


The primary focus of the article titled "10 Insights on Decentralized Clinical Trial Success" is to underscore the critical factors that drive the success of decentralized clinical trials. It highlights the significance of:
These elements collectively enhance engagement, streamline processes, and ultimately improve patient outcomes in clinical research.
Decentralized clinical trials are swiftly transforming the landscape of medical research, presenting unprecedented opportunities for patient engagement and data integrity. By leveraging advanced technologies and innovative strategies, these trials promise to enhance participant experiences while addressing long-standing challenges in recruitment and retention.
As the industry pivots towards this model, critical questions emerge:
Exploring these insights reveals not only the potential for improved patient outcomes but also underscores the essential roles of adaptability and inclusivity in shaping the future of clinical research.
bioaccess® strategically integrates the regulatory pace of Latin America, diverse patient groups from the Balkans, and efficient pathways in Australia to facilitate decentralized research studies. This innovative methodology not only permits ethical approvals within an impressive timeframe of just 4-6 weeks but also enhances patient enrollment rates by 50%. These attributes position bioaccess® as a frontrunner in the clinical research domain, underscoring its pivotal role in addressing the complexities of the Medtech landscape.
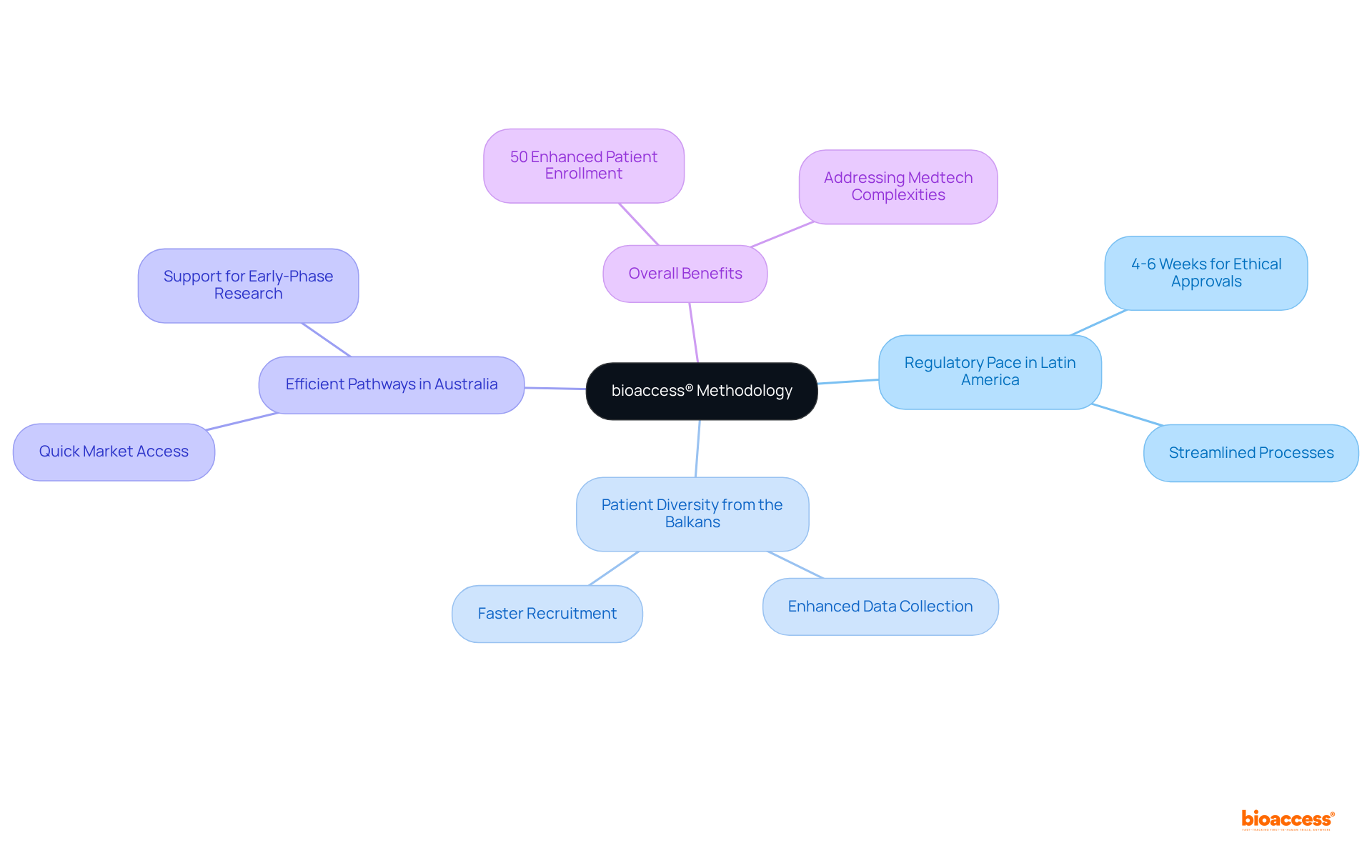
Adopting a patient-focused design in decentralized studies necessitates a thorough strategy that customizes protocols to match individuals' needs and preferences. This approach involves:
Such strategies not only enhance the user experience but also lead to significant improvements in engagement and retention rates. Research has indicated that patient-focused studies can attain retention rates as high as 85%, in contrast to only 60% in conventional environments, according to the Journal of Clinical Epidemiology.
