


The primary focus of the article '10 Essential Tips for Effective Design Verification Testing' is to delineate key strategies that significantly enhance the design verification testing process for medical technology innovators. It underscores the necessity of thorough preparation, robust test protocols, user engagement, and clear acceptance criteria. These elements are vital for ensuring compliance, improving product quality, and expediting market entry, thus reinforcing the article's relevance in the clinical research landscape.
Understanding the intricacies of design verification testing is crucial for Medtech innovators navigating the complex landscape of regulatory compliance and product development. This article explores ten essential tips that significantly enhance the effectiveness of design verification testing, ensuring products not only meet regulatory standards but also cater to user needs. However, numerous common pitfalls and challenges can derail the process. How can innovators ensure they are on the right path to successful product development?
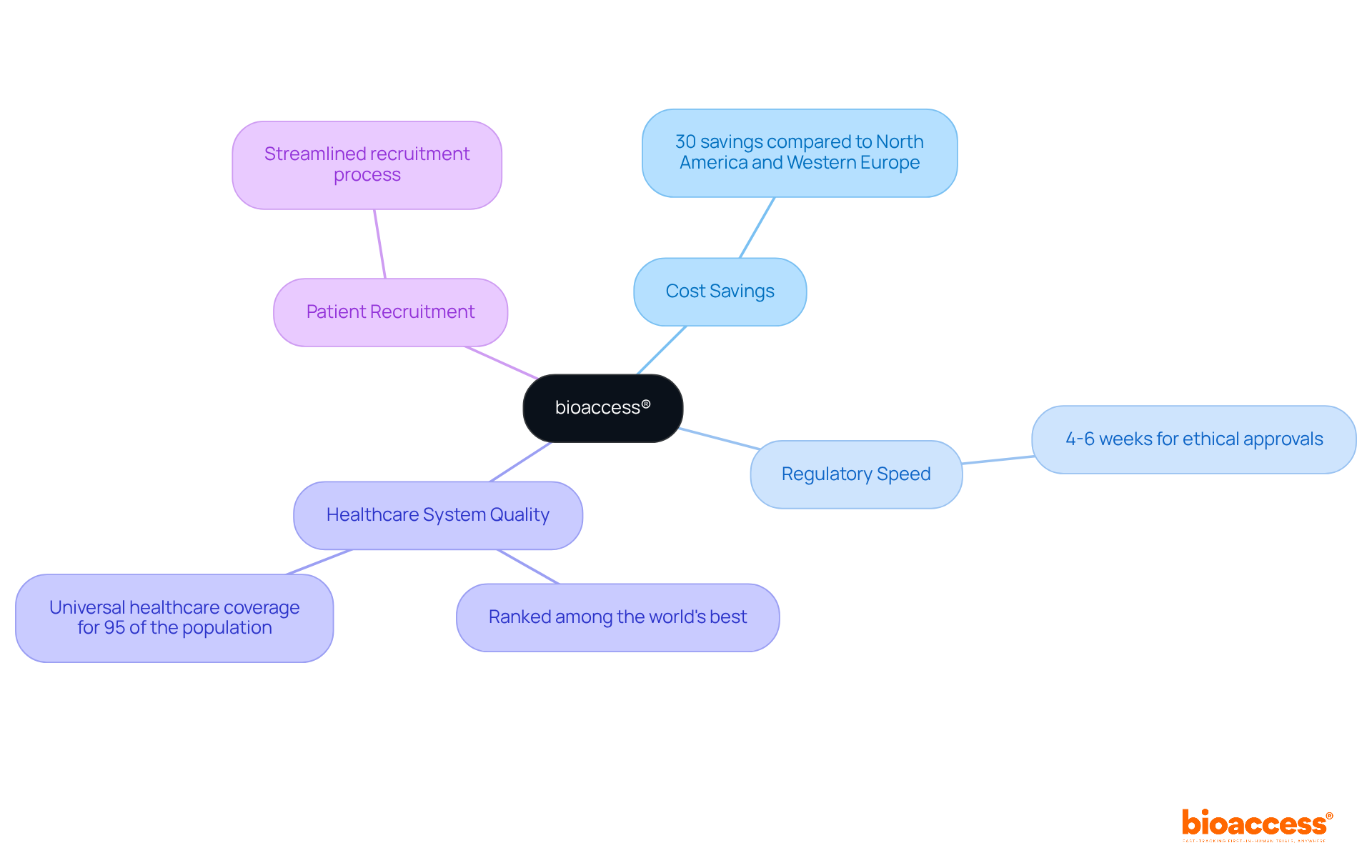
bioaccess® empowers healthcare technology innovators by providing expedited assessment services. By leveraging Colombia's competitive advantages—such as cost savings exceeding 30% compared to North America and Western Europe—bioaccess® guarantees efficient conduct of early-phase clinical research. The regulatory speed in Colombia allows for ethical approvals in just 4-6 weeks, significantly enhancing the pace of design verification testing.
With a robust healthcare system ranked among the world's best and a population of over 50 million, 95% of whom are covered by universal healthcare, patient recruitment becomes streamlined. Collaborating with bioaccess® enables innovators to navigate the complexities of clinical trials while ensuring compliance and quality throughout the process, ultimately facilitating faster product development and market entry.
