

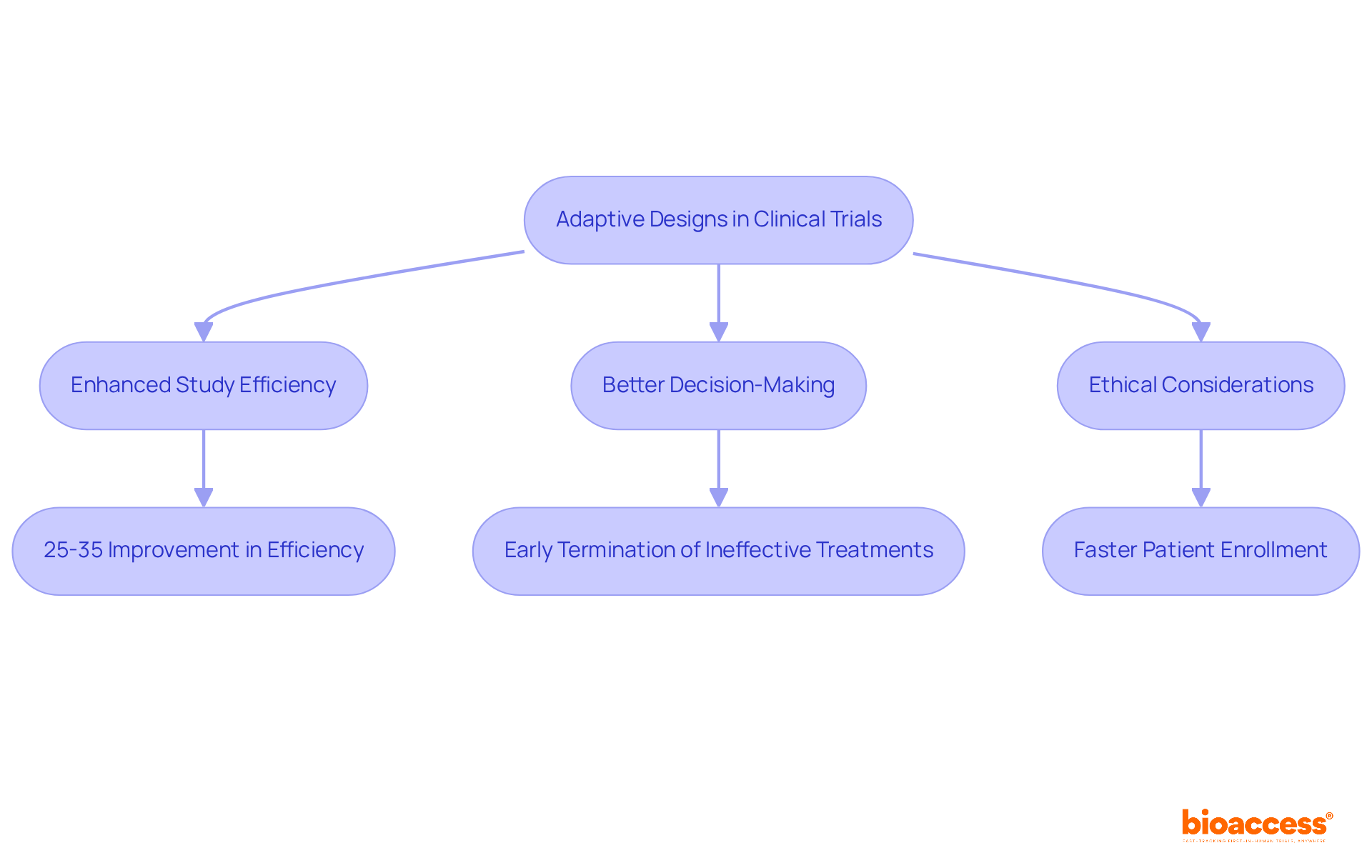
The article emphasizes the pivotal advantages of adaptive designs in clinical trials, underscoring their capacity to enhance efficiency, optimize resource allocation, and improve patient outcomes. This assertion is supported by a discussion on how adaptive designs facilitate real-time adjustments based on interim results, potentially leading to a remarkable 25-35% improvement in experimental efficiency and superior decision-making in clinical research.
Adaptive designs in clinical trials are revolutionizing the landscape of medical research. This dynamic approach enhances efficiency and patient safety, capturing the attention of researchers and stakeholders alike. By enabling real-time modifications based on interim data, these designs optimize resource allocation and significantly improve the likelihood of successful outcomes.
However, as the adoption of adaptive methodologies grows, so do the complexities and challenges associated with their implementation.
What are the key benefits that make adaptive designs a game-changer?
How can researchers navigate the hurdles to fully harness their potential?
These questions are crucial as we explore the transformative impact of adaptive designs on clinical research.
bioaccess® leverages its extensive expertise in early-phase clinical research to implement adaptive designs for clinical trials that significantly enhance study efficiency. By capitalizing on Latin America's regulatory agility and diverse patient demographics, bioaccess® adeptly utilizes adaptive designs for clinical trials, adjusting research protocols in real-time to ensure that studies align with current data and patient needs. This adaptability not only optimizes resource allocation but also increases the likelihood of favorable outcomes, with adaptive designs for clinical trials yielding a 25-35% improvement in experimental efficiency.