Furthermore, the incorporation of digital platforms—such as ENGAGE!, which includes the MyStudyManager™ Portal and eConsent solutions—has demonstrated effectiveness in promoting continuous communication and assistance. This ultimately decreases dropout rates by proactively addressing individuals' concerns.
By prioritizing patient needs and emphasizing clear communication and trust, decentralized studies can create a more inclusive and effective research environment, driving better outcomes for both participants and researchers.
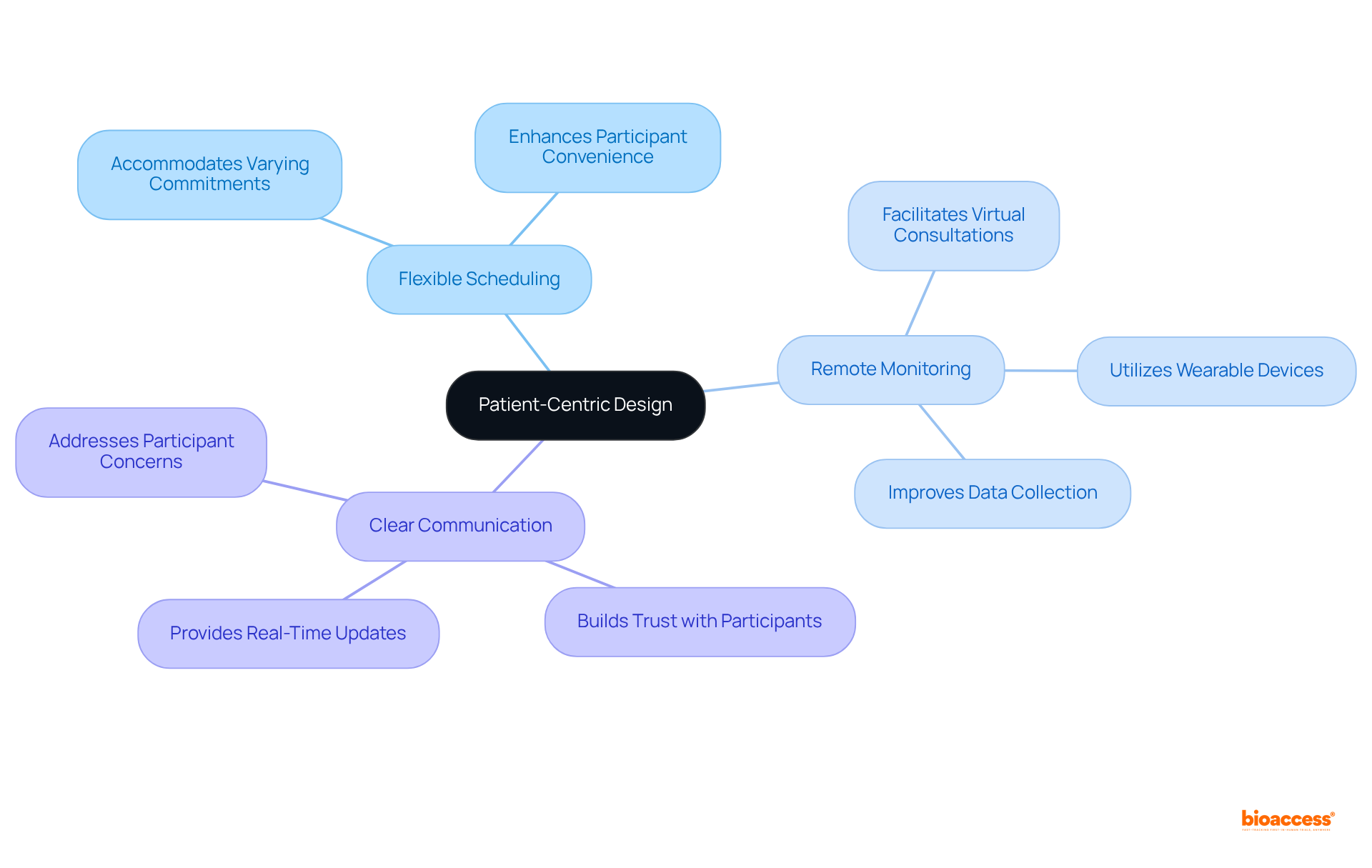
Latin America is recognized for its swift regulatory procedures, significantly expediting the endorsement and initiation of research studies. By capitalizing on these advantages, bioaccess® enhances decentralized clinical trials, ensuring they are not only efficient but also compliant with local regulations. This strategy paves a smoother pathway to market for innovative therapies.