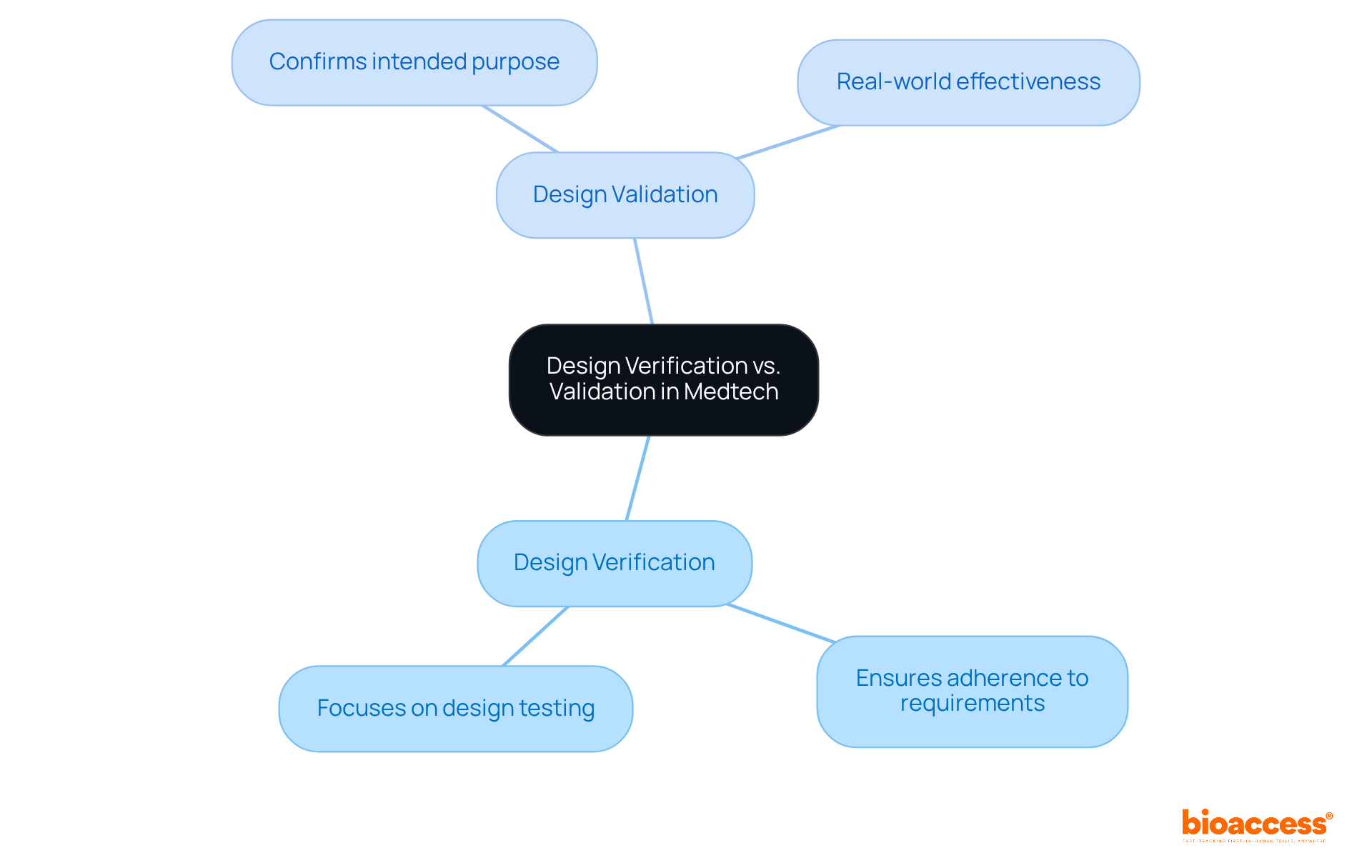
Design assessment and validation are pivotal components of the medical technology development process, particularly concerning regulatory compliance mandated by authorities such as INVIMA (Colombia National Food and Drug Surveillance Institute). Design assessment guarantees that the product adheres to specified requirements, while validation ascertains that the product effectively serves its intended purpose in real-world scenarios. Understanding this distinction is essential for Medtech innovators, as it significantly influences evaluation strategies and regulatory compliance.
Efficient evaluation procedures focus on design verification testing to ensure that the outputs align with the inputs, confirming that the product is accurately constructed before receiving approval for use. Given that INVIMA is recognized as a Level 4 health authority by PAHO/WHO, its rigorous oversight of medical devices underscores the critical importance of adhering to these assessment and validation processes to ensure safety, efficacy, and quality in product development.
