


The article delineates the critical distinctions between Phase 2a and Phase 2b clinical trials.
This differentiation is essential for informed decision-making in drug development. The outcomes from these phases significantly influence the progression to Stage 3 studies and overall therapeutic advancements, underscoring their pivotal role in the clinical research landscape.
The landscape of clinical trials is intricate, particularly when distinguishing between Phase 2a and Phase 2b studies, each serving a unique purpose in the drug development continuum. Understanding these differences is crucial for stakeholders aiming to navigate the complexities of clinical research effectively.
This article delves into the key distinctions between these phases, highlighting their respective focuses on safety and efficacy, the implications for patient demographics, and the strategic considerations that influence funding and regulatory compliance.
As the stakes rise in the race to bring innovative therapies to market, how can researchers leverage these insights to enhance trial outcomes and streamline the path to approval?
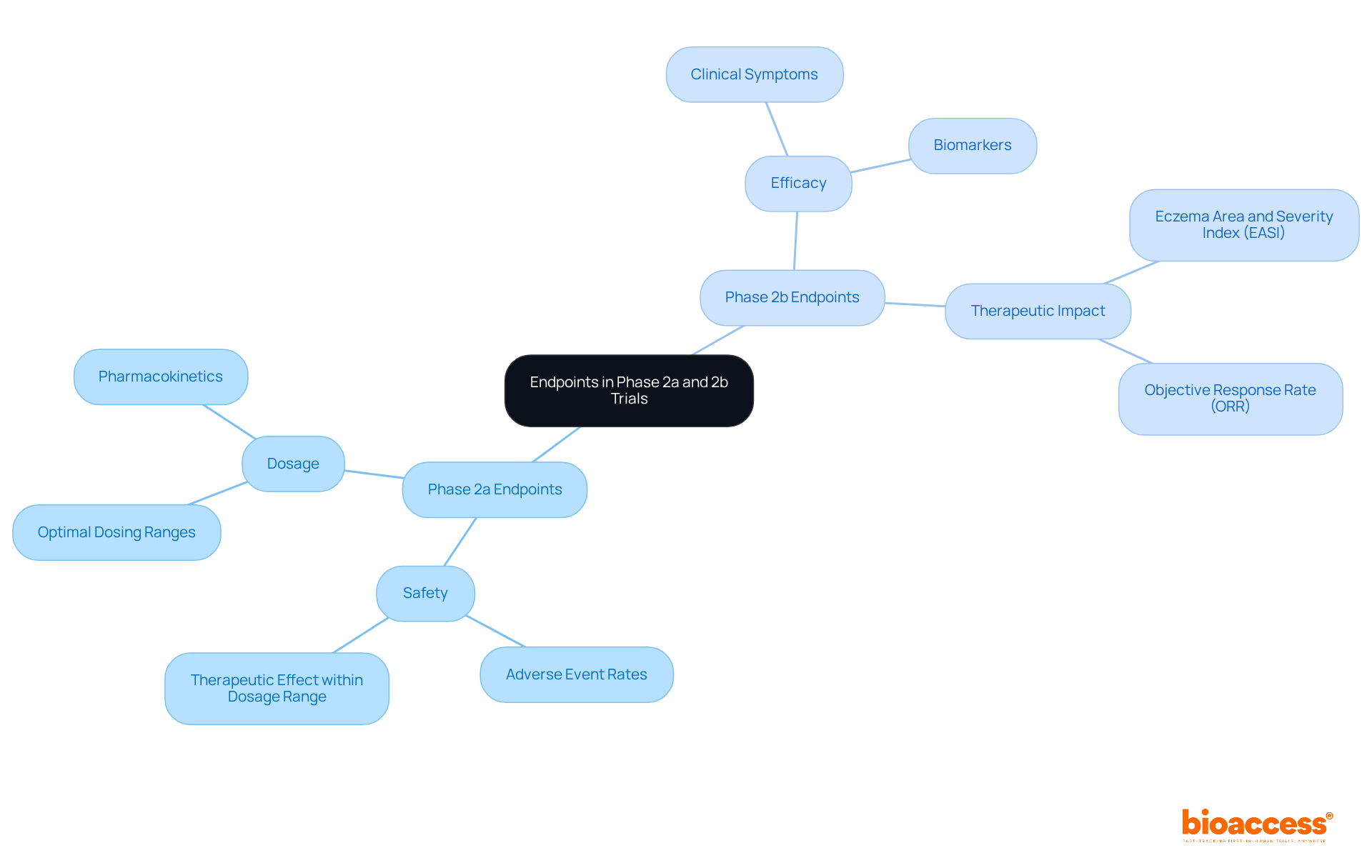
bioaccess® leverages its extensive knowledge and strategic positioning in Latin America to significantly expedite the clinical trial phase 2a 2b studies. Colombia emerges as a premier destination for first-in-human studies, providing cost savings exceeding 30% compared to North America and Western Europe, coupled with rapid regulatory processes that yield ethical approvals in just 4-6 weeks. By integrating these swift regulatory frameworks with access to a diverse patient population—over 50 million, with 95% covered by universal healthcare—bioaccess® not only shortens study timelines but also enhances the representativeness of results. This approach fosters global-first medical agility, enabling innovators in Medtech, Biopharma, and Radiopharma to adeptly navigate the complexities of research phases.
