


The landscape of clinical research is evolving rapidly, with organizations striving to meet stringent trial monitoring standards while ensuring patient safety and data integrity. In this dynamic environment, understanding the essential practices that underpin successful clinical trials is crucial for researchers and stakeholders alike. This article delves into ten vital trial monitoring standards under Halmed, highlighting how these guidelines can enhance compliance, improve study outcomes, and ultimately lead to more reliable results.
What are the key strategies that can transform clinical research and address the challenges faced by Medtech startups today? By exploring these standards, we can uncover insights that not only bolster compliance but also pave the way for innovative solutions in the Medtech landscape. As we navigate these complexities, collaboration among stakeholders becomes paramount, ensuring that we collectively rise to meet the demands of modern clinical trials.
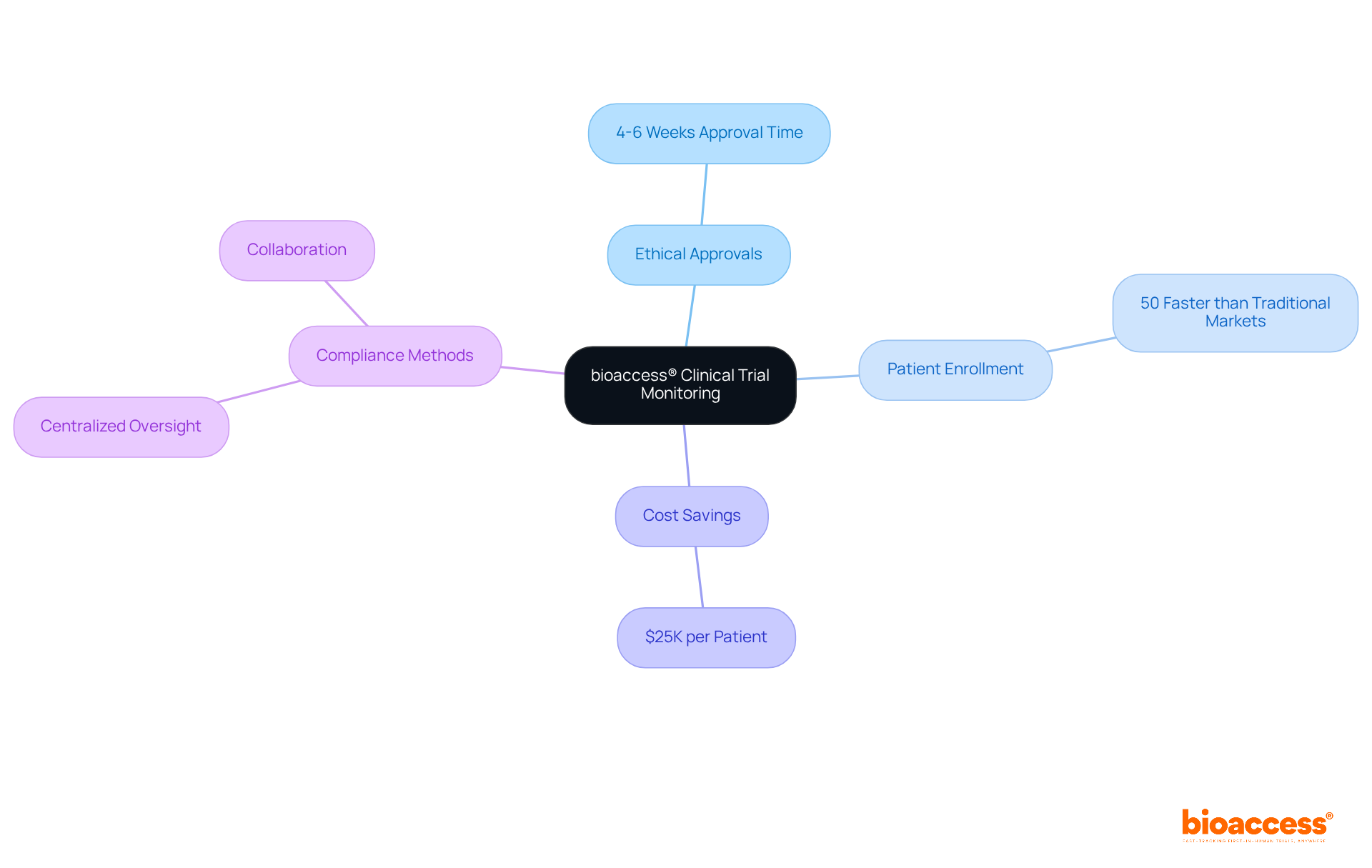
bioaccess® stands out as a leader in managing research studies, utilizing innovative practices that align with trial monitoring standards under Halmed. The organization places a strong emphasis on ethical approvals, achieving them in a remarkable 4-6 weeks. This efficiency accelerates patient enrollment by 50% compared to traditional markets, leading to substantial cost savings of $25K per patient. With extensive experience across diverse regulatory environments, bioaccess® ensures that studies prioritize patient safety and data integrity.