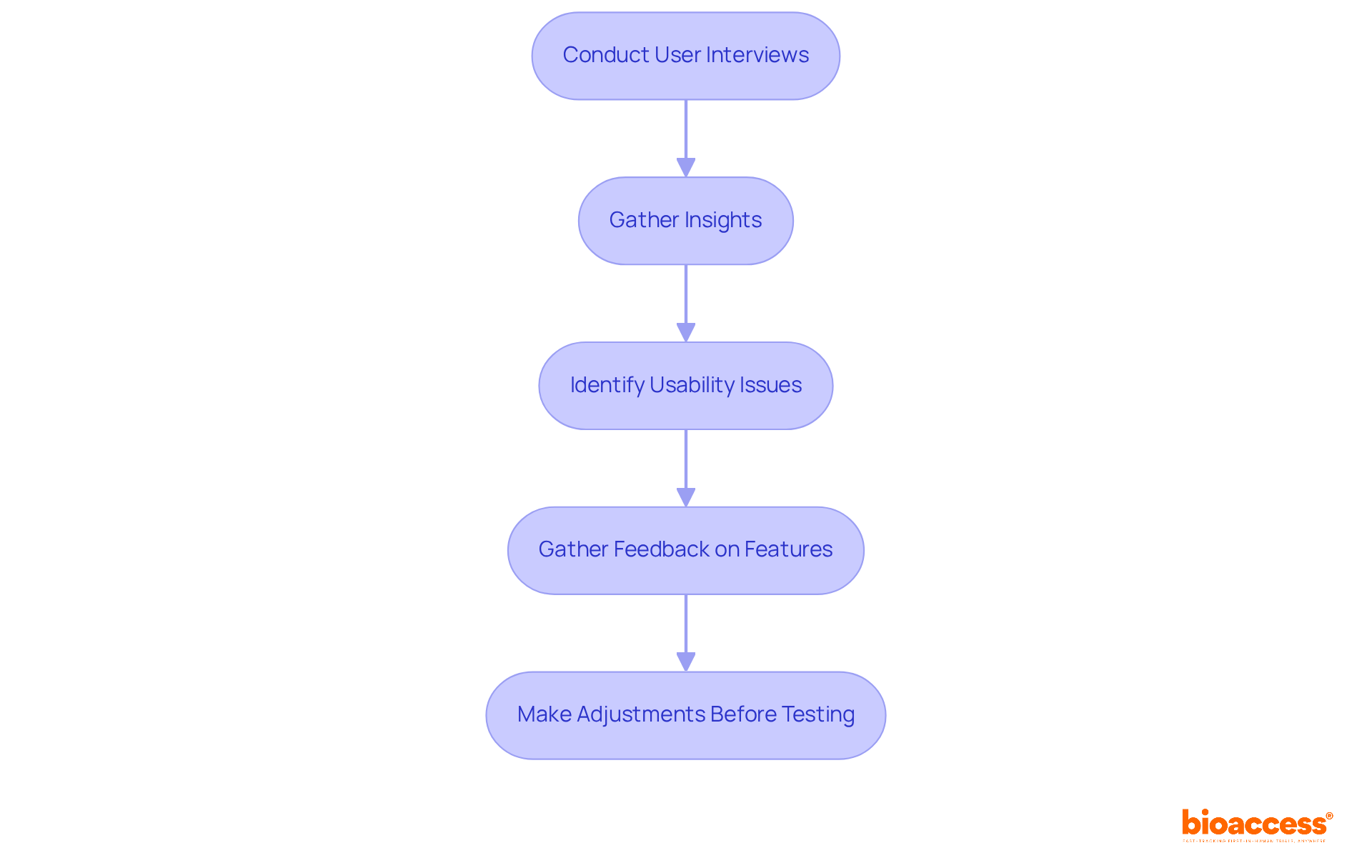
Conducting user interviews is a crucial strategy for gathering insights that inform design verification testing assessments. By engaging with end-users, Medtech innovators can identify potential usability issues and gather valuable feedback regarding specific features. This critical information can guide necessary adjustments before the design verification testing begins, ensuring that the product meets user needs. Effective user interviews should prioritize open-ended questions that foster comprehensive responses, enabling a more profound understanding of user experiences and expectations.
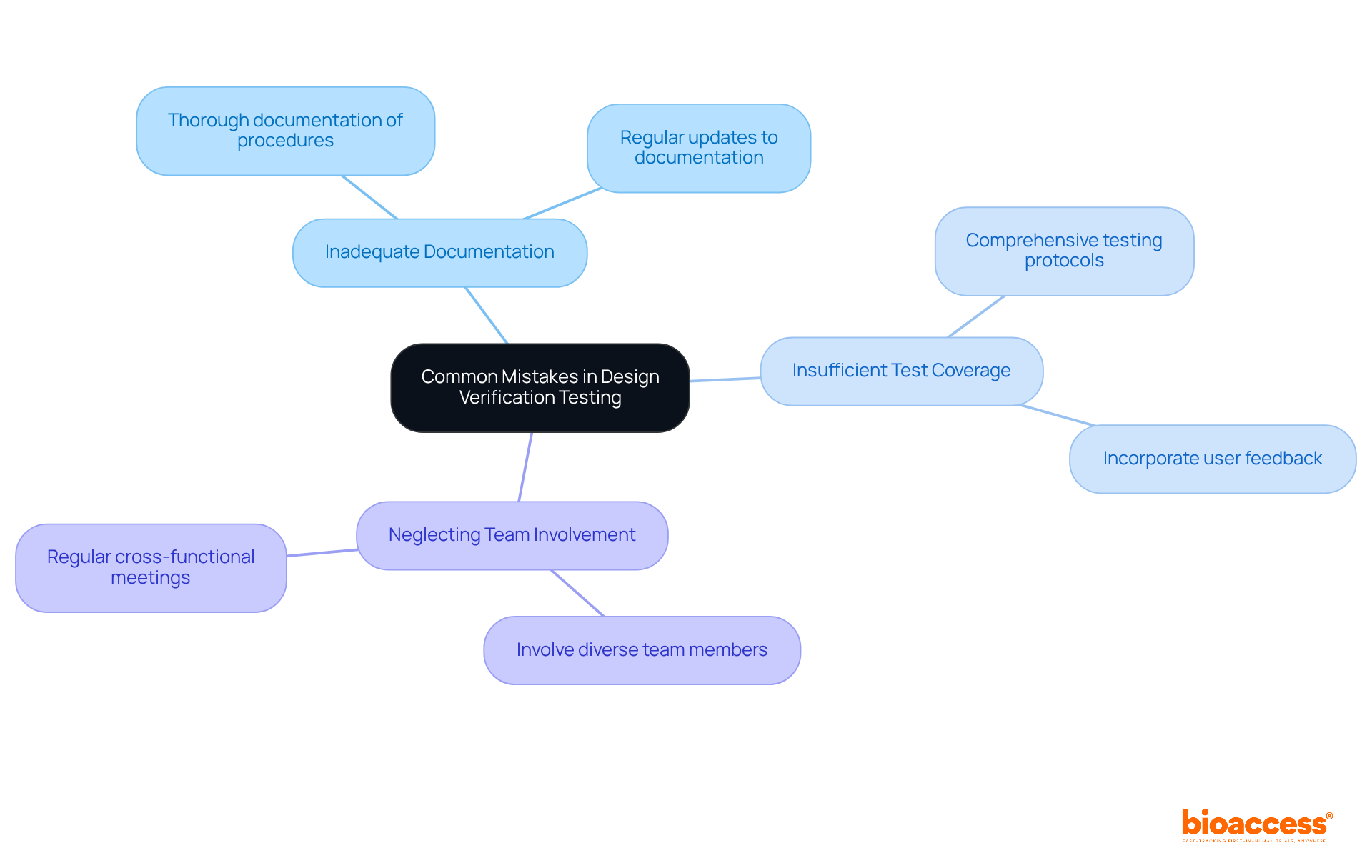
Frequent errors in assessment, such as inadequate documentation, insufficient test coverage, and neglecting to involve cross-functional teams, pose significant challenges in the Medtech landscape. To effectively prevent these pitfalls, Medtech innovators must ensure thorough documentation of all assessment procedures and results.
