


The article titled "10 Essential Steps for EU Doc Compliance in Clinical Research" highlights the crucial actions required to ensure compliance with EU documentation regulations in the realm of clinical research. It begins by emphasizing the importance of understanding EU regulations, which serves as the foundation for effective compliance. Furthermore, it stresses the necessity of:
These steps are vital for organizations aiming to navigate the complex regulatory landscape effectively and enhance the integrity of their research processes. By following these essential guidelines, clinical research entities can not only meet regulatory requirements but also foster trust and credibility in their findings.
Navigating the complex landscape of clinical research in the EU presents organizations with a multitude of regulatory challenges. The stakes are high; compliance not only influences the integrity of research but also determines how quickly innovative medical solutions can reach the market. This article explores ten essential steps that empower organizations to master EU document compliance, ensuring they meet stringent regulatory standards while fostering trust with participants.
How can organizations effectively balance the demands of compliance with the need for agility in clinical trials?
bioaccess® offers tailored solutions that empower organizations to navigate the complexities of EU doc regulations effectively. In the realm of early-stage medical research, bioaccess® stands out by ensuring that all essential documentation adheres to regulatory standards. This commitment not only accelerates the eu doc approval process but also significantly reduces the time to market for groundbreaking medical products. With over 50 pre-qualified sites activated in less than eight weeks, bioaccess® delivers FDA/EMA/MDR-ready datasets, all under centralized monitoring.
The expertise of their team is invaluable, providing clients with guidance on best practices that equip them to manage compliance requirements efficiently. As organizations face the challenges of the regulatory landscape across LATAM, Eastern Europe, and Australia, bioaccess® plays a crucial role in facilitating smooth navigation through these complexities.
In a rapidly evolving Medtech landscape, collaboration is key. By partnering with bioaccess®, organizations can ensure they are not only compliant but also positioned for success in bringing innovative medical solutions to market.
Navigating the complexities of medical research in the EU requires a solid grasp of key regulations, particularly the Trials Regulation (EU) No 536/2014 and the General Data Protection Regulation (GDPR). The Clinical Studies Regulation sets forth uniform guidelines for conducting research across EU member states, highlighting the critical need for participant consent, data protection, and stringent reporting obligations. This regulation aims to enhance clarity and security in research studies, ensuring that participants' rights are upheld.
The GDPR, effective from May 25, 2018, imposes strict guidelines on the handling of personal data, including that of trial participants. Organizations must establish a legal basis for data processing, secure informed consent, and maintain comprehensive records of their data handling practices. Compliance with GDPR is not just a legal obligation; it is vital for preserving participant trust and upholding the integrity of research.
Legal experts stress the importance of transparent communication regarding data processing activities. Jane Murphy, a specialist in data protection law, emphasizes that "transparent communication is crucial under the GDPR because without adequate information about the ins and outs of the processing activity, individuals can’t exercise their rights." This underscores the necessity for organizations to prioritize clear and open dialogue with participants about how their data will be utilized.
Recent reports indicate that adherence rates for medical studies under EU regulations are on the rise, yet challenges remain. Organizations must remain vigilant and proactive in their regulatory efforts to avert potential legal issues and ensure the successful execution of studies within the EU.
Essential documents in clinical studies—such as the study protocol, informed consent forms, ethics committee approvals, and adverse event reports—are crucial for the integrity of the research process. Organizing these documents systematically not only enhances compliance with EU doc regulations but also strengthens the overall reliability of the study. A robust EU doc management system is vital; it ensures that all records are readily accessible, up-to-date, and compliant with regulatory standards. This approach significantly reduces common documentation errors, like incomplete case histories and inconsistencies between source documents and case report forms, which can jeopardize study outcomes. Regulatory authorities emphasize that maintaining comprehensive documentation is paramount for safeguarding participant rights and ensuring the reliability of trial results. Efficient document management systems foster transparency and accountability, facilitating smooth audits and evaluations, ultimately contributing to the success of research initiatives.