Recent advancements in testing procedures include:
These advancements further streamline operations, ensuring studies are conducted efficiently and effectively. Consequently, bioaccess® achieves enrollment rates that are 50% faster than traditional markets, ultimately leading to quicker and more reliable outcomes for groundbreaking therapies. The global clinical research market is projected to reach USD 123.5 billion by 2030, underscoring the critical role of bioaccess® in this expanding landscape. Furthermore, with clinical trial phase 2a 2b being the second most costly phase after Stage III studies, bioaccess®'s services are indispensable in mitigating these expenses, ensuring that innovative therapies reach the market more swiftly.
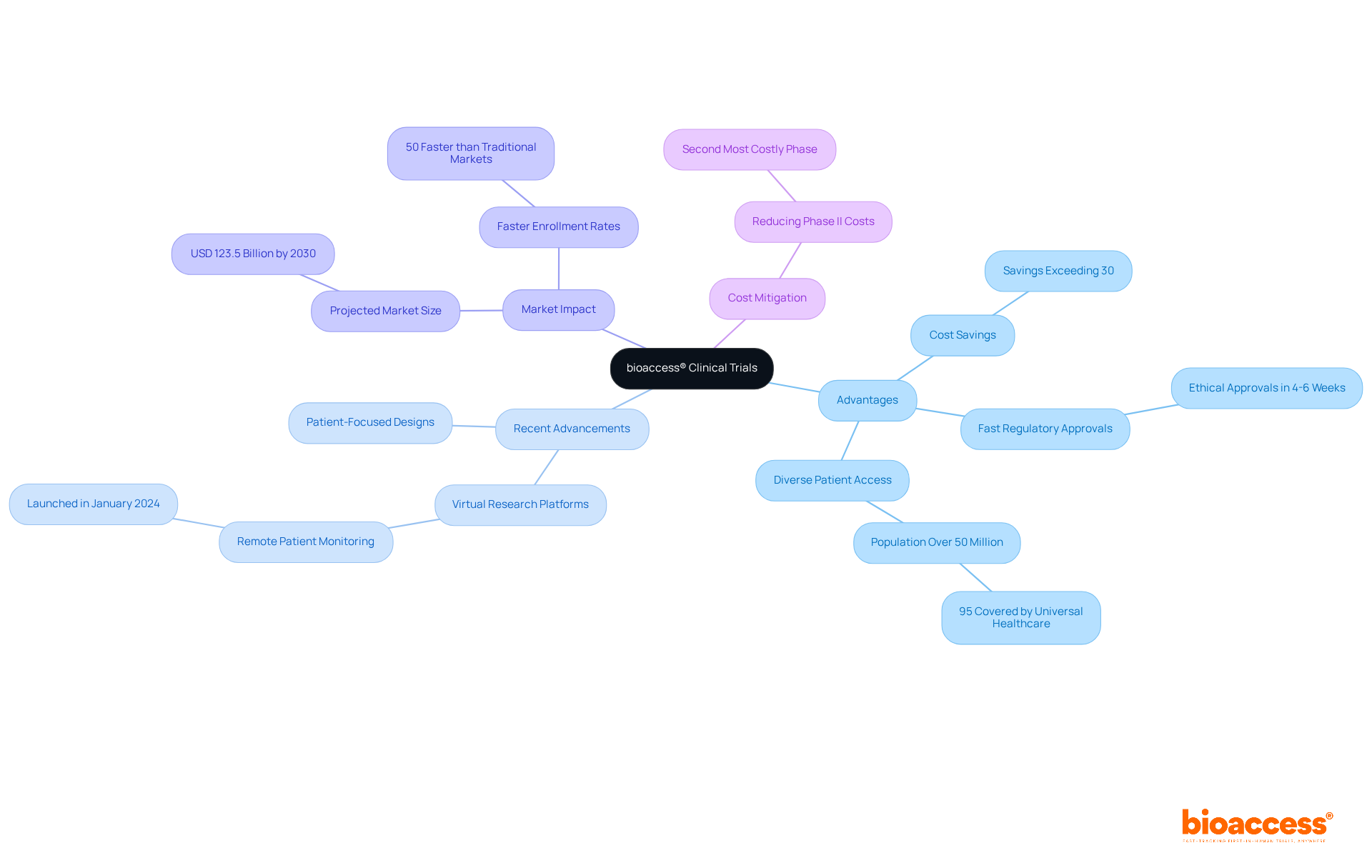
Phase 2a studies, which are part of the clinical trial phase 2a 2b, play a pivotal role in evaluating the reliability and optimal dosage of new therapies, typically involving a smaller cohort of participants, generally ranging from 50 to 200 individuals. These exploratory studies are designed to ascertain the maximum tolerated dose while meticulously monitoring for adverse effects, a critical aspect for safeguarding participant well-being. The significance of safety during this phase cannot be overstated; it serves as the foundation for understanding the drug's interaction with the human body, thereby highlighting the necessity of safety before advancing to larger efficacy studies.
Real-world examples underscore the necessity of rigorous oversight in Phase 2a studies. For instance, the BMT CTN 0601 study, which assessed the effectiveness of bone marrow transplants in children suffering from severe sickle cell disease, conducted thorough risk evaluations to monitor potential adverse events. Such proactive protective measures are vital, as evidenced by the alarming statistic that 15% of patients in various studies may experience severe negative events, necessitating robust monitoring protocols.