Involving diverse team members from engineering, regulatory, and clinical backgrounds is essential, as it provides a holistic view of the product's performance. Furthermore, regularly reviewing and updating test protocols is crucial to maintain alignment with evolving regulatory standards and user expectations.
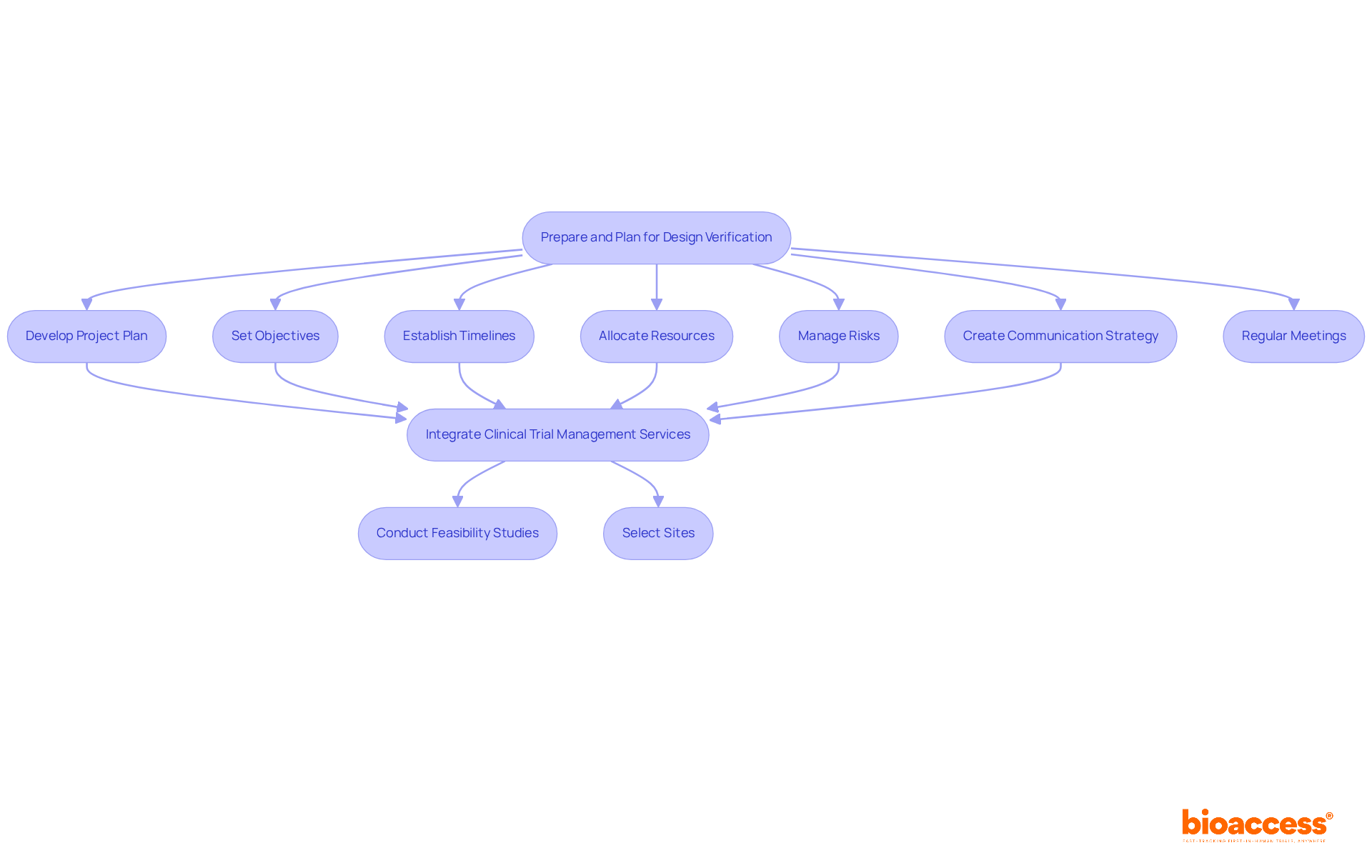
Preparation and planning are vital components of successful product assessment in the clinical research landscape. Innovators must develop a comprehensive project plan that delineates objectives, timelines, resource allocation, and risk management strategies. This plan should incorporate a robust communication strategy to keep all stakeholders informed throughout the process.
By integrating comprehensive clinical trial management services, such as feasibility studies and site selection, the planning phase can be significantly enhanced. Additionally, regularly scheduled meetings are essential to ensure that the project remains on track and that any emerging issues are addressed promptly.