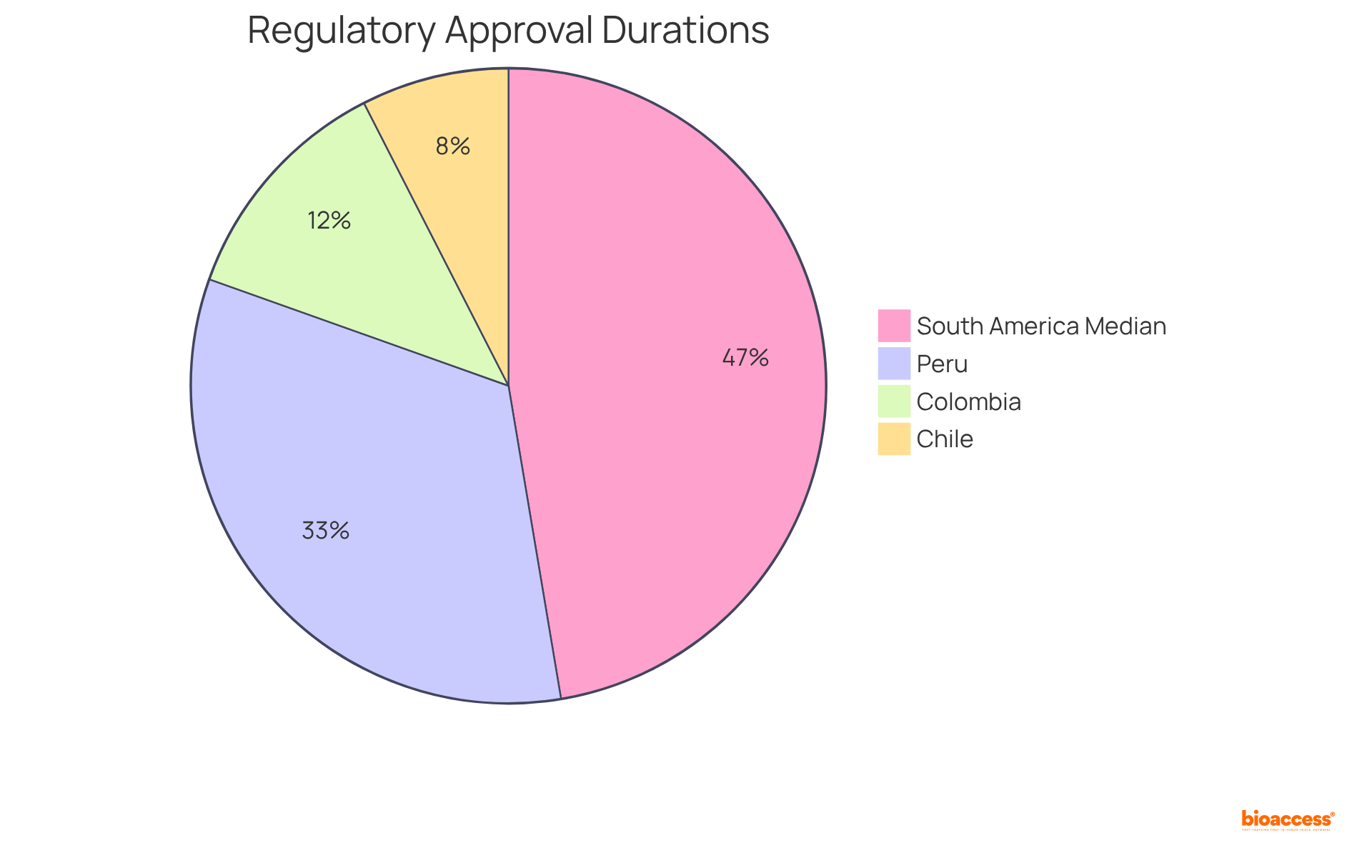
For instance, countries like Chile boast average regulatory approval durations of merely 1 to 1.5 months, while Colombia maintains its research review timelines at 60 calendar days. Peru's average time for regulatory approval is approximately 5-6 months, offering a broader perspective on the region's efficiency. Such rapid approvals are vital in a landscape where the median time for regulatory authority approval in South America can extend up to 236 days.