By integrating advanced methods that comply with trial monitoring standards under Halmed, including centralized oversight and fostering collaboration among research teams, bioaccess® not only enhances compliance but also significantly improves study outcomes. This commitment establishes a benchmark for excellence in research monitoring. With over 50 pre-qualified sites activated in under eight weeks and FDA/EMA/MDR-ready datasets, bioaccess® is dedicated to delivering swift and reliable research services tailored to the needs of Medtech startups.
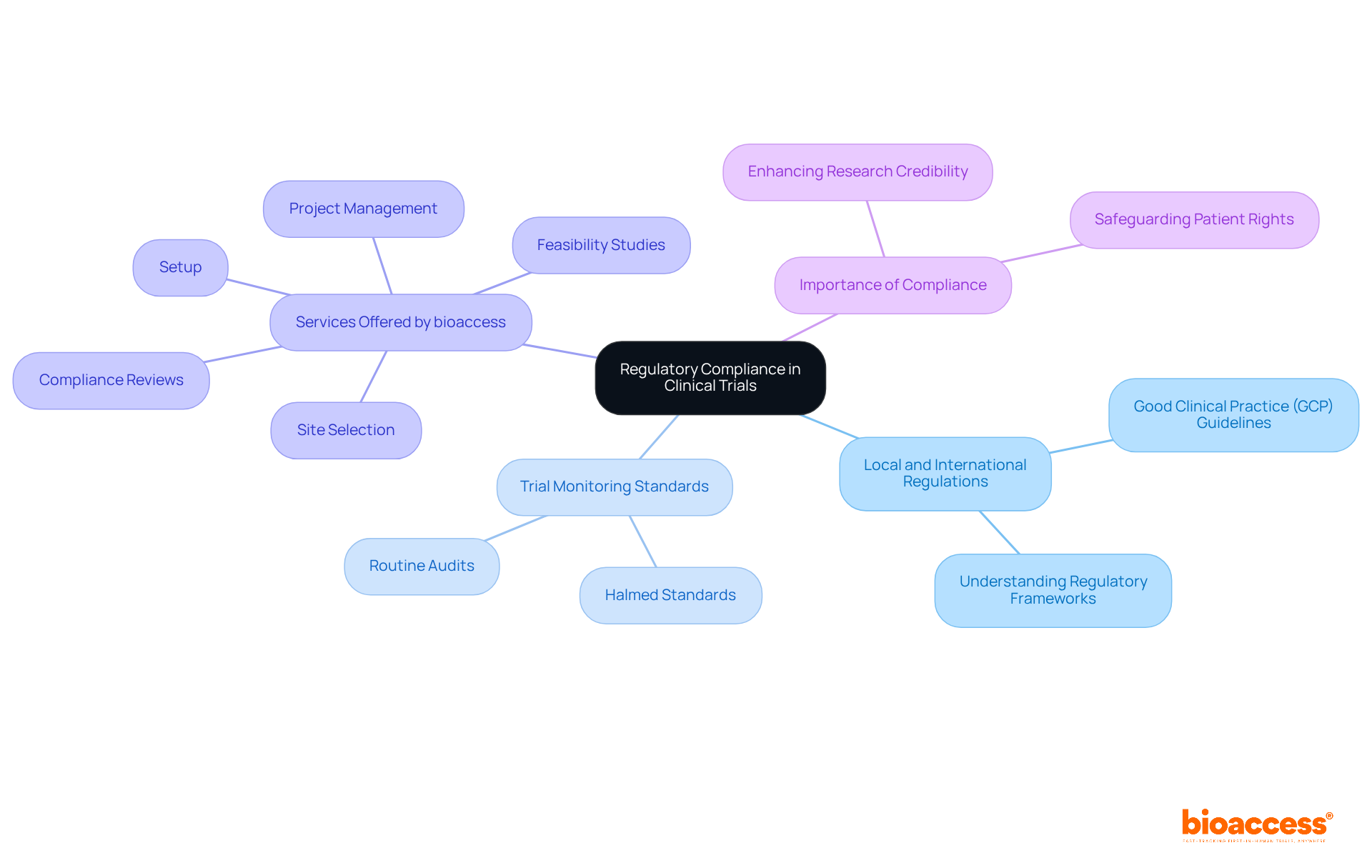
To ensure regulatory adherence in research studies, organizations must familiarize themselves with both local and international regulations, including the trial monitoring standards under Halmed. This process involves comprehensive documentation, routine audits, and thorough training for all personnel involved in the study.