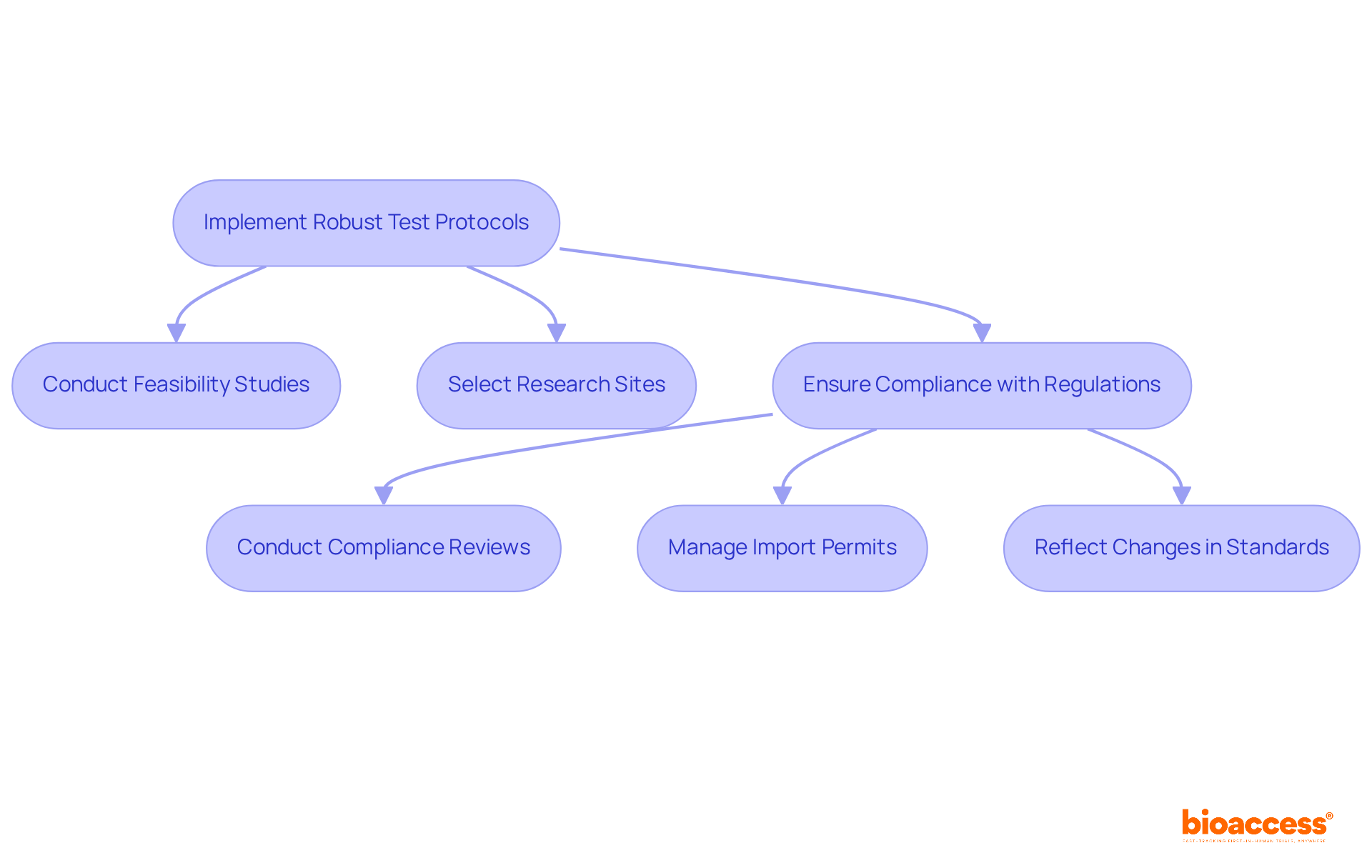
Implementing robust test protocols is vital for achieving reliable design verification testing results. These protocols must be comprehensive, clearly outlining the assessment methods, equipment, and criteria for success. In the context of clinical trials, this includes:
Furthermore, protocols should encompass:
By adhering to well-defined protocols, medical technology innovators can ensure that their design verification testing processes are consistent, reproducible, and compliant with industry regulations. This ultimately supports effective project management and reporting throughout the trial process.
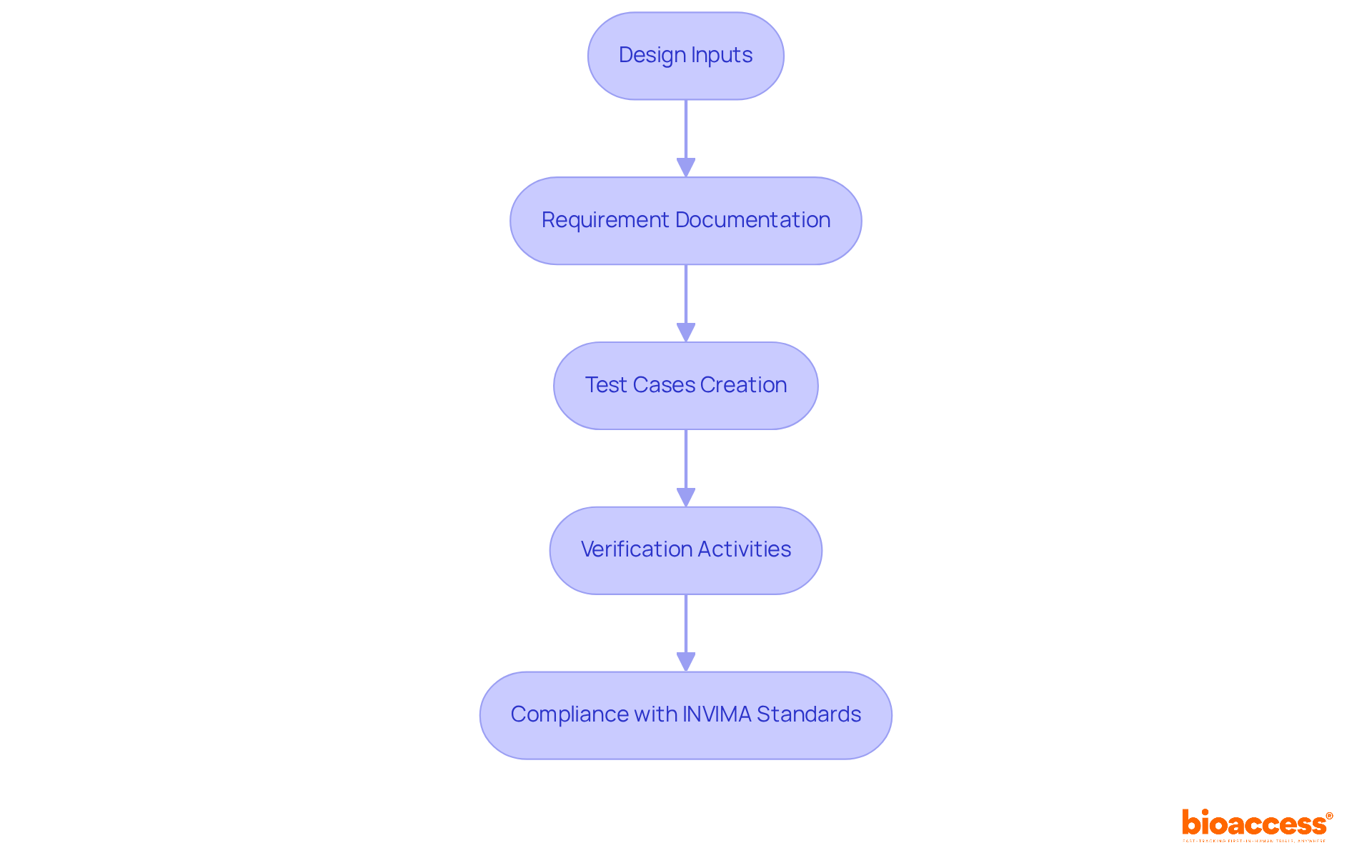
Ensuring traceability in creation is paramount; it involves meticulously documenting the relationships between inputs, outputs, and verification activities. This traceability is essential for demonstrating compliance with regulatory requirements established by authorities such as INVIMA, the Colombia National Food and Drug Surveillance Institute, which oversees the marketing and manufacturing of health products.