Clinical research leaders emphasize that adaptive designs for clinical trials facilitate better decision-making and ethical considerations by allowing for the early termination of ineffective treatments, thereby minimizing patient exposure to suboptimal interventions. As Anna Heath asserts, adaptive designs for clinical trials often include stopping rules to terminate arms that are not working, which can prevent exposing more people to ineffective or suboptimal treatments.
Looking ahead to 2025, the integration of flexible approaches is expected to continue transforming research studies, making them more responsive and effective than ever. However, the execution of adaptive designs for clinical trials may introduce complexities in planning and implementation, necessitating meticulous statistical planning and regulatory approval. With bioaccess®'s innovative approach, clients can achieve patient enrollment 50% faster and save $25K per patient with FDA-ready data, ensuring a streamlined and effective research process.
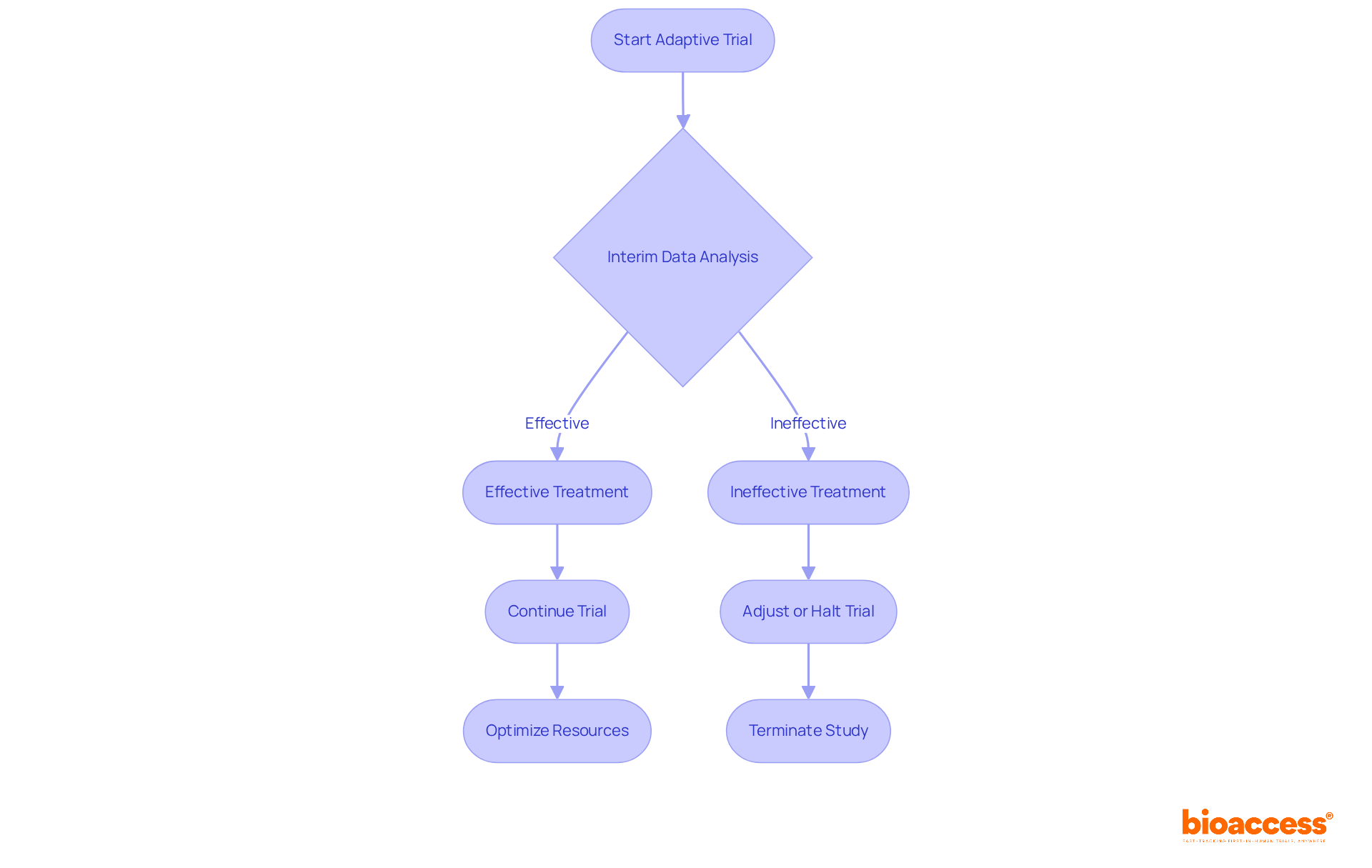
Adaptive designs for clinical trials provide considerable flexibility, allowing researchers to modify study parameters based on interim outcomes. This adaptability not only optimizes resource utilization but also minimizes patient exposure to ineffective treatments, thereby expediting the identification of successful therapies.
For instance, when early data suggests that a treatment is ineffective, the trial can be adjusted or halted, conserving both time and financial resources that would otherwise be allocated to a failing study. Notably, 41% of studies incorporated stopping thresholds for premature termination based on interim analysis, underscoring the operational efficiency of these frameworks.
Such flexibility facilitates real-time adjustments in adaptive designs for clinical trials that align with emerging data, ultimately leading to more informed decision-making in clinical research. However, it is essential to consider potential disadvantages, such as operational biases and the complexity of statistical methods, which can affect the effectiveness of adaptive approaches.