At bioaccess, we provide extensive research management services that cover:
Our deep understanding of the Latin American Medtech landscape positions us uniquely to navigate compliance challenges effectively. This not only safeguards patient rights but also bolsters the credibility of research findings.
By leveraging our services, organizations can adeptly manage the complexities of medical studies in this region. Are you ready to enhance your research capabilities and ensure compliance? Let’s collaborate to streamline your clinical research efforts.
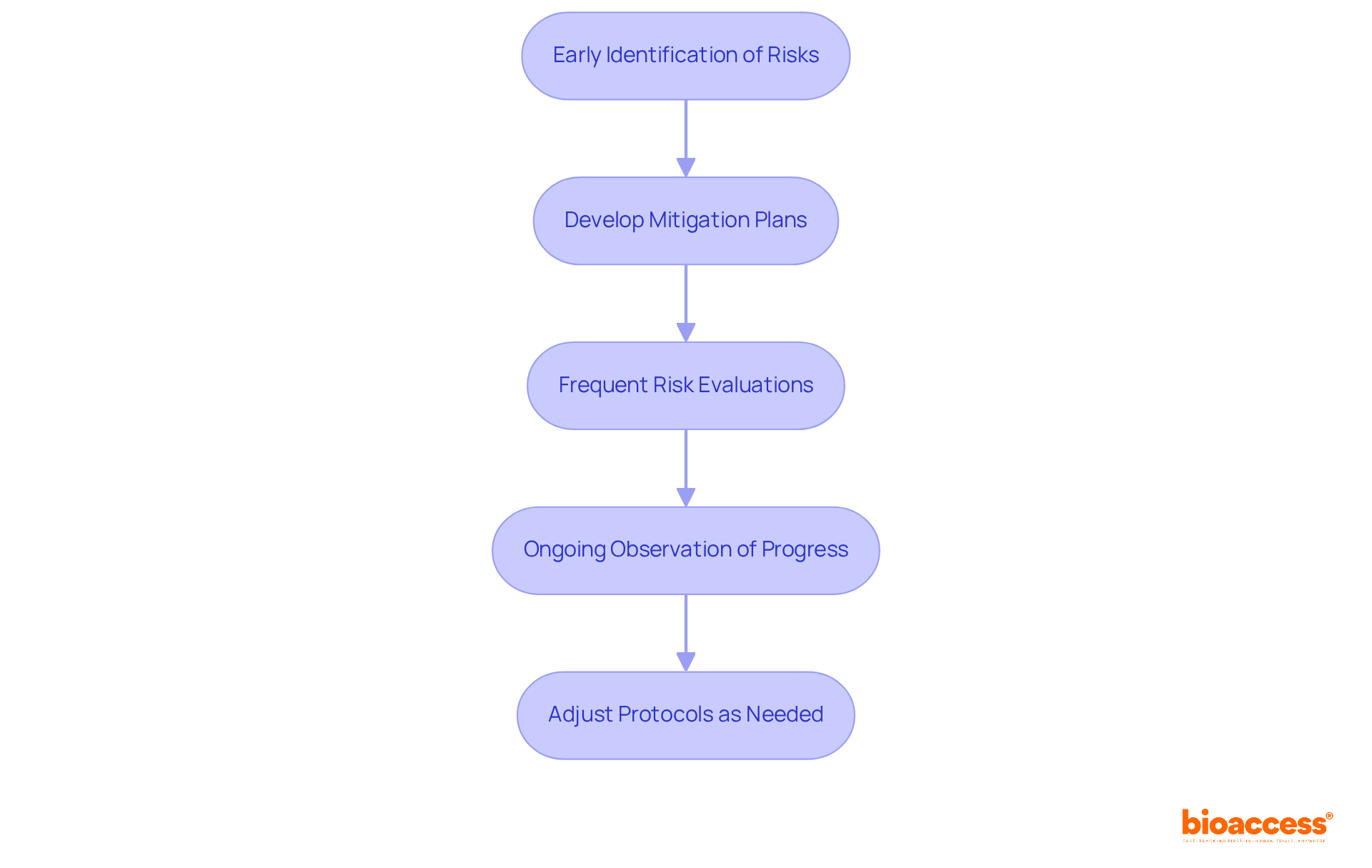
Implementing effective risk management strategies is crucial for the success of medical studies. This process begins with the early identification of potential risks, enabling teams to develop comprehensive mitigation plans. Notably, a substantial 70% of research sites reported that insufficient time for researchers is a frequent risk, underscoring the necessity for effective resource distribution.
Frequent risk evaluations and ongoing observation of progress are vital for adjusting protocols as needed. In fact, 87% of sites expressed a willingness to utilize a tailored risk management tool, indicating a strong desire to enhance their risk assessment practices. By actively managing risks, research teams can significantly improve participant safety and uphold the integrity of the information gathered. This ultimately leads to more reliable results and enhanced study performance.
As noted by industry experts, a well-structured risk management approach not only safeguards participants but also fosters a culture of accountability and transparency within research teams. This commitment to risk management is essential for navigating the complexities of clinical research and ensuring successful outcomes.