Clinical researchers emphasize that the integrity of second-stage studies hinges on effective safety protocols. Recent findings indicate that successful clinical trial phase 2a 2b not only validate drug safety and efficacy but also enhance stakeholder confidence, paving the way for future funding opportunities. As the landscape of clinical studies evolves, the adoption of advanced monitoring techniques, including adaptive study designs and real-time data analysis, is becoming increasingly essential. This progression reflects a growing awareness of the necessity for comprehensive safety assessments to mitigate risks and ensure the successful advancement of drug candidates through the development pipeline.
Moreover, common challenges such as recruitment difficulties and budget limitations must be navigated to maintain the integrity of these studies. bioaccess® offers extensive management services for studies, encompassing feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—services that are crucial for overcoming these hurdles. With a success rate of 50% during Stage II based on studies conducted from 1991 to 2015, effective safety protocols are vital for improving study outcomes. As Michael J Martens emphasizes, safeguarding patient well-being is a fundamental element in the execution of clinical trial phase 2a 2b studies, highlighting the essential role of CROs like bioaccess in managing the complexities involved.
Clinical trial phase 2a 2b studies are pivotal in assessing treatment effectiveness across a broader patient demographic, typically encompassing hundreds of participants. These studies aim to validate a drug's efficacy during the clinical trial phase 2a 2b while maintaining a stringent focus on safety. The outcomes of clinical trial phase 2a 2b studies are vital, providing the necessary evidence to determine whether a treatment possesses sufficient potential to advance to Stage 3 studies. This phase is characterized by definitive endpoints and a robust emphasis on statistical significance.
Notably, recent findings indicate that successful clinical trial phase 2a 2b can lead to significant advancements in treatment options, as evidenced by a remarkable 28.9% transition rate from clinical trial phase 2a 2b to Stage 3 in U.S. drug development between 2011 and 2020. Industry leaders underscore the importance of these studies, with one expert asserting that 'the outcomes from the clinical trial phase 2a 2b are essential in influencing the future of medication development.'