Medtech innovators must implement a traceability matrix that links requirements to corresponding test cases and results in design verification testing. Such a matrix not only aids in adhering to INVIMA's standards but also provides a clear summary of the development process, simplifying the identification and resolution of any discrepancies.
As highlighted by experts like Katherine Ruiz, who specializes in regulatory affairs for medical devices and in vitro diagnostics in Colombia, adhering to these practices is vital for maintaining product quality and ensuring safety and efficacy in the market.
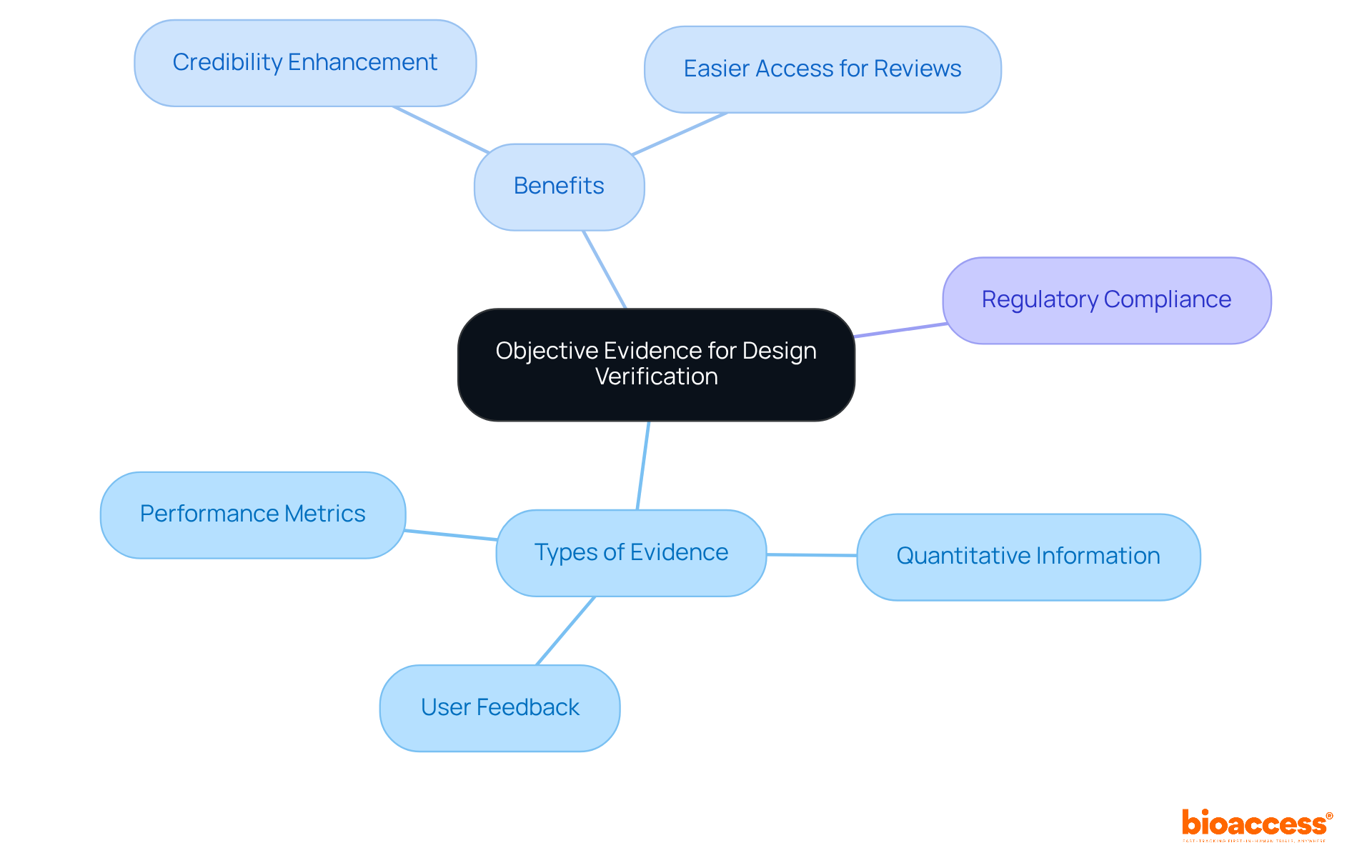
Collecting objective evidence is essential for the efficient assessment of creation within the Medtech field. This evidence encompasses quantitative information drawn from assessments, user feedback, and performance metrics. By focusing on objective criteria, Medtech innovators can provide clear and trustworthy support for their development assessment results. Moreover, maintaining a comprehensive database of evidence gathered throughout the evaluation process not only facilitates easier access during regulatory reviews and audits but also enhances the credibility of the entire assessment process.