As Edward L. Korn noted, interim monitoring is influenced by both ethical and resource considerations, promoting timely decisions that enhance study integrity.
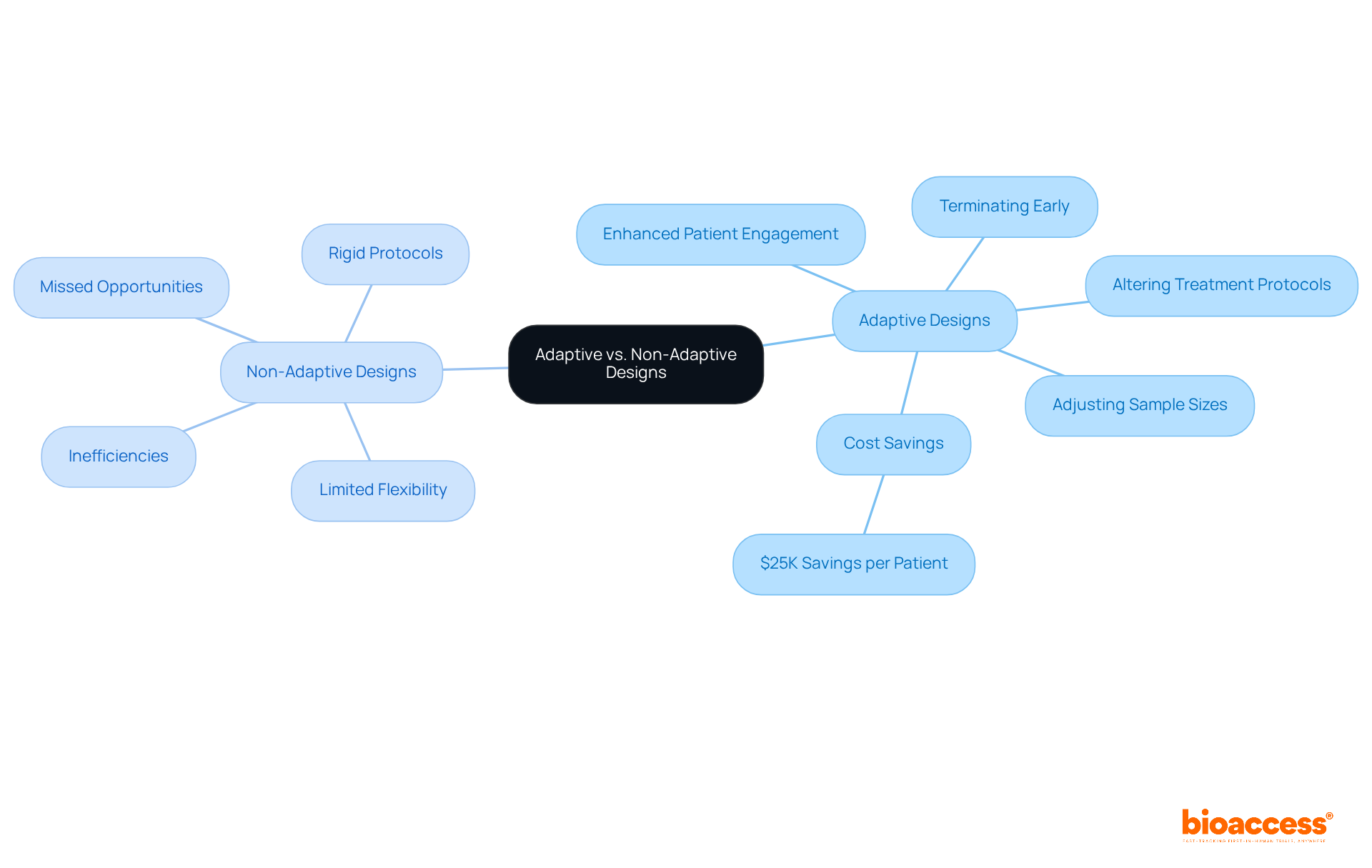
Adaptive designs for clinical trials stand in stark contrast to non-adaptive methods, especially in their ability to leverage real-time information during the testing process. Non-adaptive frameworks adhere to rigid protocols, often resulting in inefficiencies and missed opportunities for improvement. Conversely, flexible approaches facilitate modifications, such as:
This inherent adaptability not only streamlines the testing procedure but also significantly enhances the likelihood of success. For example, bioaccess® allows treatment-naive cardiology or neurology cohorts to be enrolled 50% faster than traditional Western sites, resulting in substantial savings of $25K per patient with FDA-ready data—no rework, no delays. The ability to implement real-time adjustments can expedite enrollment and decrease dropout rates, ultimately fostering greater patient engagement and retention.
As medical studies evolve, the adoption of adaptive designs for clinical trials is becoming increasingly vital, empowering researchers to respond dynamically to new information and optimize results effectively. The implications of this shift are profound, as adaptive designs for clinical trials are set to revolutionize the field of medical research, making studies more efficient and aligned with patient needs. Notably, statistics reveal that 70% of clinical study protocols necessitate two to three modifications throughout the research duration, underscoring the critical need for adaptability.