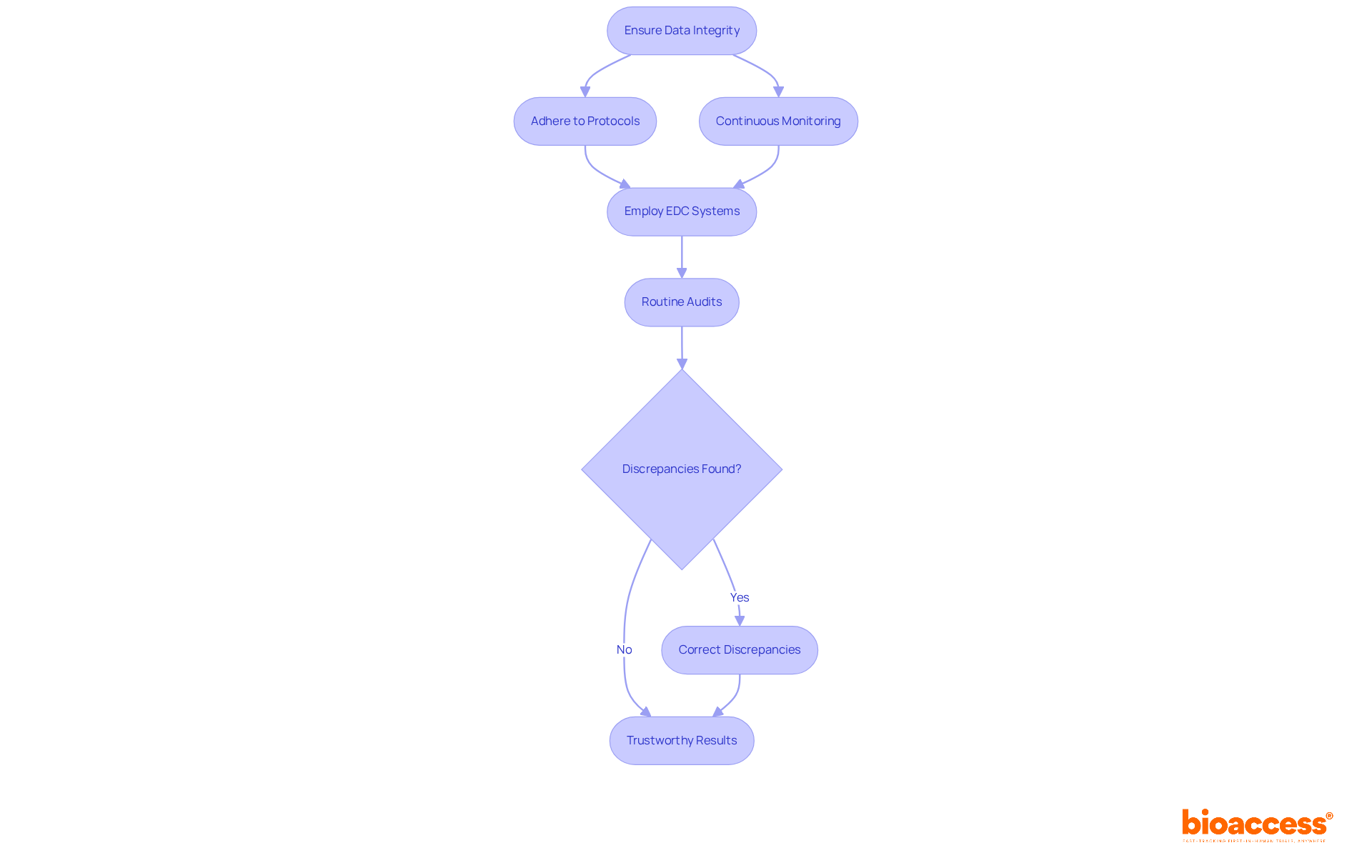
Ensuring information integrity throughout clinical trials is paramount. It necessitates strict adherence to established protocols and continuous monitoring of information collection processes. Employing validated electronic data capture (EDC) systems is crucial; these systems improve precision, simplify entry, and strengthen security.
Routine audits are essential for verifying information integrity, enabling the identification and correction of discrepancies in real time. Research indicates that experiments using EDC systems encounter fewer information errors and enhanced overall efficiency. Notably, there’s a correlation between EDC sophistication and experiment success rates. By prioritizing information integrity, researchers can produce trustworthy results that enhance medical knowledge while maintaining patient safety and regulatory compliance.
The effects of compromised data integrity can be severe, potentially leading to incorrect conclusions about drug safety and effectiveness. This highlights the significance of upholding strict standards throughout the research process.
What challenges do you face in ensuring data integrity in your clinical trials?