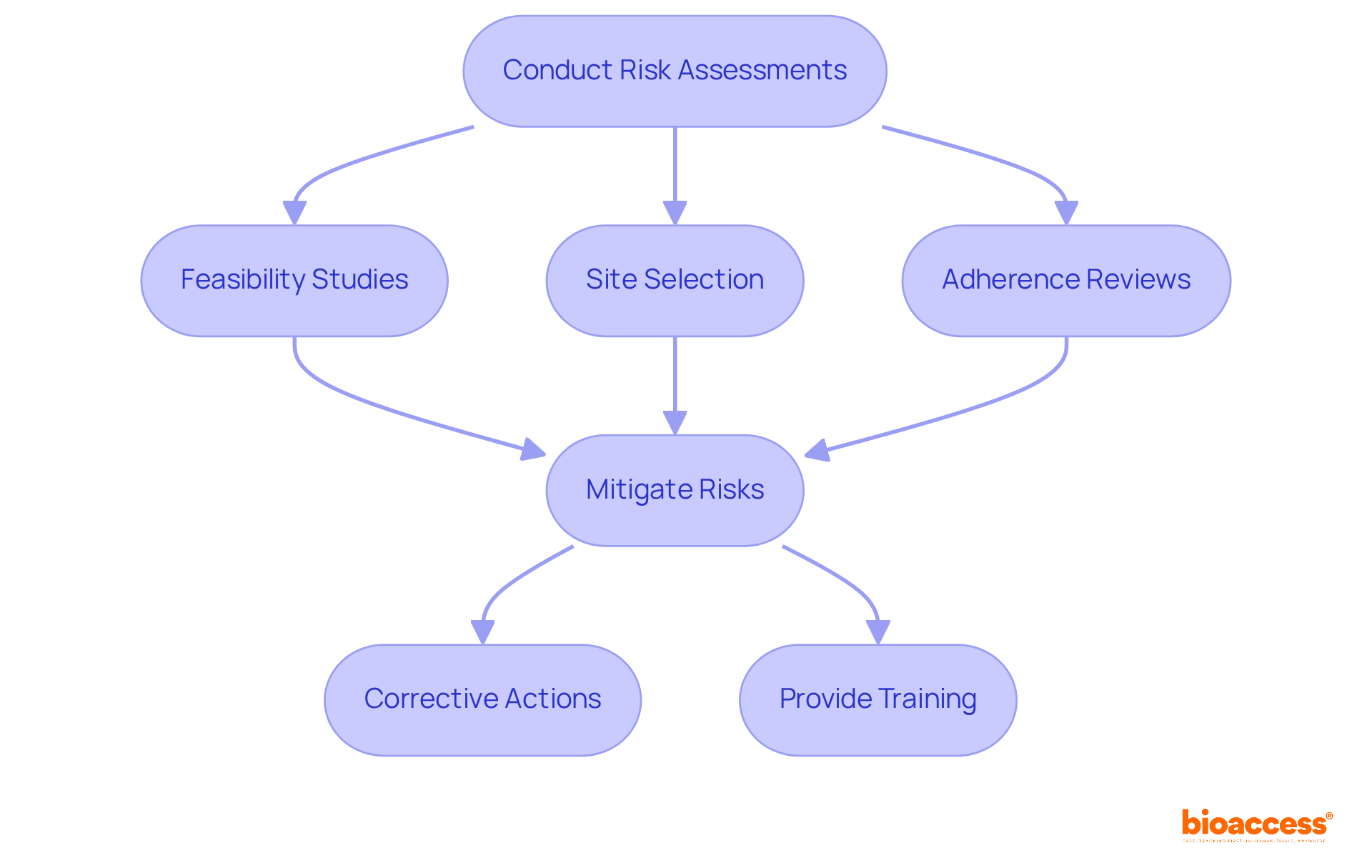
Conducting regular risk assessments is essential for pinpointing potential non-compliance areas in clinical research. This critical process involves evaluating various factors, including:
to ensure that all documentation and team competencies meet regulatory standards. By identifying risks early, organizations can take corrective actions and provide necessary training, effectively mitigating these risks. This proactive approach is vital for maintaining compliance throughout the study lifecycle, especially concerning:
Establishing a strong training program is essential in the realm of clinical research, particularly in light of EU doc regulations and adherence protocols. Regular workshops and refresher courses are vital; they keep staff informed about the latest regulatory changes and underscore the importance of adherence in their daily activities. By integrating insights from our extensive clinical trial management services—such as feasibility studies, site selection, reviews, trial setup, import permits, project management, and reporting—your team will be well-equipped to uphold high regulatory standards. This approach not only enhances knowledge but also fosters a proactive adherence culture within your organization.