Furthermore, as one specialist articulated, "Clinical studies are improved, quicker, more economical with big data," which highlights the advantages of flexible structures that rely on real-time information. Additionally, real-time monitoring reduces reliance on self-reported data, enhancing data accuracy and contributing to the overall success of flexible studies.
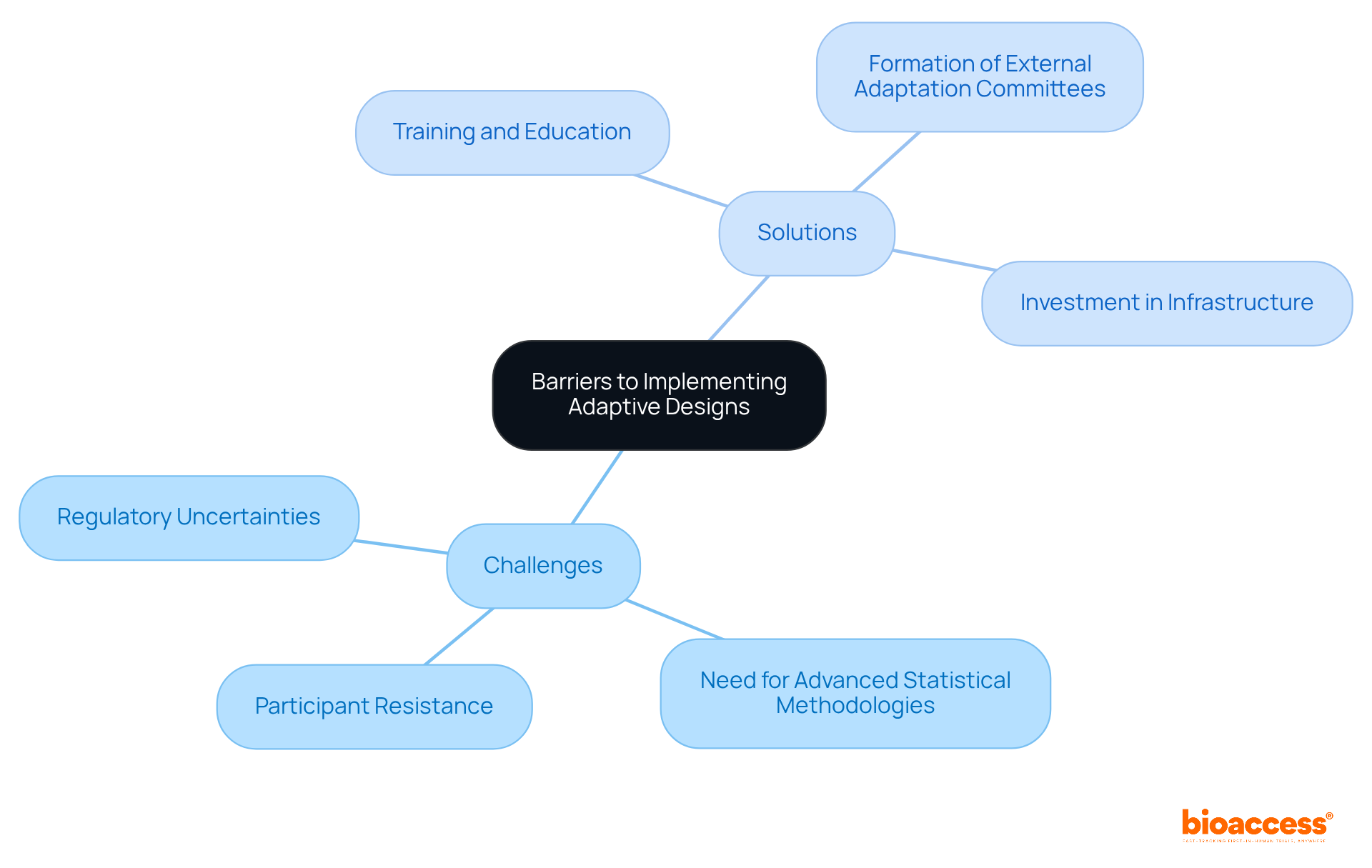
Applying adaptive designs for clinical trials presents significant advantages, yet it is fraught with challenges. Key barriers include:
For instance, the FDA has raised concerns regarding the control of type I error rates in flexible trials, complicating the regulatory approval processes. Additionally, many participants lack familiarity with flexible approaches, leading to resistance against transitioning from conventional frameworks.
To address these challenges, organizations must prioritize training and education, fostering a culture of understanding around adaptive designs for clinical trials. This entails ensuring that all team members grasp the implications of these methodologies on study integrity and patient safety. The NeuroNEXT network, for example, underscores the importance of infrastructure and training to support adaptive designs for clinical trials in neuroscience research, highlighting the need for comprehensive frameworks that facilitate their implementation.
Moreover, the formation of external adaptation committees can enhance oversight and transparency, ensuring that modifications based on interim data are both ethically sound and statistically valid. By investing resources in these areas, organizations can overcome the obstacles to flexible structures, ultimately leading to more effective and ethical research studies.