As the landscape of medical research evolves, the efficacy demonstrated in clinical trial phase 2a 2b studies continues to serve as a cornerstone for the progression of innovative therapies. Furthermore, the integration of comprehensive study management services—such as feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—is crucial for the successful execution of these studies.
Companies like bioaccess® leverage their expertise in managing various study types, including Early-Feasibility and First-In-Human studies, to ensure that clinical research not only meets regulatory standards but also positively impacts local economies through job creation, economic growth, and healthcare improvements.

The patient groups in the clinical trial phase 2a 2b exhibit significant differences that are crucial to understanding clinical research dynamics. Stage 2a studies, part of the clinical trial phase 2a 2b, typically involve a smaller, more uniform group, concentrating on safety and dosage evaluation. In contrast, clinical trial phase 2a 2b studies expand the participant group to include a varied demographic, which encompasses different age ranges, genders, and health statuses. This diversity is essential for assessing treatment responses among various populations, thereby enhancing the applicability of study outcomes.
Research has demonstrated that positive results in the clinical trial phase 2a 2b assessments are pivotal for advancing to Stage 3 evaluations, where broader demographic representation can considerably influence regulatory decisions. The FDA emphasizes the significance of demographic variety in research studies, stating that 'the involvement of diverse populations is essential for comprehending how various groups react to treatments.' Moreover, ensuring varied demographics in the clinical