Furthermore, the region's ability to meet subject enrollment criteria solidifies its status as the world's fourth-largest market for research studies. With a median rating of 4 for the education level of local doctors involved in clinical studies, the region benefits from a well-educated workforce, enhancing the quality of research.
As Henry L. Gómez noted, 'Perceived strengths were the prevailing education levels in personnel, the interest of patients and researchers in taking part in studies...' By leveraging these rapid regulatory processes, bioaccess® empowers Medtech, Biopharma, and Radiopharma innovators to expedite their breakthroughs in decentralized clinical trials, achieving enrollment rates that are 50% faster than traditional markets.
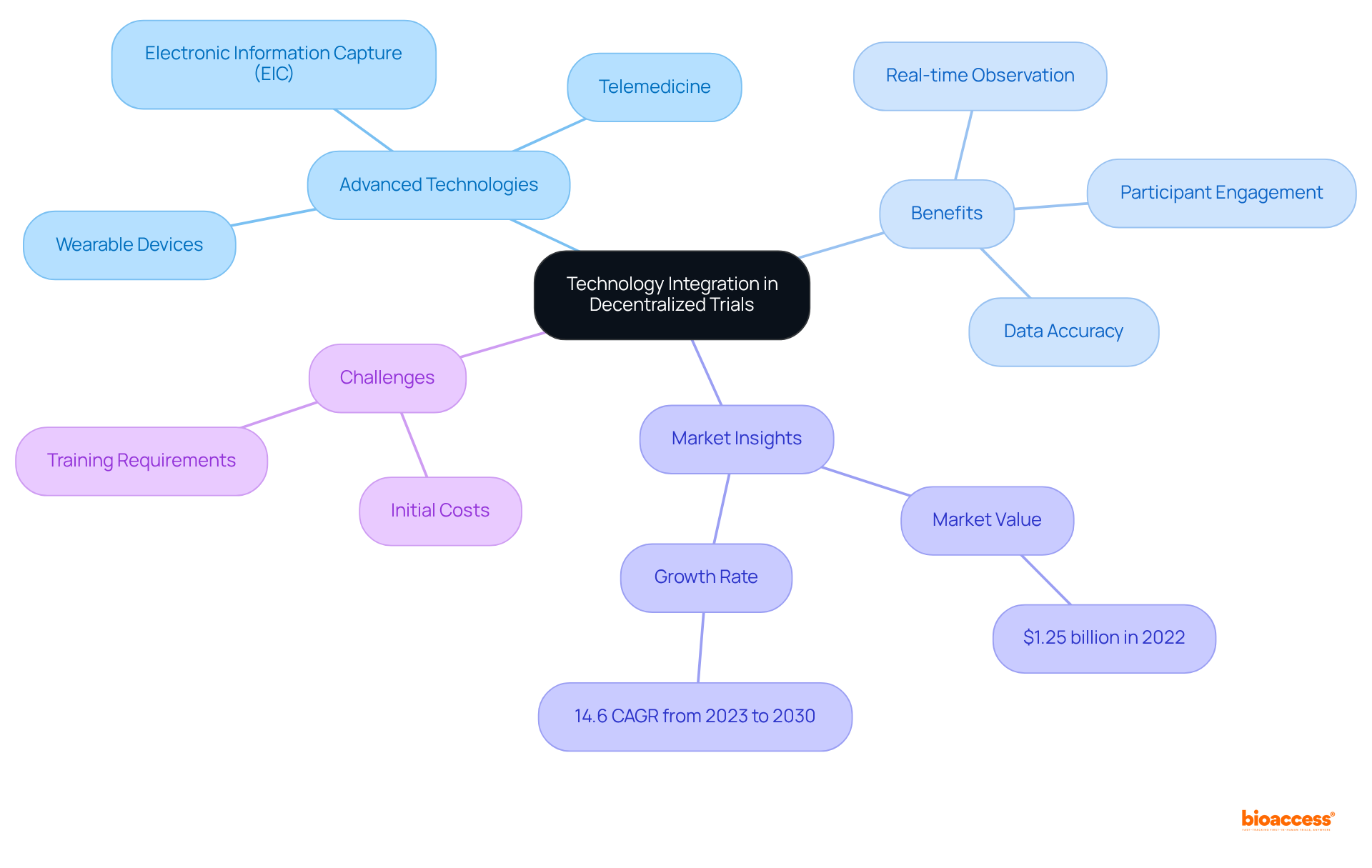
The incorporation of advanced technologies such as electronic information capture (EIC), telemedicine, and wearable devices is revolutionizing information gathering in decentralized studies. EDC systems optimize information management by facilitating real-time observation and input, significantly alleviating the burden on participants while enhancing accuracy. This enhancement is vital for maintaining regulatory compliance and ensuring the integrity of studies.