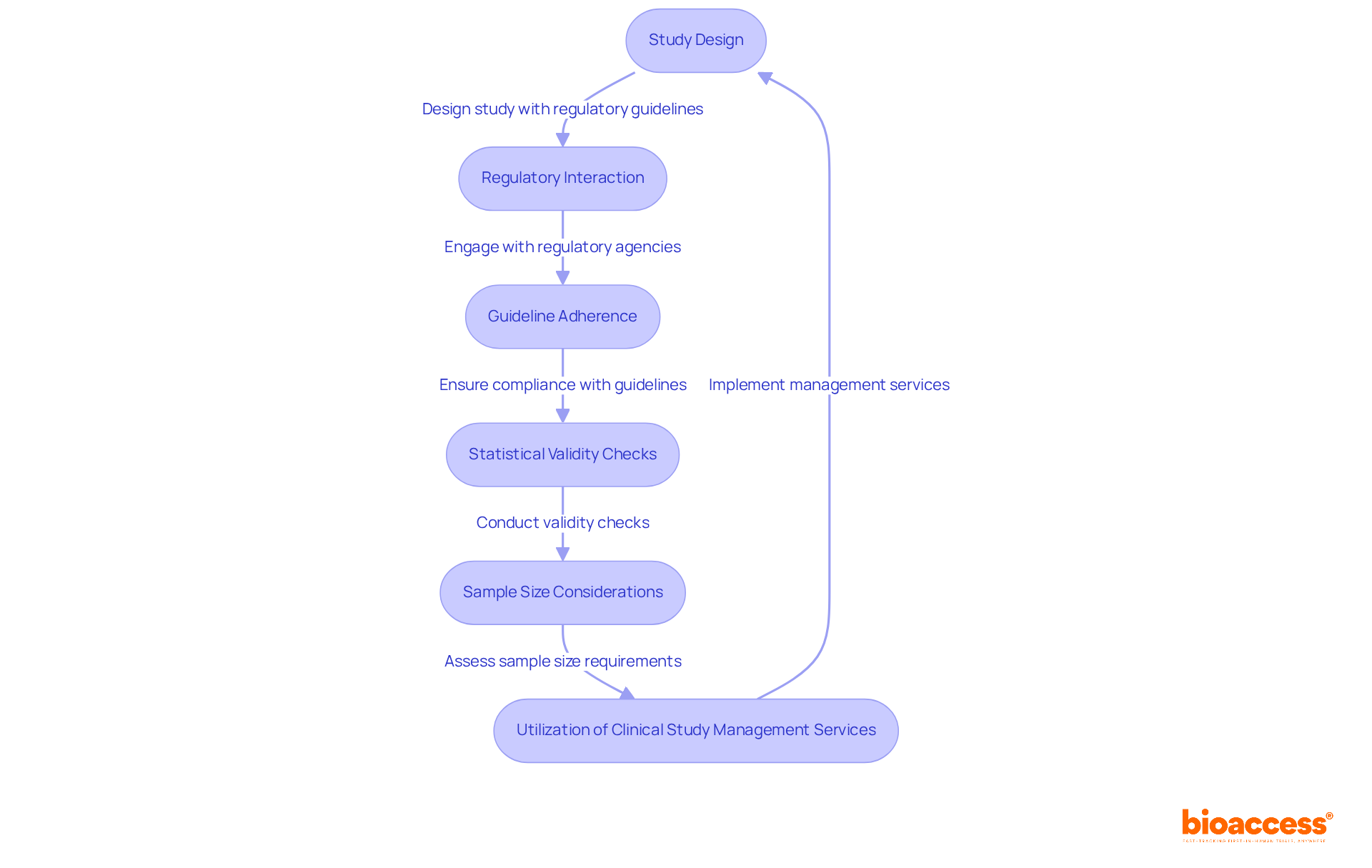
Navigating regulatory considerations is crucial for the effective execution of flexible approaches in clinical studies. Regulatory bodies, including the FDA and EMA, have established specific guidelines that organizations must follow to ensure compliance. These guidelines highlight the importance of comprehensive documentation of the adaptive development process, which includes pre-planned modifications and detailed statistical analyses. For instance, adherence to the Adaptive approaches CONSORT Extension (ACE) checklist revealed that the average compliance score was merely 13.9 out of 26, underscoring considerable deficiencies in reporting quality across various studies.
Interacting with regulatory agencies early in the study design process is essential. This proactive approach not only facilitates smoother approvals but also helps in addressing potential compliance issues before they arise. Recent FDA recommendations have emphasized the significance of valid statistical techniques in flexible studies, particularly to uphold the integrity of results and reduce the likelihood of type I errors. Furthermore, the EMA has voiced concerns about the sufficiency of sample sizes in certain flexible studies, emphasizing the necessity for strong statistical power to reach dependable conclusions.
Effective management of flexible study regulations can be illustrated by research that successfully employed integrated Phase II/III approaches, merging components of both stages to enhance drug development. These patterns have demonstrated potential in improving efficiency while complying with regulatory standards. As adaptive designs for clinical trials continue to gain momentum, it is crucial for organizations to stay informed about changing guidelines and best practices to enhance their research strategies.
To effectively tackle these regulatory challenges, organizations can utilize extensive clinical study management services provided by bioaccess. These services encompass:
By employing these services, organizations can more effectively manage the intricacies of flexible studies, ensuring compliance and improving the integrity of their research results. Moreover, it is vital to acknowledge the challenges linked to adaptive frameworks, such as possible biases and issues in statistical inference, which must be meticulously handled to ensure study integrity.