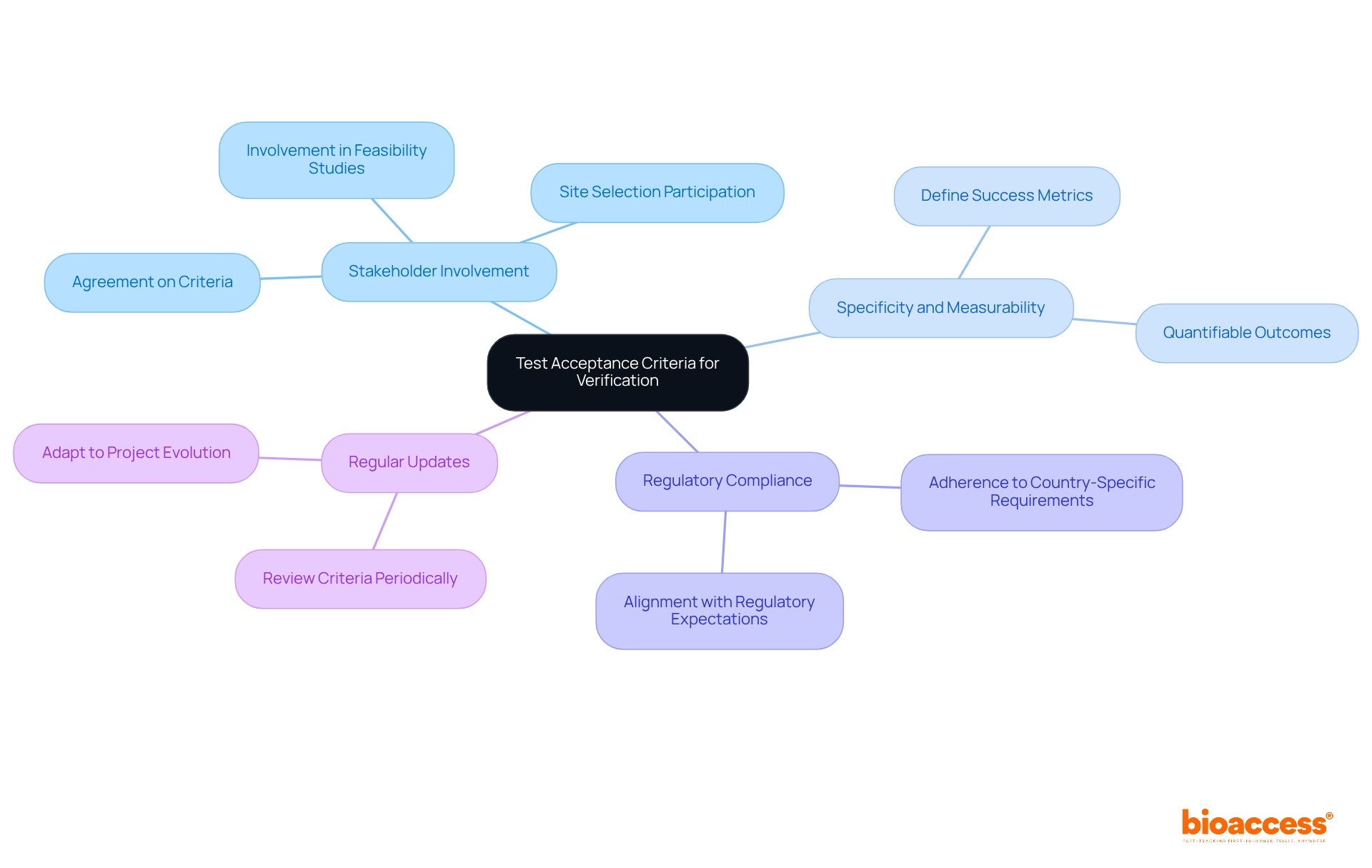
Establishing clear evaluation acceptance criteria is essential for assessing the effectiveness of the development assessment process, especially within the realm of clinical trials. These criteria must be specific, measurable, and mutually agreed upon by all stakeholders involved in the project, including those tasked with feasibility studies and site selection. By delineating what defines a successful outcome, medical technology innovators can ensure that their evaluation processes align with regulatory expectations and user needs, particularly in adherence to country-specific requirements.
Regularly reviewing and updating these criteria is crucial to maintain their relevance as the project evolves, thereby ensuring that all facets of trial setup, project management, and reporting are comprehensively addressed.