EDC systems are adaptable to various study sizes and protocols, positioning them as versatile tools across different trial contexts. They have largely replaced traditional paper methods, leading to faster information collection and improved quality, ultimately accelerating the introduction of new therapies to the market. Notably, the global market for EDC systems was valued at $1.25 billion in 2022, with a projected compound annual growth rate of 14.6% from 2023 to 2030, highlighting their increasing significance in clinical research.
Furthermore, the utilization of telemedicine facilitates remote consultations and monitoring, thereby enhancing participant engagement and information gathering. Wearable devices, such as fitness trackers, continuously collect real-time health data, providing researchers with invaluable insights into patient conditions. Collectively, these technologies not only enhance the precision of study information but also contribute to creating a more efficient and participant-friendly research environment.
However, it is essential to consider challenges such as high initial costs and the necessity for extensive training when implementing EDC systems. Additionally, the FDA's recent recommendations regarding digital health technologies for remote data collection underscore the importance of these tools in the evolving landscape of medical studies.
Decentralized clinical trials leverage access to diverse patient groups, especially in regions such as the Balkans and Latin America. Engaging a wide range of participants enhances the representativeness of studies, ensuring that results resonate with the broader population. This inclusivity is essential, as varied demographics can significantly impact treatment responses and outcomes.
For instance, diversity among research participant groups is crucial for assessing the relevance of individual differences. Moreover, integrating diverse patient perspectives can lead to improved recruitment and retention strategies, ultimately elevating the quality of research.
Successful instances of inclusive practices in decentralized clinical trials include community engagement initiatives that build trust and collaboration, as exemplified in the case study on 'Community Engagement in Clinical Trials.' By prioritizing representation in medical research, organizations fulfill their ethical obligations while advancing scientific progress that benefits all segments of society.
It is also noteworthy that only 22% of Americans reported having clinical research mentioned to them by a doctor or healthcare professional, highlighting the pressing need for outreach and education to ensure diverse participation.
Streamlining operational processes in decentralized studies is crucial for optimizing workflows, leveraging digital tools for effective data management, and enhancing communication among stakeholders. This approach minimizes redundancies and boosts coordination, ultimately leading to quicker and more dependable results. By addressing these operational challenges, bioaccess® positions itself as a leader in the Medtech landscape, providing solutions that meet the evolving needs of clinical research.
Informed consent stands as a cornerstone of ethical medical research, particularly in decentralized studies where participant involvement is paramount. The integration of digital platforms into the consent process not only streamlines the procedure but also enhances transparency, fostering a sense of security among participants. This trust is vital; research indicates that 84% of individuals are more likely to participate in studies if the consent process is conducted remotely, underlining the importance of effective communication and informed consent in building confidence.
Keri Henderson, Vice President of Information Technology, notes that participants in clinical studies exhibit a greater propensity to engage when provided with remote options, reiterating the critical role of informed consent in this setting. For instance, a 2018 study revealed that all patients who opted for a decentralized study expressed satisfaction with the technology employed, including electronic informed consent (eIC) and telemedicine visits. Such positive experiences can profoundly influence participant willingness and retention.
Furthermore, as digital technology becomes increasingly woven into clinical studies, it is imperative to address potential concerns surrounding privacy and operational complexities. By ensuring that informed consent processes are both clear and accessible, researchers can alleviate these concerns and bolster trust among participants, ultimately contributing to the success of decentralized clinical trials.
