


The article presents ten innovative clinical trial study designs that are pivotal in accelerating research outcomes.
By integrating strategies such as:
These methodologies significantly enhance participant engagement, streamline processes, and improve the overall efficiency of clinical research. This ultimately leads to faster and more effective therapeutic developments, underscoring the critical role of innovation in the Medtech landscape.
Innovative clinical trial study designs are revolutionizing the landscape of medical research, paving the way for faster and more efficient pathways to market new therapies. By leveraging cutting-edge methodologies, researchers can streamline processes while enhancing patient engagement and safety. As the demand for quicker results intensifies, a pivotal question emerges: how can these innovative designs be effectively implemented to surmount traditional barriers in clinical research, ensuring both speed and quality in the pursuit of medical advancements?
bioaccess® stands at the forefront of research innovation, employing unique clinical trial study designs that significantly accelerate the research process. By harnessing the regulatory agility of Latin America, the diverse populations in the Balkans, and the streamlined pathways in Australia, bioaccess® not only expedites research studies but also enhances their overall effectiveness.
This groundbreaking methodology enables ethical approvals within an impressive timeframe of just 4-6 weeks and achieves enrollment rates that are 50% faster than traditional markets. Furthermore, with over 80% of potential participants actively seeking research studies online, bioaccess® effectively engages patients in the research process.
Decentralized medical studies, a cornerstone of bioaccess®'s strategy, can achieve retention rates as high as 85%, compared to 60% in conventional settings. Such advancements position bioaccess® as a vital partner for Medtech, Biopharma, and Radiopharma innovators eager to accelerate their market entry.
With a proven track record spanning over 20 years in Medtech, bioaccess® specializes in clinical trial study designs, managing:
This ensures comprehensive research management services, encompassing:
Additionally, bioaccess® leverages pre-qualified networks to activate sites swiftly, enhancing the overall efficiency of the research process. As bioaccess® states, "This innovative methodology not only permits ethical approvals within an impressive timeframe of just 4-6 weeks but also boosts enrollment rates by 50%.
Basket studies empower researchers to evaluate a single therapy across diverse groups of individuals possessing specific biomarkers or genetic traits. This design proves particularly advantageous in oncology, where therapies can be tailored to distinct tumor types. By categorizing individuals into 'baskets' based on shared characteristics, researchers streamline data collection, thereby accelerating clinical trials and potentially expediting the approval process for effective therapies.
With bioaccess®, researchers can enroll treatment-naive cardiology or neurology cohorts 50% faster than traditional Western sites, achieving substantial cost savings of $25K per individual through FDA-ready data—eliminating rework and delays. This innovative method not only hastens the identification of promising treatments but also enhances the likelihood of success across varied patient demographics.
Recent studies, including the Basket of Baskets (BoB) experiment, underscore the effectiveness of this approach, having successfully treated over 170 individuals and demonstrating its capacity to deliver personalized therapies. Moreover, the cost-effective nature of basket studies can lead to a 20-30% reduction in total research expenses, making them an appealing option for medical research in Argentina.
As the landscape of therapeutic agents evolves, the adoption of clinical trial study designs, especially basket study designs, is gaining prominence in oncology, where precision medicine is crucial. Furthermore, bioaccess® offers comprehensive management services for studies, encompassing:
These elements are vital for fostering innovative therapies and improving patient outcomes. This emphasizes the importance for researchers to contemplate the broader implications of basket studies in their endeavors.