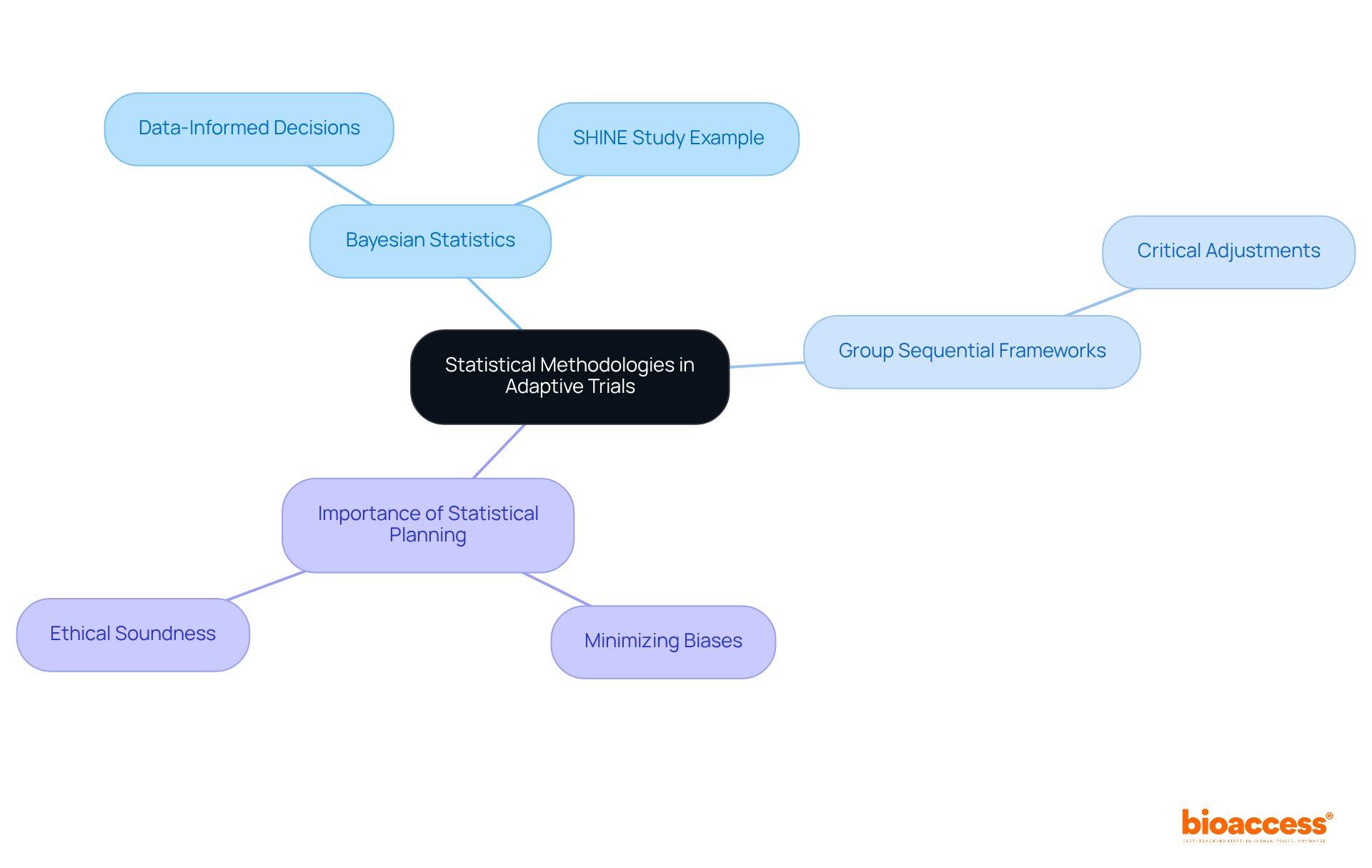
Adaptive studies are grounded in rigorous statistical methodologies that uphold the validity of their findings. Bayesian statistics, paired with group sequential frameworks, empower researchers to make data-informed decisions as information accumulates throughout the study. This approach not only enhances the integrity of the research but also allows for critical adjustments in response to emerging data. For instance, the SHINE study utilized a Bayesian design, which facilitated earlier conclusions regarding futility compared to traditional methods, thereby demonstrating the effectiveness of adaptive designs for clinical trials.
Effective statistical planning is paramount to minimizing biases and ensuring that study outcomes are scientifically robust and ethically sound. Within the realm of bioaccess's expertise, these statistical methodologies are particularly relevant for studies such as Early-Feasibility Studies and First-In-Human Studies, where timely and informed decision-making can significantly impact the success of the research. The growing recognition of Bayesian techniques in medical studies underscores their capacity to enhance study efficiency and significance, making them indispensable in the evolving landscape of flexible medical evaluations.