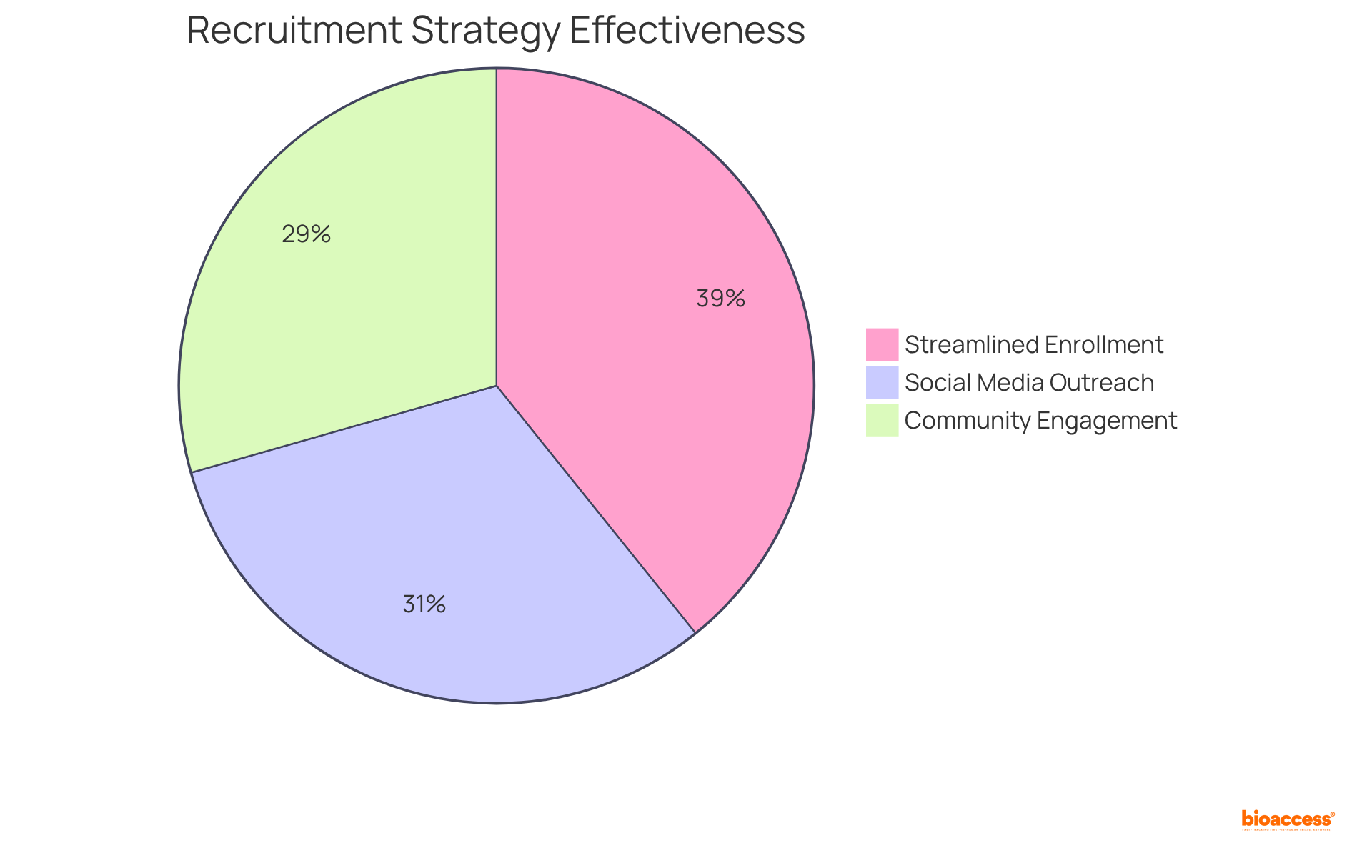
To effectively overcome recruitment obstacles in decentralized clinical trials, it is essential to implement strategies that utilize social media, involve community organizations, and streamline enrollment processes. Notably, over 80% of potential contributors actively seek research studies online, underscoring the necessity for bioaccess® to establish a robust digital presence that enhances outreach and engages prospective contributors.
By leveraging platforms like Power, researchers can effectively connect with patients interested in research studies, thereby increasing diversity and accessibility. Furthermore, community service events have demonstrated effectiveness, achieving a completion rate of 75% among prescreened individuals. This illustrates how bioaccess® can engage local communities to strengthen recruitment efforts.
Streamlining enrollment procedures not only alleviates the burden on individuals but also enhances the attractiveness of studies, as evidenced by the 100% completion rate achieved through referrals—strategies that bioaccess® actively employs to boost retention and engagement.
By prioritizing these strategies, bioaccess® can significantly improve enrollment rates and ensure a diverse participant base, ultimately contributing to the success of decentralized clinical trials.