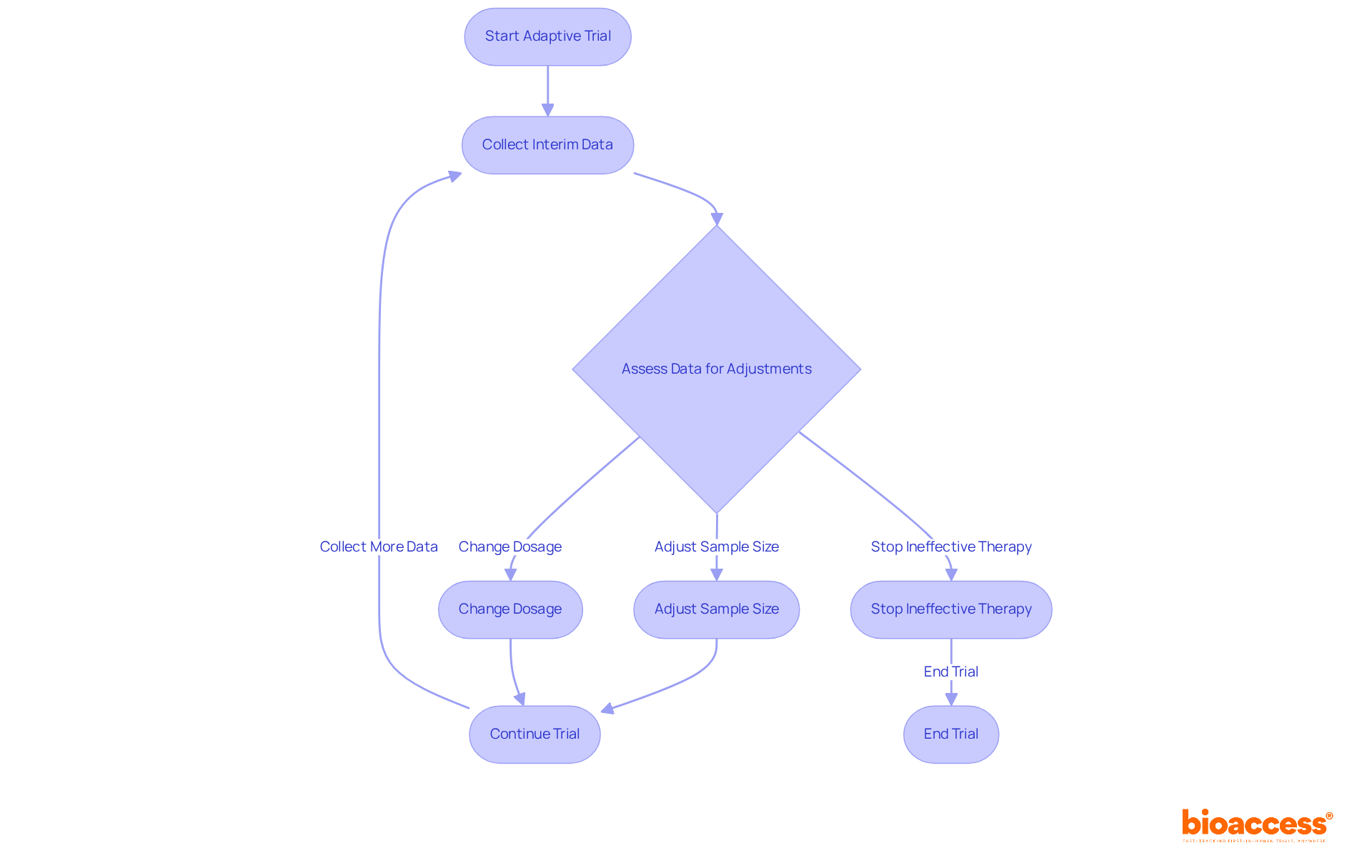
Clinical trial study designs, such as adaptive trial designs, empower researchers to implement real-time adjustments to trial protocols based on interim data, significantly enhancing the efficiency of clinical research. This flexibility permits essential modifications, such as adjusting sample sizes, changing dosage amounts, or even stopping ineffective therapies early in the process. For instance, the continuous reassessment model (CRM) has demonstrated superior accuracy in estimating the maximum tolerated dose compared to traditional methods, ensuring that participants receive optimal treatment while minimizing exposure to ineffective therapies.
Current trends indicate a growing adoption of clinical trial study designs, with a notable increase in their utilization from 2006 to 2021, particularly in 2020 when 41 new cases were recorded. These designs not only simplify the research process but also emphasize patient safety, as they enable faster decision-making and resource distribution. Indeed, research indicates that clinical trial study designs utilizing adaptive methods can result in a mean decrease of 22% in sample size, further enhancing the efficiency of the study.