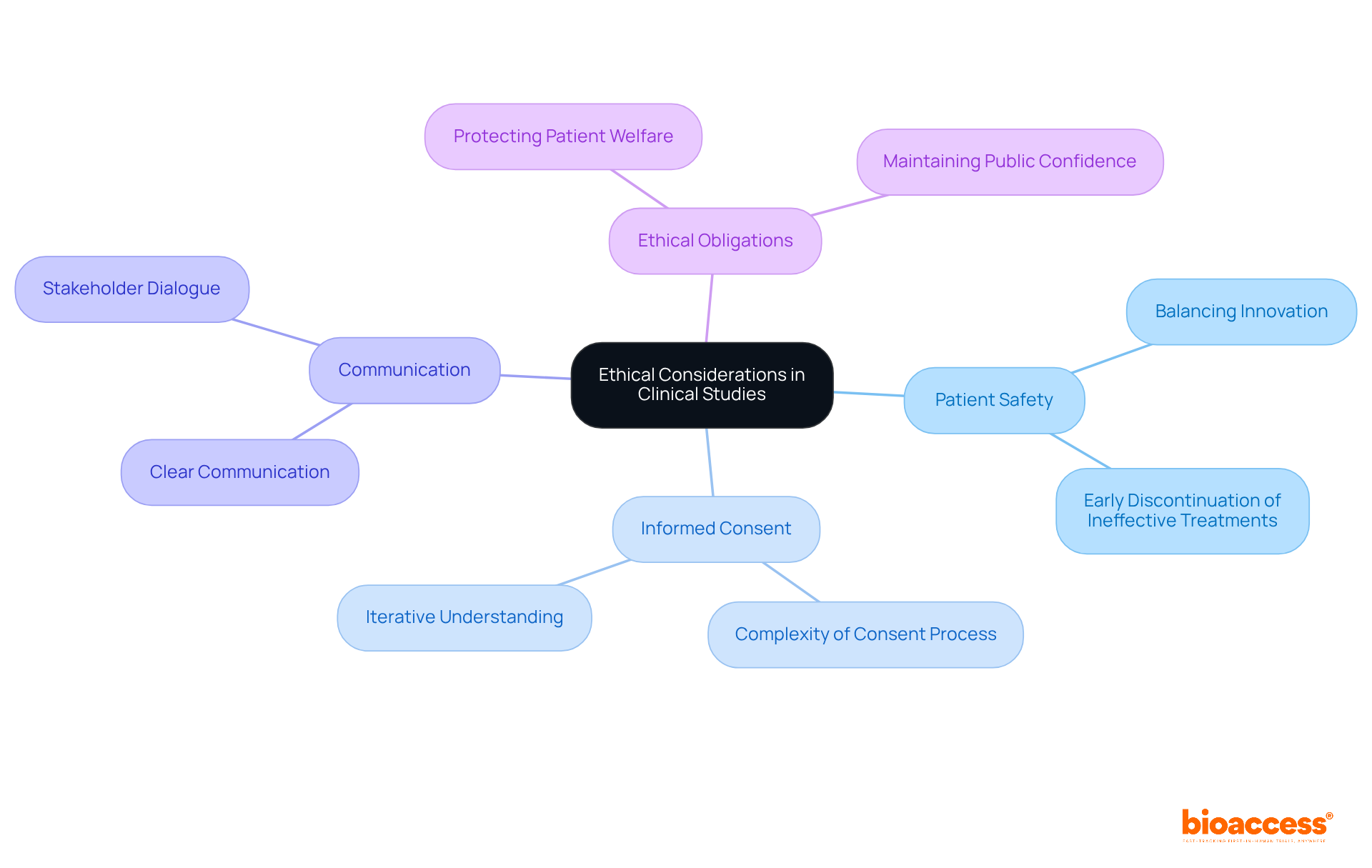
Ethical factors are essential in flexible clinical studies, as these frameworks can complicate issues related to patient safety and informed consent. Researchers must prioritize clear communication, ensuring that participants are fully informed about the trial's flexible nature and any associated risks.
For instance, flexible designs can enhance patient safety by allowing for the early discontinuation of ineffective treatments, thereby minimizing exposure to unproven therapies. However, this innovation must be balanced with the ethical obligation to protect patient welfare, which is crucial for maintaining public confidence in medical research.
The complexities of informed consent in flexible studies necessitate ongoing dialogue among stakeholders to ensure that participants understand their involvement and the implications of study modifications. Ultimately, the goal is to foster an environment where innovation in medical research does not compromise patient safety.
Handling logistics in adaptive designs for clinical trials presents unique challenges, primarily due to the necessity for real-time data analysis and the potential for protocol changes. Organizations must ensure their infrastructure is robust enough to accommodate these dynamic requirements, which include:
A proactive drug supply management plan is crucial, incorporating forecasting simulations and contingency strategies to address potential supply chain issues while adapting to varying treatment demands across multiple sites. This complexity is underscored by the fact that over 50 clinical research sites may be active concurrently, necessitating a flexible approach to logistics management.
Effective project management and clear communication are vital in navigating these operational intricacies. The creation of a completely integrated data flow process, as observed in successful responsive experiments, guarantees that data is extracted, analyzed, and reported swiftly, enhancing the performance of the responsive algorithm. Furthermore, the execution of daily data assessments and automated inquiries can greatly improve data quality, allowing precise computations of randomization probabilities. By encouraging cooperation between researchers and statisticians early in the study process, organizations can ensure that suitable statistical methods are utilized, further enhancing study outcomes.
Despite the benefits of adaptive designs for clinical trials, the uptake remains low due to cultural and organizational obstacles, such as hierarchical systems and a risk-averse mentality. Overcoming these challenges necessitates a change in viewpoint and a dedication to adopting innovative experimental approaches, including adaptive designs for clinical trials. Instruction and development initiatives for sponsors, researchers, and regulatory bodies are vital to enhance comprehension and trust in flexible methodologies, ultimately aiding their wider application in medical research.