Decentralized clinical trials are revolutionizing patient care by enhancing access to innovative treatments and minimizing participation barriers. By enabling patients to engage in studies from the comfort of their homes, these investigations not only improve retention rates but also facilitate more comprehensive data collection.
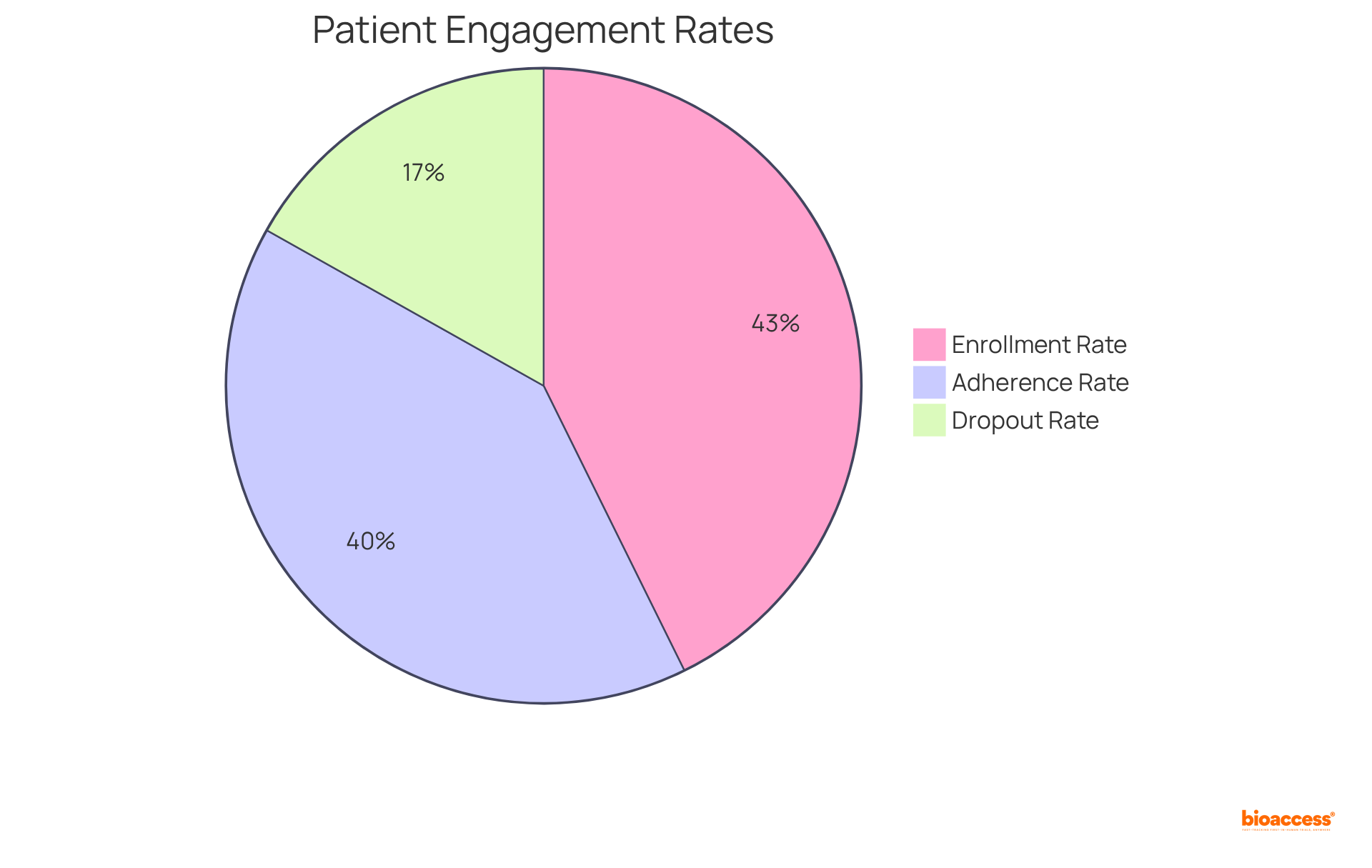
For example, a telehealth-delivered home-based prehabilitation program for cancer patients achieved an impressive 76% enrollment rate and a 72% adherence rate, clearly illustrating the efficacy of remote participation in sustaining patient engagement.
Furthermore, the shift towards decentralized studies significantly alleviates the travel burden on participants, with research indicating that 44% of participants find traveling to study clinics burdensome. This highlights the growing challenges associated with traditional participation and underscores the importance of patient-centric strategies.