trial phase 2a 2b studies not only complies with regulatory requirements but also fosters progress in medical science that benefits a wider patient population. Historically, women and ethnic minorities have been underrepresented in clinical studies, underscoring the necessity for enhanced diversity to ensure that study results resonate with all segments of the population.

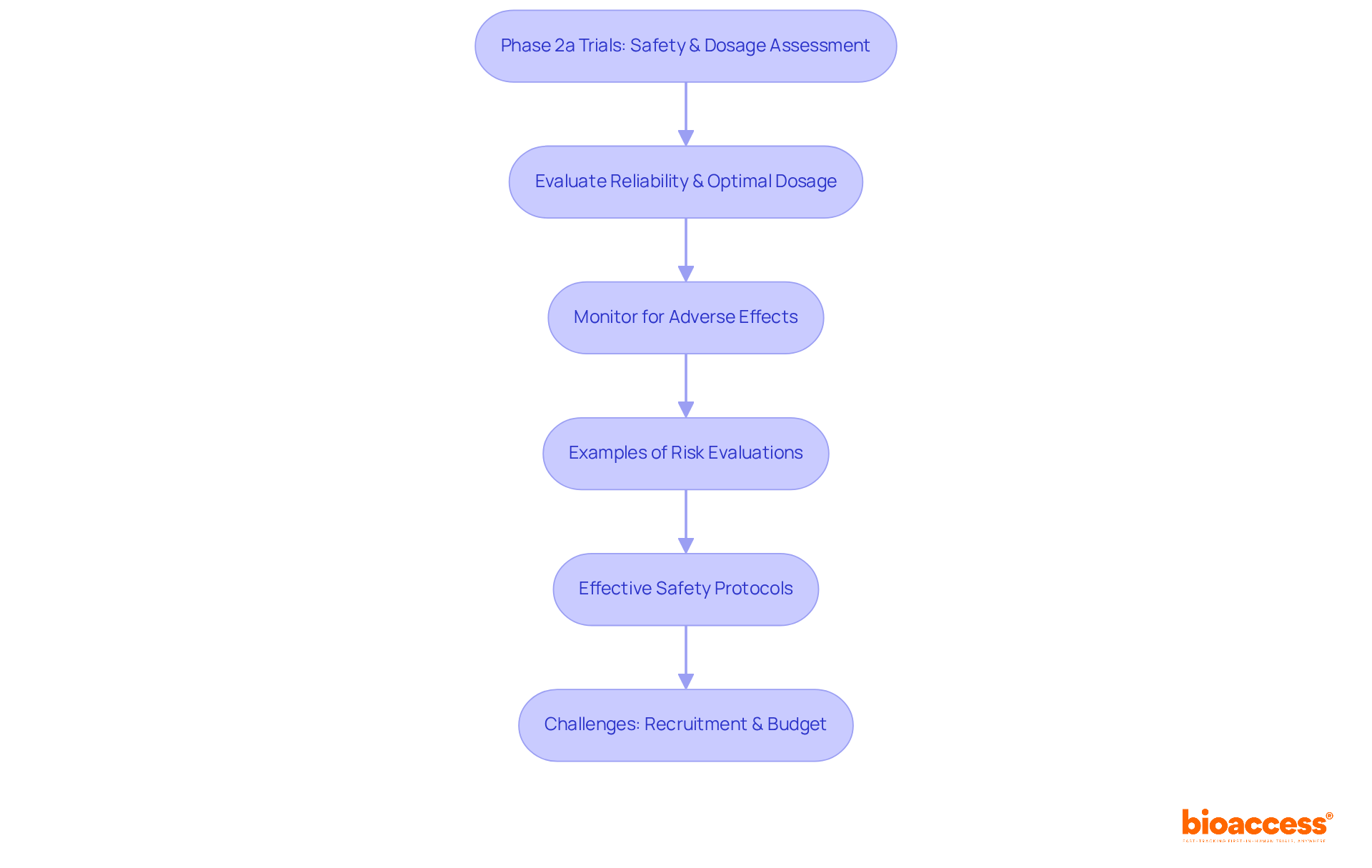
The design guidelines for clinical trial phase 2a 2b are tailored to their specific objectives, underscoring their relevance in clinical research. In clinical trial phase 2a 2b studies, a dose-escalation approach is often employed to identify the optimal dosage, allowing researchers to assess tolerability and efficacy while determining the most effective amount for further investigation. Conversely, clinical trial phase 2a 2b studies predominantly utilize randomized controlled designs, comparing the experimental treatment against a placebo or standard of care. This structured approach in the clinical trial phase 2a 2b is crucial for assessing the treatment's effectiveness and safety profile, ensuring that the results are both statistically valid and reliable.
Current trends indicate a growing preference for randomized designs in clinical trial phase 2a 2b studies, as these designs enhance the reliability of results and mitigate biases associated with single-arm research. Notably, approximately 33% of medications in the clinical trial phase 2a 2b advance to Stage 3, highlighting the importance of meticulous study design in facilitating promising treatments. As Dan Sargent from the Mayo Clinic articulates, "The most significant concern with utilizing historical controls to evaluate PFS or OS in a single-arm phase II study of an experimental treatment is that the historical controls may not accurately reflect the anticipated outcome of the experimental patients." This statement emphasizes the necessity of selecting appropriate primary endpoints and employing robust designs to minimize risks in clinical trial phase 2a 2b.