Encouraging transparent communication among stakeholders is crucial for the success of medical studies. Regular updates, collaborative platforms, and a clear understanding of each team member's role within the clinical study management framework are essential components. Transparency not only facilitates addressing regulatory hurdles but also mitigates recruitment challenges that medical device startups frequently encounter. By leveraging bioaccess®'s expertise in site feasibility, study setup, investigator selection, compliance reviews, and project management, teams can significantly enhance their communication strategies.
Frequent meetings and open channels for input can greatly improve teamwork and productivity. This collaborative approach ensures that the study remains on track and complies with the trial monitoring standards under halmed required for successful medical device research. As the Medtech landscape evolves, the importance of effective communication and collaboration cannot be overstated. The next steps involve integrating these practices to foster a more cohesive and efficient research environment.
Investing in the training and education of personnel engaged in research studies is crucial for upholding high standards of practice. Regular workshops, online courses, and hands-on training sessions should cover essential topics such as:
This efficient training not only boosts employee confidence in discussing studies with prospective participants but also significantly enhances the overall quality of research.
For instance, programs that incorporate applied learning techniques - like case studies and role-playing - have proven effective in improving knowledge retention and the practical application of Good Clinical Practice (GCP) principles. Furthermore, integrating technology into training can provide flexible learning options, ensuring that staff stay updated on evolving regulations and best practices. By prioritizing comprehensive education strategies, organizations can mitigate risks associated with protocol deviations and cultivate a culture of compliance and excellence in research.
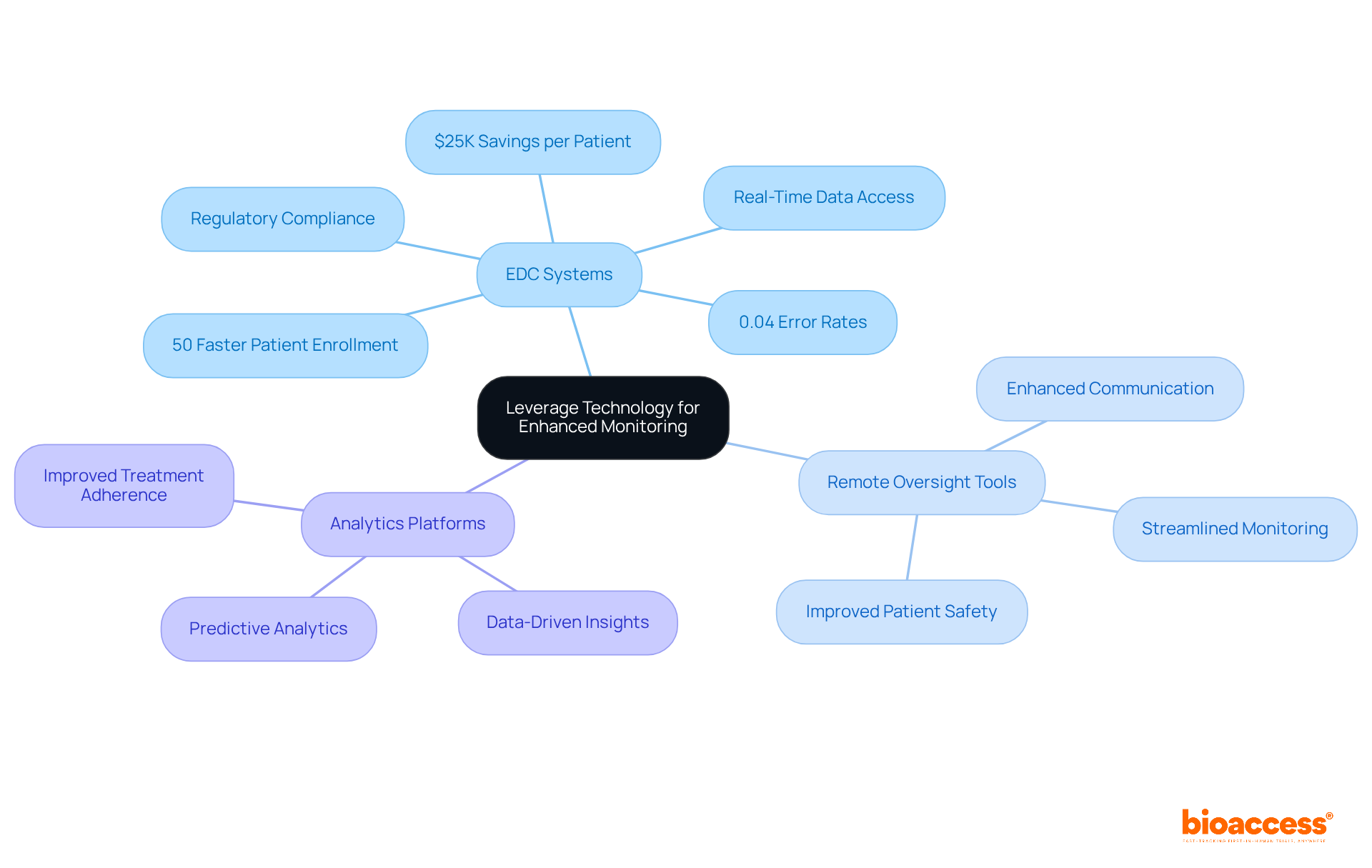
Utilizing electronic data capture (EDC) systems, remote oversight tools, and analytics platforms is essential for enhancing monitoring in clinical studies. These technologies facilitate real-time tracking of trial progress, enabling teams to communicate effectively while maintaining the integrity of information. Notably, trials that implement EDC systems have experienced a 50% faster patient enrollment rate compared to traditional methods, significantly boosting operational efficiency.