The successful execution of flexible strategies hinges on a robust commitment to training and education for clinical research teams. This commitment encompasses a comprehensive understanding of statistical techniques, regulatory frameworks, and ethical considerations inherent to flexible studies. Engaging in workshops, online classes, and collaborative learning initiatives cultivates essential knowledge within organizations, empowering team members to adeptly navigate the complexities of flexible study designs. Notably, educational programs that emphasize practical applications and case studies significantly bolster confidence and proficiency in implementing flexible methodologies.
For instance, initiatives fostering collaboration between researchers and statisticians have proven effective in ensuring the appropriate statistical methods are employed, thereby facilitating a smoother execution of experiments. As organizations prioritize the development of this expertise, they position themselves to harness the full potential of flexible structures, ultimately yielding more effective and ethical clinical research outcomes.
Furthermore, with 292 flexible frameworks employed across 236 trials, the demand for thorough training becomes increasingly critical. As Mahatma Gandhi aptly stated, 'Live as if you were to die tomorrow. Learn as if you were to live forever,' highlighting the significance of continuous learning in this dynamic field.
To effectively implement training initiatives, organizations should contemplate integrating flexible concepts into their academic curricula and offering workshops that showcase successful case studies.
The outlook for flexible frameworks in medical research is undeniably promising, marked by an increasing embrace and application across diverse treatment fields. As technology advances and regulatory frameworks evolve, these designs are becoming increasingly sophisticated, enhancing both flexibility and efficiency in research. Notably, the integration of digital tools and data analytics streamlines processes, enabling real-time adjustments based on interim results. This progression is evidenced by a significant rise in flexible experiments, particularly in 2020, underscoring a shift towards more responsive methodologies.
Organizations that leverage these innovations are poised to excel in accelerating drug development and improving patient outcomes, thereby transforming the research landscape. Industry leaders assert that adaptive designs not only curtail costs and time but also amplify the probability of trial success, establishing them as a crucial element of future clinical strategies.
Adaptive designs for clinical trials represent a transformative approach that enhances the efficiency and effectiveness of clinical research. By allowing real-time modifications based on interim data, these designs not only optimize resource allocation but also improve patient safety and engagement. As demonstrated throughout the article, adaptive designs significantly increase the likelihood of successful outcomes, making them a vital component of modern clinical research strategies.
Key insights highlight the flexibility of adaptive designs, enabling researchers to adjust study parameters and terminate ineffective treatments early, thereby conserving time and resources. The comparison between adaptive and non-adaptive designs underscores the advantages of flexibility, with adaptive frameworks proving to be more responsive to emerging data and patient needs. Furthermore, addressing the challenges related to regulatory compliance, statistical methodologies, and ethical considerations is essential for successful implementation.
As the landscape of clinical research continues to evolve, embracing adaptive designs is critical for organizations aiming to improve patient outcomes and streamline drug development processes. The future of clinical trials lies in the ability to adapt and respond swiftly to new information, ultimately fostering a more effective and ethical research environment. Organizations are encouraged to invest in training and education to build expertise in adaptive methodologies, ensuring they can navigate the complexities and seize the opportunities these innovative designs present.
What is bioaccess® and how does it enhance clinical trials?
bioaccess® leverages its expertise in early-phase clinical research to implement adaptive designs for clinical trials, significantly enhancing study efficiency by adjusting research protocols in real-time based on current data and patient needs.
What are adaptive designs for clinical trials?
Adaptive designs allow researchers to modify study parameters based on interim outcomes, optimizing resource utilization and minimizing patient exposure to ineffective treatments, leading to quicker identification of successful therapies.
How do adaptive designs improve study efficiency?
Adaptive designs can yield a 25-35% improvement in experimental efficiency by enabling early termination of ineffective treatments and allowing for real-time adjustments that align with emerging data.
What ethical considerations are associated with adaptive designs?
Adaptive designs facilitate better decision-making by allowing for the early termination of ineffective treatments, minimizing patient exposure to suboptimal interventions, and incorporating stopping rules to prevent further exposure to ineffective treatments.
What challenges might arise when implementing adaptive designs?
The execution of adaptive designs may introduce complexities in planning and implementation, requiring meticulous statistical planning and regulatory approval.
How does bioaccess® accelerate patient enrollment and reduce costs?
bioaccess® achieves patient enrollment 50% faster and saves $25K per patient with FDA-ready data, ensuring a streamlined and effective research process.
What are the key differences between adaptive and non-adaptive designs?
Adaptive designs allow for real-time modifications such as adjusting sample sizes and treatment protocols, while non-adaptive designs adhere to rigid protocols, often resulting in inefficiencies.
What percentage of clinical study protocols require modifications?
Statistics reveal that 70% of clinical study protocols necessitate two to three modifications throughout the research duration, highlighting the critical need for adaptability.
How do adaptive designs affect patient engagement and retention?
The ability to implement real-time adjustments in adaptive designs can expedite enrollment and decrease dropout rates, ultimately fostering greater patient engagement and retention.
What role does big data play in adaptive clinical trials?
Big data enhances the efficiency and economy of clinical studies, allowing for improved, quicker results by relying on real-time information and reducing reliance on self-reported data.