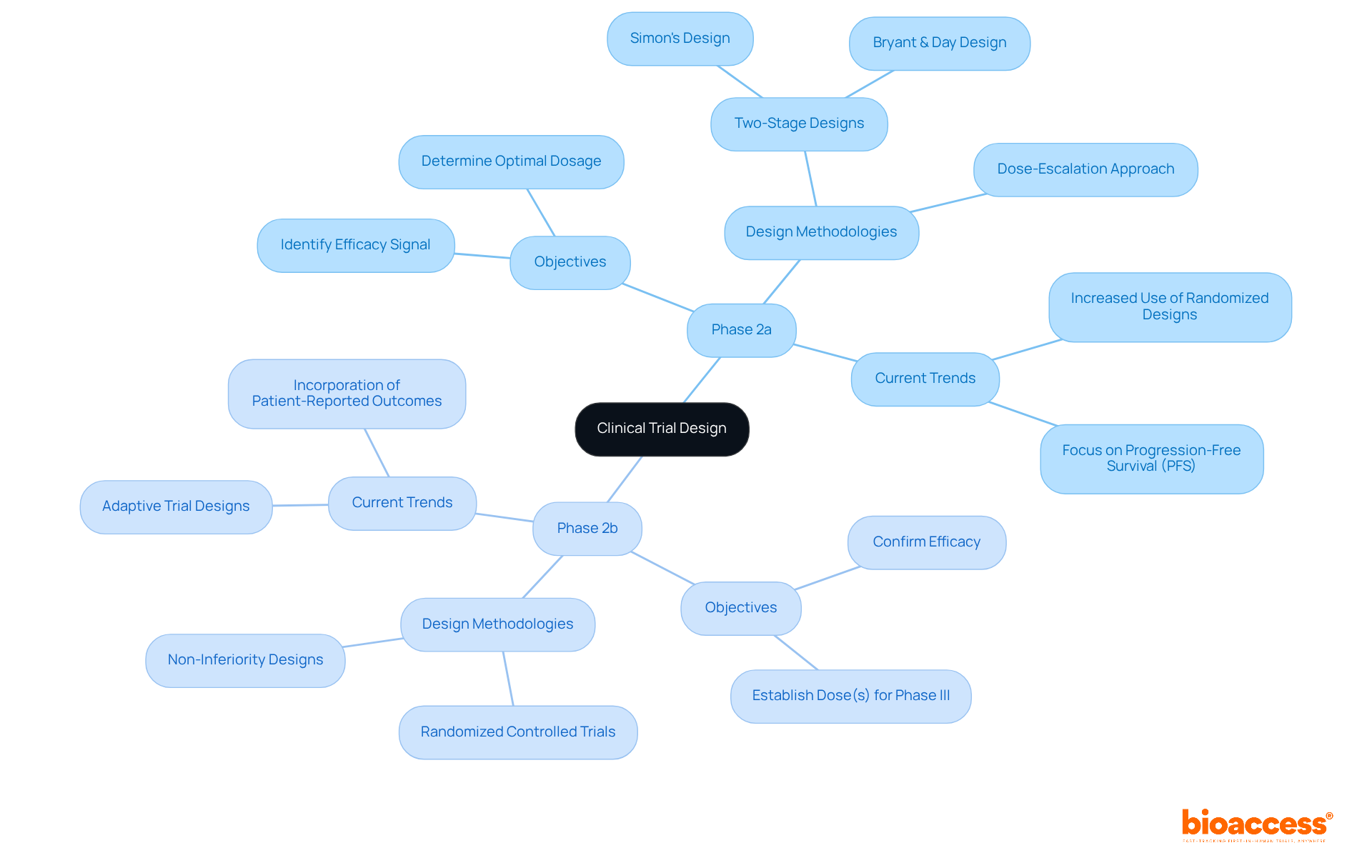
Endpoints in clinical trial phase 2a 2b studies primarily concentrate on safety and dosage, often evaluated through adverse event rates and pharmacokinetic information. These studies typically involve fewer participants and serve as exploratory investigations to ascertain optimal dosing ranges. Conversely, in the clinical trial phase 2a 2b, studies pivot towards efficacy endpoints, such as improvements in clinical symptoms or relevant biomarkers, which are critical for evaluating the treatment's impact on patient health. For instance, the REZOLVE-AD study demonstrated significant progress in the Eczema Area and Severity Index (EASI) scores across various dosing groups, underscoring the importance of efficacy endpoints in justifying progression to the third stage.
Furthermore, safety endpoints in the clinical trial phase 2a 2b are vital for identifying potential risks, ensuring that the drug achieves its therapeutic effect within a permissible dosage range. Clinical researchers emphasize that accurately assessing efficacy in the clinical trial phase 2a 2b studies is crucial for determining a drug's viability and guiding regulatory discussions. Bioaccess plays an essential role in refining study designs, guaranteeing that research is conducted efficiently and effectively, from feasibility assessments and site selection to compliance reviews and project management. This integration of advanced statistical methods bolsters the robustness of findings, ultimately facilitating informed decision-making in the drug development process.