With bioaccess®, organizations can realize savings of $25K per patient and access FDA-ready information, ensuring no rework or delays. Moreover, organizations that leverage EDC can achieve error rates as low as 0.04%, highlighting the critical role of automation in reducing data entry errors.
As the landscape of medical studies evolves, integrating EDC solutions not only streamlines oversight processes but also enhances compliance with trial monitoring standards under halmed, ultimately leading to improved patient outcomes and more reliable research results.
Additionally, bioaccess® is dedicated to accelerating medical device research services in Latin America, overseeing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, further underscoring its commitment to expediting studies in Medtech and Biopharma.
To enhance the quality and reliability of clinical trials, embracing continuous improvement in oversight practices is essential. This means consistently reviewing and updating observation protocols based on feedback and new insights. Post-trial evaluations play a vital role, enabling organizations to assess the effectiveness of their strategies and identify areas for improvement. For example, the error rate for major efficacy was 0.15% for risk-based monitoring (RBM) compared to 0.28% for classic monitoring (CM), underscoring the impact of effective monitoring practices.
At bioaccess, our comprehensive clinical study management services encompass:
These capabilities ensure that assessments are conducted effectively and in accordance with local regulations, ultimately contributing to job creation, economic development, and healthcare enhancement in local economies. By actively soliciting input from team members, we foster a collaborative environment where best practices can be shared and implemented. Staying informed about industry developments ensures that our monitoring protocols remain aligned with the trial monitoring standards under halmed. As Dr. John-Arne Rottingen stated, "Clinical trials are a critical part of getting interventions and products to the people that need them most." By cultivating a culture of ongoing enhancement, organizations can significantly elevate the integrity and outcomes of their research. Furthermore, implementing structured feedback mechanisms, such as regular team meetings to discuss monitoring challenges and successes, can further enhance the effectiveness of monitoring protocols.
Enhancing patient involvement in clinical studies is crucial for achieving successful research outcomes. A multifaceted approach centered on effective communication, educational resources, and robust support systems tailored to patient needs is essential. By prioritizing these strategies, organizations can significantly improve patient satisfaction and retention rates.
