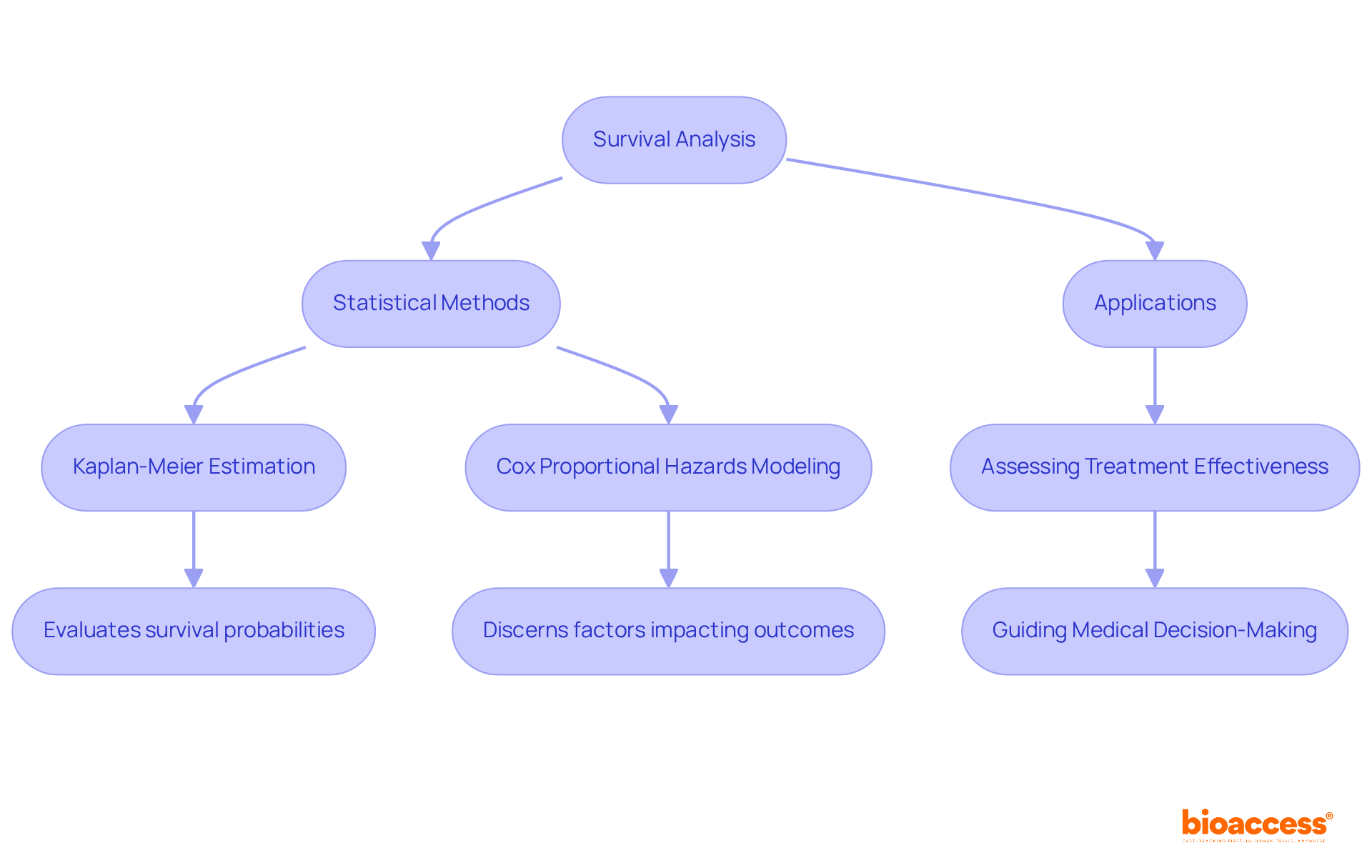
Survival analysis encompasses a suite of statistical methods essential for examining time-to-event data, such as the duration until a patient encounters a specific event, including death or disease progression. Techniques such as Kaplan-Meier estimation and Cox proportional hazards modeling are frequently utilized to evaluate survival probabilities and discern factors that impact outcomes. Understanding these methodologies is crucial for assessing the effectiveness of treatments over time, thereby guiding informed medical decision-making. The insights garnered from survival analysis not only enhance statistics clinical research but also foster collaboration among stakeholders aiming to address significant healthcare challenges.