At bioaccess, our extensive clinical study management services, which support the execution of clinical trial study designs, include:
Specialists in the area highlight the significance of openness and thorough preparation in adaptive studies. As one noted, 'The best way to minimize operational bias is through rigorous planning and transparency.' This dedication to transparency guarantees that modifications are well-justified and that the integrity of the process is upheld.
In 2025, the emphasis on immediate modifications in clinical trial study designs continues to progress, with scientists increasingly acknowledging the importance of flexible approaches. By utilizing these innovative designs and the strong support from bioaccess, medical studies can not only achieve quicker results but also improve the overall success rate, ultimately benefiting patient outcomes and advancing medical research.
First-in-human (FIH) studies are essential for evaluating the safety and initial efficacy of new medical interventions. Typically involving a small group of 10 to 30 participants, these studies aim to gather critical information about how an intervention functions in humans for the first time. The importance of FIH studies has increased in 2025, as they are acknowledged for their capacity to provide early insights into a treatment's viability, which is vital for informing subsequent clinical trial study designs.
Conducting FIH studies in regions with expedited regulatory processes, such as those provided by bioaccess®, can significantly shorten the timeline for introducing innovative therapies to the market. For example, the regulatory speed in Latin America allows for ethical approvals in just 4 to 6 weeks, facilitating quicker enrollment compared to traditional markets. This efficiency not only accelerates the development process but also enhances the likelihood of successful outcomes. Insights from Steve Garchow emphasize the importance of understanding local market dynamics and regulatory landscapes, which can further optimize the process.
Current success rates for FIH studies in Medtech and Biopharma are promising, with numerous experiments demonstrating favorable safety profiles and initial efficacy. Notable examples include trials that have led to groundbreaking therapies, underscoring the regulatory advantages of conducting studies in regions with streamlined procedures. These preliminary evaluations are crucial for determining the future direction of medical development and shaping clinical trial study designs, ultimately benefiting patients and fostering health innovation. Furthermore, it is imperative that informed consent is obtained from all participants and that studies are overseen by an institutional review board (IRB) to ensure ethical compliance. The collaboration with LATAM CRO experts, as exemplified by Dr. John B. Simpson's work on Avinger's OCT-guided atherectomy research in Cali, Colombia, further highlights the significance of local partnerships in navigating the complexities of the Latin American Medtech landscape.
Early-feasibility studies (EFS) serve as crucial initial investigations that assess the safety and functionality of new medical devices prior to extensive patient testing. These studies typically involve a small cohort of participants, concentrating on gathering essential data that informs subsequent development phases.
Conducting EFS in regions with favorable regulatory frameworks, such as Latin America, enables innovators to validate their concepts swiftly and effectively. This strategic approach not only accelerates the timeline for groundbreaking medical technologies but also enhances the likelihood of successful market entry.
For example, Avantec Vascular Corporation has selected Latin America for its first-in-human study of an innovative vascular device, with bioaccess® providing support in selecting a principal investigator and managing regulatory submissions.
With over 20 years of experience in overseeing clinical studies, bioaccess® offers extensive services, including site selection, compliance assessments, and project management, all of which are vital for the success of EFS.
As the landscape of EFS evolves, current trends indicate a growing emphasis on reducing timelines and improving outcomes, positioning EFS as an invaluable tool for Medtech innovators striving to bring their devices to market more rapidly. Innovators acknowledge that embracing EFS fosters more informed decision-making and ultimately leads to better patient outcomes.
Multi-arm studies represent a robust type of clinical trial study designs that facilitate the concurrent evaluation of several intervention arms within a single investigation. This approach empowers researchers to compare the efficacy of various interventions, maximizing resource efficiency and significantly reducing the time required to gather comparative data. By employing multi-arm studies, clinical researchers can swiftly identify the most effective therapies, thereby streamlining the development process and accelerating the journey to market for successful solutions.
The advantages of multi-arm clinical trial study designs are particularly pronounced in therapeutic fields with numerous intervention options. For instance, multi-arm multi-stage (MAMS) studies not only enable the assessment of multiple active therapies against a single control group but also allow for the early termination of ineffective treatments. This ethical strategy enhances patient safety by reallocating resources to more promising interventions.