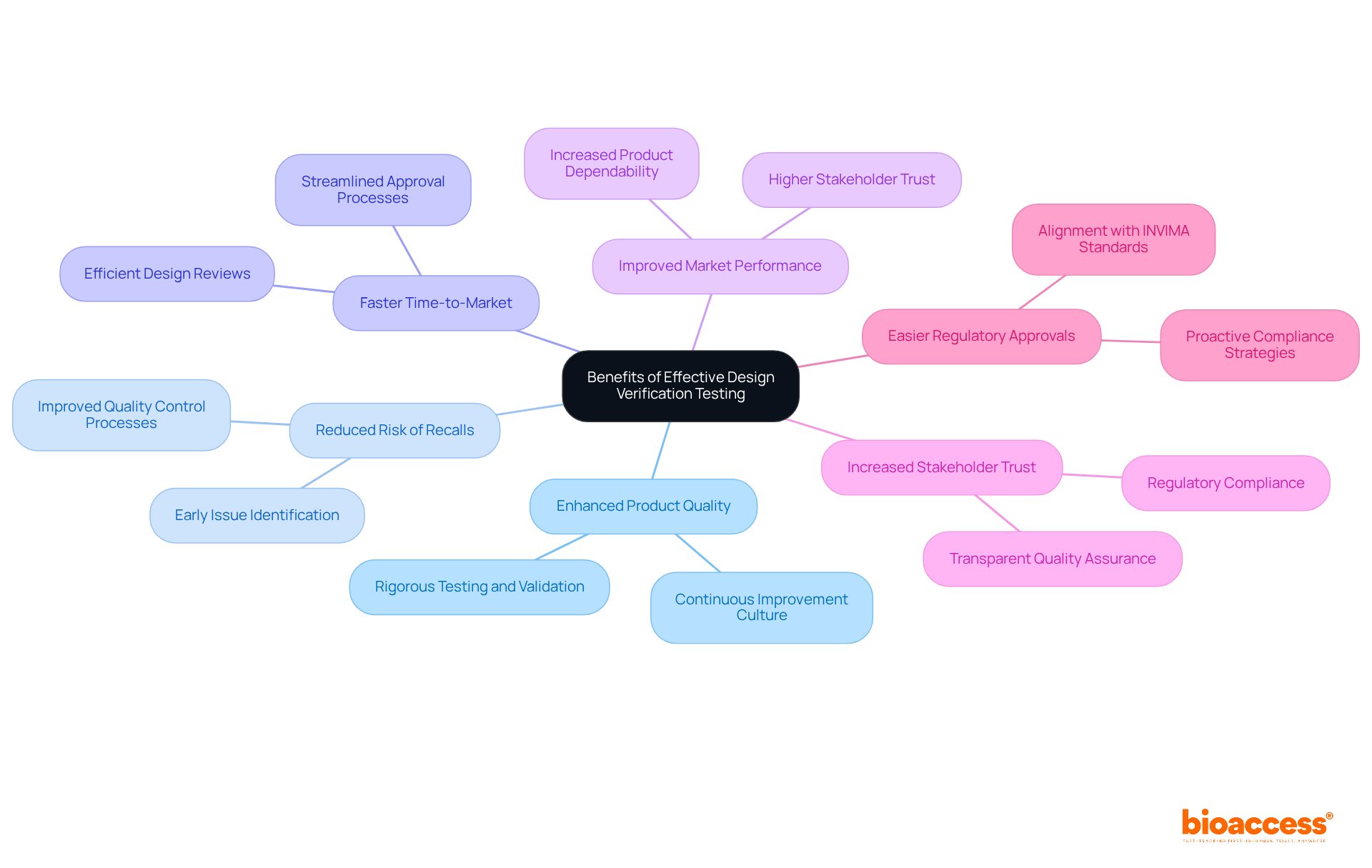
Efficient design verification testing is pivotal for Medtech innovators, providing numerous advantages that significantly enhance product quality and market success. By implementing design verification testing to identify potential issues early in the development process, companies can mitigate the risk of costly recalls and expedite their time-to-market.
Studies indicate that organizations implementing stringent design verification testing procedures experience a notable increase in product dependability, which directly correlates with improved market performance. Furthermore, a thorough assessment of the layout fosters stakeholder trust and facilitates smoother regulatory approvals, which are essential for navigating the complexities of the healthcare environment, particularly in the context of design verification testing.
INVIMA, recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, ensures that medical devices comply with rigorous safety, efficacy, and quality standards, thereby influencing the development assessment processes for medical technology firms.
Industry leaders assert that investing in robust design verification testing not only results in superior product outcomes but also bolsters patient safety and satisfaction. By acknowledging and prioritizing these benefits, Medtech companies like bioaccess can strategically position themselves for success in an increasingly competitive market.
Efficient design verification testing is paramount for Medtech innovators committed to enhancing product quality and ensuring compliance with regulatory standards. By grasping the intricacies of design verification and validation, utilizing user insights, and implementing robust testing protocols, organizations can significantly streamline their development processes. The significance of meticulous planning, clear acceptance criteria, and maintaining traceability is critical, as these elements directly contribute to the safety, efficacy, and market readiness of medical technologies.
Throughout this article, key strategies such as avoiding common pitfalls, gathering objective evidence, and establishing comprehensive test protocols have been underscored as essential for successful design verification testing. By concentrating on these areas, Medtech companies can mitigate risks, bolster stakeholder trust, and accelerate their time-to-market. Furthermore, collaborating with experienced partners like bioaccess® can offer invaluable support in navigating the complexities of clinical trials and regulatory compliance.
In conclusion, prioritizing effective design verification testing not only leads to superior product outcomes but also promotes patient safety and satisfaction. As the Medtech landscape continues to evolve, adopting these best practices will empower innovators to excel in a competitive environment. It is imperative for companies to invest in these strategies now to secure their future position and ultimately enhance healthcare outcomes for all.
What services does bioaccess® provide for medtech innovators?
bioaccess® offers expedited assessment services to healthcare technology innovators, focusing on early-phase clinical research to facilitate faster product development and market entry.
How does bioaccess® leverage Colombia's advantages for design verification testing?
bioaccess® utilizes Colombia's competitive advantages, including cost savings exceeding 30% compared to North America and Western Europe, and a regulatory speed that allows for ethical approvals in just 4-6 weeks.
What is the significance of the healthcare system in Colombia for patient recruitment?
Colombia has a robust healthcare system ranked among the world's best, with over 50 million people, 95% of whom are covered by universal healthcare, making patient recruitment more streamlined.
What is the difference between design verification and validation in medtech?
Design verification ensures that a product adheres to specified requirements, while validation confirms that the product effectively serves its intended purpose in real-world scenarios. Both are crucial for regulatory compliance.
Why is understanding the distinction between design verification and validation important for medtech innovators?
Understanding this distinction influences evaluation strategies and regulatory compliance, which are essential for ensuring safety, efficacy, and quality in product development.
What role does INVIMA play in the medical device evaluation process?
INVIMA (Colombia National Food and Drug Surveillance Institute) oversees the evaluation of medical devices and is recognized as a Level 4 health authority by PAHO/WHO, emphasizing the importance of adhering to assessment and validation processes.
How can user interviews enhance design verification testing?
Conducting user interviews helps medtech innovators gather insights to identify potential usability issues and gather feedback on specific features, guiding necessary adjustments before testing begins.
What approach should be taken when conducting user interviews for design verification?
Effective user interviews should prioritize open-ended questions that encourage comprehensive responses, allowing for a deeper understanding of user experiences and expectations.