Randomization serves as a fundamental method in research design, systematically allocating participants to treatment or control groups to eliminate selection bias. This approach guarantees that every participant has an equal opportunity to be assigned to any group, which fosters comparability and strengthens the validity of the study's findings. By effectively mitigating bias, randomization not only enhances the evidence generated from clinical studies but also reinforces the reliability of the conclusions drawn from the data.
A well-executed large randomized controlled trial (RCT) should exhibit no systematic error, with random error minimized below 0.05, thereby ensuring robust results. For example, consider a block size of 4 with two groups (A and B); possible arrangements such as AABB, ABAB, BBAA, or BABA illustrate how block randomization can maintain balance in treatment groups, further reducing the risk of bias.
The significance of randomization is highlighted by its capacity to facilitate double blinding, which is essential for minimizing biases linked to participant and investigator expectations. As observed in studies like the TASTE and NORSTENT trials, effective randomization practices yield more reliable and generalizable findings. However, it is crucial to acknowledge that RCTs may not be practical for small populations. Consequently, researchers must meticulously plan their randomization procedures to ensure balance and minimize bias.

Ethical factors in statistical analysis are paramount, encompassing various methods designed to guarantee the integrity and reliability of study results. These factors include:
Upholding these ethical standards is essential for fostering trust in statistics clinical research, ultimately ensuring that the outcomes benefit both patients and the broader medical community. By prioritizing these principles, researchers can navigate the complexities of the Medtech landscape and address key challenges effectively.

The integration of essential statistics in clinical research is pivotal for ensuring that studies are efficient, reliable, and ultimately beneficial for patient care. By leveraging advanced statistical methodologies, organizations like bioaccess® are revolutionizing the landscape of medical research, enhancing both the speed and accuracy of clinical trials. This emphasis on statistical rigor not only promotes trustworthy data but also fosters a more inclusive representation of diverse patient populations, which is critical for addressing health disparities.
Throughout the article, various statistical techniques such as descriptive statistics, inferential statistics, and survival analysis have been highlighted for their roles in improving clinical trial outcomes. Key insights include:
These elements collectively underscore the necessity of robust statistical frameworks in clinical research, enabling researchers to make informed decisions that can significantly impact patient health.
In conclusion, advancements in statistical analysis within clinical research not only enhance the validity of findings but also ensure that medical innovations are developed with a commitment to ethical standards and patient welfare. As the field continues to evolve, embracing these essential statistics will be crucial for driving forward medical progress and achieving equitable health outcomes for all. Engaging with these principles not only improves the quality of research but also serves as a call to action for stakeholders to prioritize statistical integrity in their clinical endeavors.
What is bioaccess® and its role in clinical research?
bioaccess® is a leader in medical research that leverages essential statistical techniques to streamline processes and enhance outcomes in clinical research. They focus on accelerating approval and enrollment stages to ensure studies are efficient and yield trustworthy data.
How does bioaccess® improve recruitment and retention in clinical trials?
Through a collaboration with Caribbean Health Group and GlobalCare Clinical Trials, bioaccess® has achieved over a 50% reduction in recruitment time and impressive retention rates of 95% in clinical trials.
What is the significance of descriptive statistics in clinical research?
Descriptive statistics summarize essential characteristics of patient populations, such as age and gender, which are vital for contextualizing medical trials and ensuring accurate representation of broader populations.
How has gender representation been addressed in clinical research?
From 2014 to 2021, women represented an average of 51% of participants in FDA-approved medications, highlighting the importance of gender representation in research and the need to analyze demographics to refine methodologies.
What are some current trends in health conditions related to statistics clinical research?
Trends indicate that 10.1% of U.S. adults with diagnosed diabetes reported severe vision difficulty or blindness, and 39.2% had chronic kidney disease, emphasizing the necessity for targeted research that addresses specific health conditions.
Why is it important to include diverse patient populations in clinical trials?
Including diverse patient populations enhances trial design, bolsters the validity of findings, and addresses health inequalities, which are crucial for advancing medical research and achieving equitable health outcomes.
What are inferential statistics and their importance in clinical trials?
Inferential statistics allow researchers to make predictions and generalizations about a population based on sample data. Techniques like hypothesis testing and confidence intervals help evaluate the effectiveness of new treatments and ensure trial outcomes are reliable and actionable.
In what types of trials is bioaccess® involved?
bioaccess® is involved in various types of trials, including Early-Feasibility, First-In-Human, and Pivotal Trials, utilizing rigorous statistical methodologies to support informed decision-making in patient care.