Recent trends in multi-arm clinical trial study designs reveal a notable increase in their adoption, with 2025 witnessing a surge in studies that utilize adaptive designs. These studies are crafted to be adaptable, permitting the inclusion of new intervention arms based on preliminary evaluations that assess intervention potential. This flexibility is crucial for addressing various research inquiries within one protocol, ultimately boosting the efficiency of discovering effective solutions.
Successful examples of clinical trial study designs highlight the efficiency of multi-arm studies. MAMS studies, for example, have been instrumental in refining numerous competing therapies to a select few that exhibit the greatest potential, thus expediting the decision-making process regarding treatment effectiveness. With bioaccess®'s FDA-ready data, researchers can enroll treatment-naive cardiology or neurology groups 50% faster than conventional methods, realizing substantial savings of $25K for each individual. This efficiency is achieved through streamlined processes and real-time data access, which enhance decision-making and resource allocation.
As the landscape of medical research continues to evolve, the implementation of clinical trial study designs, supported by bioaccess®, is expected to play a pivotal role in advancing medical innovations. To fully leverage the benefits of these studies, medical researchers should consider integrating bioaccess® into their study frameworks to enhance participant recruitment and optimize resource utilization.
Pragmatic studies are fundamentally designed to evaluate the efficacy of interventions in real-world environments, offering insights that are directly pertinent to medical practice. Unlike conventional studies, which often operate within regulated settings, pragmatic experiments embrace diverse participant groups and standard clinical processes. This approach enables researchers to collect data on treatment effectiveness in everyday situations, thereby informing healthcare decisions and enhancing outcomes for patients.
Current trends reveal an increasing focus on patient population diversity within pragmatic studies, underscoring the necessity for research that reflects the complexities of real-world healthcare. Successful examples of pragmatic studies demonstrate their capacity to generate actionable insights, bridging the gap between research and medical practice. Gracy Crane, for instance, emphasized that pragmatic studies aim to ascertain whether a therapy is effective in varied, real-world contexts—an inquiry often left unanswered by conventional randomized controlled trials (RCTs) due to their regulated nature and homogeneous populations. The 2025 SCOPE Summit highlighted the importance of these studies in evaluating treatment efficacy across diverse environments, showcasing their potential to shape medical protocols and enhance patient care.
As the landscape of medical research evolves, pragmatic studies are increasingly recognized for their role in assessing interventions in alignment with routine healthcare practice. This transformation not only boosts the relevance of clinical research but also promotes a more patient-centric approach, ultimately advancing medical knowledge and improving health outcomes. Nevertheless, challenges to the genuine implementation of pragmatic studies, such as onboarding community clinics and training research-naive providers, remain significant hurdles that must be addressed.
Platform studies represent a creative framework that facilitates the concurrent assessment of various therapies under a single protocol. This innovative method streamlines the research process by permitting the inclusion or exclusion of intervention arms based on interim results. By utilizing platform studies, researchers can efficiently evaluate the efficacy of different interventions, thereby minimizing the time and resources required to introduce new therapies to the market. This design proves particularly advantageous in rapidly evolving fields such as oncology, where multiple treatment options are frequently explored concurrently.
Decentralized research studies (DCTs) leverage advanced technology to create a more patient-focused research environment, enabling participants to engage in studies from their homes or local healthcare facilities. This innovative methodology significantly enhances access for individuals, particularly those in remote areas or facing mobility challenges. By alleviating travel difficulties and offering greater flexibility, DCTs can improve patient recruitment and retention rates, addressing the alarming statistic that nearly 85% of studies struggle to maintain sufficient participants, with an average dropout rate of approximately 30%.
The integration of digital tools for data collection and monitoring not only streamlines the process but also boosts overall efficiency, leading to improved outcomes for participants. Comprehensive research study management services, including feasibility assessments, compliance evaluations, and study setup provided by bioaccess, are crucial in ensuring the effective execution of DCTs. For instance, Walgreens' experience over three years in conducting trials illustrates how the incorporation of DCTs into pharmacy operations has significantly enhanced access and participation in research, resulting in superior enrollment and retention outcomes.