In the ever-evolving Medtech landscape, understanding these elements is crucial. As challenges arise, Bioaccess plays a pivotal role in navigating them effectively. Collaboration and continuous learning are key to maintaining compliance and achieving success in clinical research.

Leverage management software to automate documentation processes, track regulatory metrics, and enhance communication among team members. These advanced tools not only ensure the timely completion of compliance-related tasks but also make documentation readily accessible for audits. By embracing technology, organizations can significantly reduce the administrative burden associated with regulatory management. In fact, industry specialists highlight that AI can lead to substantial cost reductions in regulatory operations over time.
Moreover, research shows that 70% of healthcare entities are actively considering the use of generative AI, reflecting a growing trend towards automation in the sector. As healthcare organizations increasingly adopt these technologies, they are witnessing improved efficiency and productivity. AI notably accelerates the processing and analysis of regulatory data, allowing oversight personnel to focus on more complex issues rather than routine tasks. This transition is vital for effectively navigating the evolving regulatory landscape.
For instance, groups utilizing AI's predictive analytics capabilities have reported enhanced compliance outcomes, demonstrating the tangible benefits of these tools. As the Medtech landscape continues to evolve, the integration of such technologies is not just advantageous; it is essential for staying ahead in clinical research.
Conducting regular internal audits is crucial for assessing compliance with EU doc regulations and internal protocols in clinical research. These audits must thoroughly evaluate documentation, processes, and team performance. By proactively identifying areas for improvement, organizations can implement corrective actions before external evaluations, significantly enhancing compliance and minimizing the risk of penalties. For instance, organizations that have established a systematic internal audit process have reported a remarkable increase in compliance rates, with some achieving up to a 50% faster resolution of identified issues. Furthermore, auditors emphasize that a well-defined audit strategy not only fosters accountability but also cultivates a culture of continuous improvement within teams.
Recent findings in medical studies indicate that sites classified as 'Satisfactory'—those with no critical remarks and fewer than seven significant observations—benefit from regular audits, which help maintain high standards and readiness for external evaluations. This proactive approach not only safeguards against regulatory breaches but also bolsters the integrity of research outcomes. Incorporating comprehensive research trial management services, such as trial setup and import permits, can further enhance the effectiveness of these audits. It's important to note that failing to comply with regulations can result in follow-up audits if a site is deemed 'Unsatisfactory,' highlighting the critical nature of these internal assessments.
Establishing open channels of communication with regulatory agencies is essential for navigating the complexities of adherence in clinical research. Engaging with regulators not only clarifies adherence expectations but also keeps organizations informed about upcoming regulatory changes. By fostering these relationships, businesses can gain invaluable support and guidance throughout the regulatory landscape.
Effective communication strategies, such as regular updates and consultations, are vital for building trust and transparency. This proactive approach significantly enhances adherence success; organizations that maintain strong ties with regulators are better positioned to adapt to evolving requirements.