Additionally, the average dropout rate across all research studies stands at 30%, emphasizing the financial implications of poor retention and the urgent need for improved strategies in decentralized studies.
By leveraging telehealth and virtual technologies, a decentralized clinical trial can enhance patient health and well-being, ultimately leading to better outcomes and more reliable data integrity. This innovative model not only addresses the hurdles faced during conventional study involvement but also aligns with the increasing demand for convenience among potential participants, making decentralized clinical trials a promising avenue for future medical research.
Decentralized clinical trials are poised to revolutionize the landscape of medical research, driven by rapid technological advancements and evolving regulatory frameworks. This shift is expected to position the decentralized clinical trial approach as the norm, significantly enhancing patient engagement and improving the quality of data collected.
Automation in clinical studies is becoming essential for boosting efficiency, with automation tools simplifying operations, thereby reducing costs and operational burdens. For instance, in a Phase II study on Parkinson’s disease, 80% of data was submitted via mobile applications, demonstrating technology's capacity to facilitate real-time data collection and patient involvement.
As the healthcare data landscape expands—projected to achieve a compound annual growth rate of 36% by 2025—these innovations will foster more efficient and effective research methodologies. The transition towards decentralized clinical trials not only promises to accelerate medical research but also aims to deliver more personalized and effective therapies, ultimately enhancing patient outcomes and safety.
Additionally, bioaccess® ensures that enrollment in decentralized clinical trials occurs 50% faster than in traditional markets, highlighting the efficiency of these trials. As Nicholas Borys aptly noted, 'Clinical trials are better, faster, cheaper with big data,' emphasizing the pivotal role of technology in shaping the future of clinical research.
Decentralized clinical trials signify a transformative shift in medical research, emphasizing patient-centric approaches and leveraging technology to enhance efficiency and engagement. By integrating diverse patient populations and streamlining regulatory processes, these trials not only accelerate the research timeline but also improve the overall experience for participants. Insights shared highlight how organizations like bioaccess® are leading the charge in this evolution, ensuring that decentralized trials can address the complexities of modern healthcare.
The multifaceted benefits of decentralized trials are evident, including:
The focus on operational efficiency, ethical considerations, and effective recruitment strategies underscores the importance of adapting to the changing landscape of clinical research. Each of these elements contributes to a robust framework that enhances the quality of research while prioritizing participant well-being.
As the future of clinical trials unfolds, embracing decentralized methodologies is crucial for fostering inclusivity and improving patient outcomes. Ongoing advancements in technology and a commitment to ethical practices will shape a new era of medical research that is more responsive to the needs of diverse populations. Stakeholders are encouraged to actively engage with these insights and consider how they can implement strategies that support the growth and success of decentralized clinical trials, ultimately leading to breakthroughs that benefit all.
What is bioaccess® and how does it contribute to decentralized clinical trials?
bioaccess® integrates the regulatory pace of Latin America, diverse patient groups from the Balkans, and efficient pathways in Australia to facilitate decentralized research studies. It allows for ethical approvals in just 4-6 weeks and enhances patient enrollment rates by 50%, positioning itself as a leader in the clinical research domain.
How does patient-centric design enhance engagement in decentralized trials?
A patient-centric design customizes protocols to meet individual needs and preferences, offering flexible scheduling, remote monitoring technologies, and clear communication. This approach leads to improved engagement and retention rates, with studies showing retention rates of up to 85% compared to 60% in traditional settings.
What role do digital platforms play in decentralized trials?
Digital platforms like ENGAGE!, which includes the MyStudyManager™ Portal and eConsent solutions, promote continuous communication and support, helping to address participants' concerns proactively. This can lead to decreased dropout rates and a more inclusive research environment.
What are the advantages of conducting decentralized trials in Latin America?
Latin America is known for its swift regulatory procedures, with countries like Chile and Colombia offering rapid approval times of 1-1.5 months and 60 days, respectively. This efficiency helps bioaccess® ensure compliance and expedites the initiation of research studies, paving smoother pathways to market for innovative therapies.
How does the education level of local doctors impact clinical research in Latin America?
The region benefits from a well-educated workforce, with a median rating of 4 for the education level of local doctors involved in clinical studies. This enhances the quality of research and supports the region's status as a significant market for research studies.
What is the significance of rapid regulatory approvals in decentralized clinical trials?
Rapid regulatory approvals are crucial in reducing the time it takes to bring innovative therapies to market. bioaccess® leverages these processes to achieve enrollment rates that are 50% faster than traditional markets, benefiting Medtech, Biopharma, and Radiopharma innovators.