As we approach 2025, the shift towards DCTs is expected to accelerate, driven by the demand for more inclusive and accessible research methodologies. Industry leaders emphasize that DCTs not only facilitate participation but also empower patients by granting them greater control over their involvement in research studies. This transition to a more decentralized model is vital for overcoming the challenges that have historically impeded recruitment and retention in clinical trial study designs.
Data-informed designs in medical studies leverage real-time insights and analytics to enhance decision-making during the research process. This proactive method not only improves the overall efficiency of research trials but also reduces risks, significantly speeding up the time to market for new therapies.
By utilizing sophisticated data gathering and evaluation methods, researchers can:
With bioaccess®, researchers can enroll treatment-naive cardiology or neurology groups 50% quicker than conventional Western locations, achieving $25K savings per individual with FDA-ready data—no rework, no delays.
Prioritizing data-driven methodologies empowers clinical researchers to improve patient outcomes, ensuring that innovative treatments are delivered to those in need more rapidly.
As we move towards 2025, the integration of real-time data analytics is expected to become even more critical, with trends indicating a shift towards patient-centric models that leverage real-world evidence for more inclusive and representative trials.
The exploration of innovative clinical trial study designs unveils a transformative approach to medical research, underscoring the urgent need for faster and more efficient pathways to bring new therapies to market. By embracing these cutting-edge methodologies, researchers can significantly enhance participant engagement, streamline processes, and ultimately improve patient outcomes.
Throughout the article, several key designs have been highlighted, including decentralized trials, adaptive designs, and multi-arm studies. Each of these approaches offers unique advantages, such as quicker enrollment, real-time adjustments, and the ability to evaluate multiple treatments simultaneously. The role of organizations like bioaccess® is crucial in supporting these methodologies, providing the expertise and resources necessary for faster ethical approvals and effective study management.
As the landscape of clinical research continues to evolve, the embrace of these innovative study designs becomes essential for advancing medical knowledge and enhancing healthcare delivery. Researchers are encouraged to integrate these methodologies into their trials, leveraging the insights and efficiencies they offer to drive meaningful change in patient care and therapeutic development.
What is bioaccess® and what does it focus on?
bioaccess® is a research innovation company that employs unique clinical trial study designs to accelerate the research process, focusing on enhancing the effectiveness of clinical trials through regulatory agility and diverse populations.
How does bioaccess® expedite the clinical trial process?
bioaccess® achieves ethical approvals in just 4-6 weeks and enrolls participants 50% faster than traditional markets, leveraging the regulatory environment in Latin America, the Balkans, and streamlined pathways in Australia.
What types of studies does bioaccess® specialize in?
bioaccess® specializes in various study designs, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies.
What services does bioaccess® offer for clinical trial management?
bioaccess® provides comprehensive research management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting.
What are basket clinical trials and their advantages?
Basket clinical trials allow researchers to evaluate a single therapy across diverse groups with specific biomarkers or genetic traits, particularly in oncology. This design accelerates data collection and the approval process for effective therapies.
How does bioaccess® enhance the efficiency of basket studies?
bioaccess® enables researchers to enroll treatment-naive cohorts in cardiology or neurology 50% faster than traditional sites, achieving significant cost savings and eliminating delays.
What are adaptive trial designs and their benefits?
Adaptive trial designs allow researchers to make real-time adjustments to trial protocols based on interim data, enhancing efficiency by enabling modifications like sample size changes or early stopping of ineffective therapies.
What are the trends in the adoption of adaptive trial designs?
There has been a growing adoption of adaptive trial designs, with a notable increase in utilization from 2006 to 2021, particularly in 2020, emphasizing patient safety and faster decision-making.
How does bioaccess® support adaptive trial designs?
bioaccess® provides extensive clinical study management services that support the execution of adaptive trial designs, including feasibility studies, site selection, compliance reviews, and project management.
What is the significance of transparency in adaptive studies?
Transparency and rigorous planning are crucial in adaptive studies to minimize operational bias and ensure that modifications are justified, maintaining the integrity of the research process.