Regulatory affairs professionals assert that nurturing these connections is not just advantageous but critical for ensuring that clinical trials comply with all necessary standards and expectations.

Establishing channels for team members to provide input on regulatory processes and challenges is crucial.
Regularly reviewing this feedback allows organizations to identify trends and areas for improvement.
By cultivating an environment of transparent communication, entities can enhance their adherence practices and proactively address issues.
This approach not only fosters collaboration but also strengthens the overall regulatory framework, ensuring that challenges in clinical research are met with informed solutions.
To ensure ongoing adherence to EU doc regulations, it is essential to regularly review updates from regulatory bodies and industry publications. Subscribing to newsletters, attending conferences, and participating in professional groups can provide vital insights into regulatory changes.
For instance, organizations like ICON have streamlined their workflows based on insights gained from the EU Clinical Trials Regulation (CTR) implementation. This highlights the importance of adjusting adherence strategies to meet evolving requirements.
Staying informed not only boosts compliance rates but also positions organizations to respond proactively to regulatory shifts, ultimately fostering a more efficient clinical trial environment.

Navigating the complex landscape of EU document compliance in clinical research is crucial for organizations looking to effectively bring innovative medical solutions to market. By grasping and implementing the necessary steps outlined in this article, organizations can bolster their compliance efforts and ensure adherence to vital regulations, ultimately protecting participant rights and upholding the integrity of their studies.
Key insights include:
Additionally, utilizing technology and fostering strong communication with regulatory bodies are essential strategies that ease navigation through the regulatory framework. By actively engaging in these practices, organizations can markedly reduce compliance risks and enhance their overall research outcomes.
In summary, the commitment to EU document compliance transcends mere regulatory obligation; it is a foundational element of successful clinical research. Organizations are urged to adopt a proactive stance, continuously monitor regulatory changes, and cultivate a culture of compliance within their teams. By doing so, they not only meet current standards but also position themselves advantageously for future challenges in the evolving Medtech landscape.
What is bioaccess® and what services does it offer?
bioaccess® provides tailored solutions to help organizations navigate EU documentation regulations effectively, particularly in early-stage medical research. They ensure that essential documentation adheres to regulatory standards, accelerating the EU doc approval process and reducing time to market for medical products.
How does bioaccess® support compliance with EU regulations?
bioaccess® offers expert guidance on best practices for managing compliance requirements, helping organizations navigate the regulatory landscape across regions like LATAM, Eastern Europe, and Australia.
What are the key EU regulations relevant to clinical research?
The key regulations include the Trials Regulation (EU) No 536/2014, which provides guidelines for conducting research, and the General Data Protection Regulation (GDPR), which governs the handling of personal data of trial participants.
Why is participant consent important in EU medical research?
Participant consent is critical as it upholds participants' rights and is a requirement under the Trials Regulation, ensuring that individuals are informed about their involvement in research and how their data will be used.
What are the main obligations under the GDPR for organizations conducting clinical research?
Organizations must establish a legal basis for data processing, obtain informed consent, and maintain comprehensive records of their data handling practices to comply with GDPR.
What role does documentation play in EU compliance for clinical studies?
Comprehensive documentation, including study protocols and consent forms, is essential for ensuring compliance with EU regulations, safeguarding participant rights, and enhancing the reliability of research outcomes.
How can organizations improve their document management for EU compliance?
By implementing a robust EU document management system that ensures all records are accessible, up-to-date, and compliant with regulatory standards, organizations can reduce documentation errors and facilitate smooth audits.
What is the significance of transparent communication regarding data processing under GDPR?
Transparent communication is crucial for individuals to understand how their data will be used, allowing them to exercise their rights effectively. This is emphasized by legal experts as essential for maintaining participant trust and integrity in research.
What challenges do organizations face in adhering to EU regulations for medical studies?
Despite rising adherence rates, organizations must remain vigilant and proactive to avoid potential legal issues and ensure successful execution of studies within the EU.