Key Strategies for Patient Engagement:
Organizations that embrace patient engagement report 30% higher retention rates (source: European Medicines Agency). This commitment to patient-centered practices ensures the collection of high-quality data, ultimately leading to more successful research outcomes. The principle 'Nothing about us, without us, is for us' emphasizes the necessity of viewing patients as partners in research. To further enhance engagement, researchers should actively listen to patient feedback and adapt their strategies accordingly.
Effective post-trial monitoring and follow-up, guided by trial monitoring standards under halmed, are essential for assessing long-term outcomes and gathering participant feedback in clinical research. This process typically involves a combination of surveys, interviews, and health assessments to evaluate the sustained effects of interventions. For example, studies indicate that maintaining contact with participants significantly enhances the credibility of findings and fosters trust within the community.
Consider the COBIN study, which achieved an impressive 83% retention rate at the five-year follow-up. This underscores the importance of ongoing engagement in research. Best practices for follow-up in medical research, guided by trial monitoring standards under halmed, include:
By implementing these strategies, researchers can improve data quality and strengthen ethical standards in clinical trials, ultimately benefiting both participants and the broader healthcare landscape.
The significance of adhering to trial monitoring standards under Halmed is paramount in the field of clinical research. By embracing these standards, organizations can significantly enhance their research capabilities, ensuring that ethical considerations, regulatory compliance, and patient safety are prioritized throughout the study lifecycle.
Key insights discussed highlight the importance of:
Moreover, investing in staff training and leveraging technology for enhanced monitoring are vital components that contribute to the success of clinical trials. Organizations that adopt these best practices not only improve their operational efficiency but also build trust within the research community and among participants.
In conclusion, a commitment to continuous improvement in monitoring practices is essential for advancing clinical research. By actively engaging patients and implementing thorough post-trial follow-up, researchers can collect high-quality data and enhance the credibility of their findings. Embracing these essential trial monitoring standards under Halmed will ultimately lead to more reliable outcomes, benefiting both the medical community and the patients it serves.
What is bioaccess® and what role does it play in clinical trial monitoring?
bioaccess® is a leader in managing research studies, focusing on innovative practices that align with trial monitoring standards under Halmed. They emphasize ethical approvals and enhance patient enrollment efficiency.
How quickly can bioaccess® achieve ethical approvals for clinical trials?
bioaccess® can achieve ethical approvals in a remarkable 4-6 weeks.
What are the benefits of bioaccess®'s approach to patient enrollment?
Their approach accelerates patient enrollment by 50% compared to traditional markets, resulting in substantial cost savings of $25,000 per patient.
How does bioaccess® ensure the safety and integrity of clinical studies?
bioaccess® ensures patient safety and data integrity by integrating advanced methods that comply with trial monitoring standards, including centralized oversight and collaboration among research teams.
What services does bioaccess® provide for regulatory compliance in clinical trials?
bioaccess® offers extensive research management services, including feasibility studies, site selection, compliance reviews, setup, and project management.
Why is understanding local and international regulations important for clinical trials?
Familiarity with regulations is crucial for ensuring regulatory adherence, safeguarding patient rights, and bolstering the credibility of research findings.
What is the significance of risk management in clinical research according to bioaccess®?
Effective risk management is essential for identifying potential risks early, developing mitigation plans, and improving participant safety, which leads to more reliable results and enhanced study performance.
What percentage of research sites reported insufficient time for researchers as a frequent risk?
A substantial 70% of research sites reported that insufficient time for researchers is a frequent risk.
How do research teams feel about using tailored risk management tools?
87% of sites expressed a willingness to utilize a tailored risk management tool to enhance their risk assessment practices.
What impact does a well-structured risk management approach have on clinical research?
A well-structured risk management approach safeguards participants, fosters accountability and transparency within research teams, and is essential for navigating the complexities of clinical research.