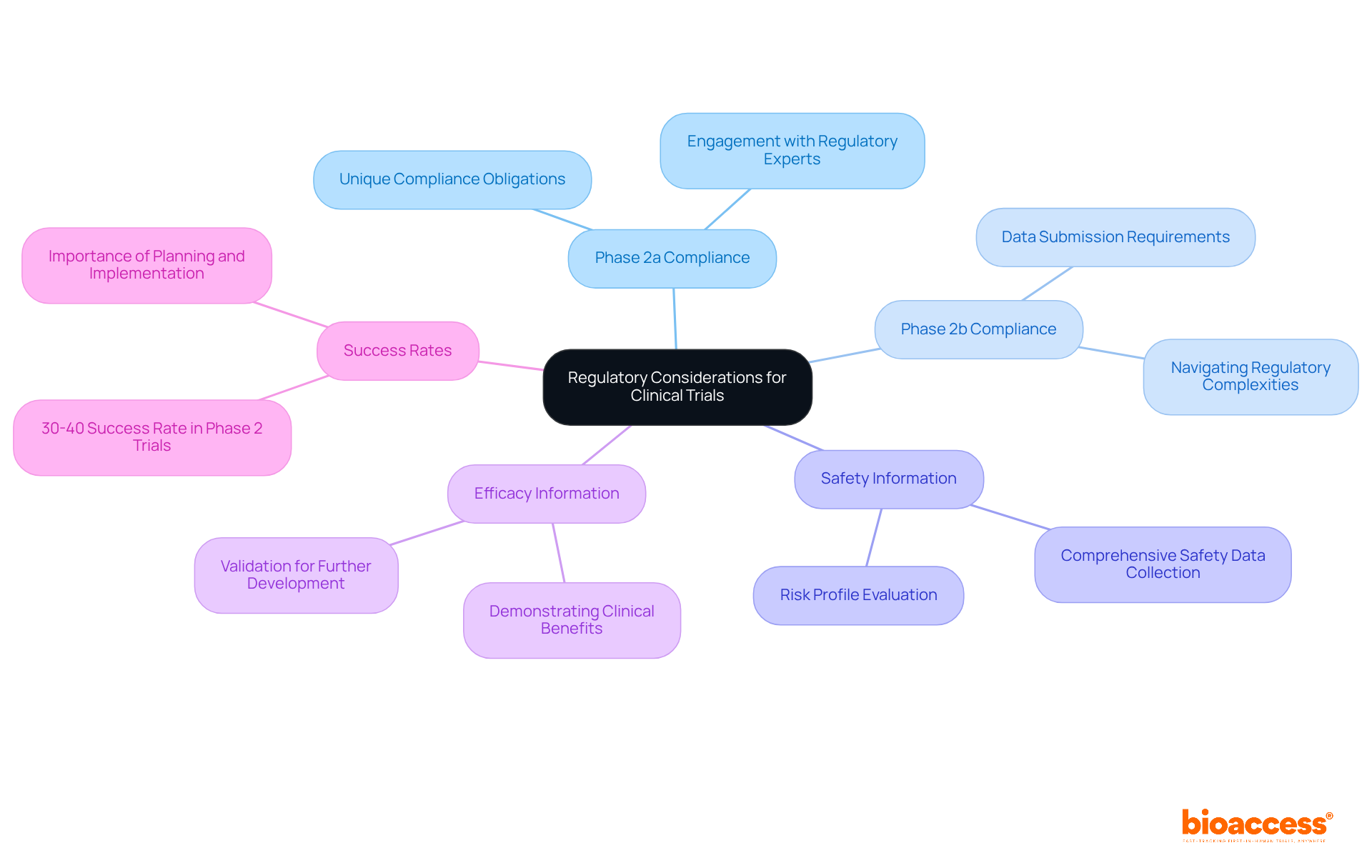
Navigating the regulatory environment is crucial for the success of the clinical trial phase 2a 2b, as each phase presents unique compliance obligations set by health authorities. Clinical trial phase 2a 2b studies generally concentrate on collecting comprehensive safety information, which is essential for evaluating the drug's risk profile. In contrast, clinical trial phase 2a 2b studies shift the focus toward showcasing extensive efficacy information, which is vital for validating additional development. Statistics indicate that only around 30%-40% of drugs effectively succeed in the clinical trial phase 2a 2b, underscoring the significance of careful planning and implementation to fulfill these regulatory standards.
Compliance challenges are prevalent in clinical trial phase 2a 2b, particularly in ensuring that all data submitted aligns with the varying requirements of different regulatory bodies. Engaging with regulatory experts early in the process can facilitate smoother navigation through these complexities. As Robert Emmitt aptly stated, 'Accountability is the way we measure responsibility,' emphasizing the necessity for rigorous adherence to compliance standards throughout the process. By comprehending and tackling these regulatory subtleties, clinical research organizations like bioaccess® can improve study advancement and reduce setbacks in obtaining essential approvals.
Funding and resource distribution exhibit notable variations between experiments in clinical trial phase 2a 2b. Stage 2a studies, part of the clinical trial phase 2a 2b, are characterized by their exploratory nature and smaller participant groups, generally incurring lower expenses that average between $7 million and $20 million in total. These studies focus on determining initial effectiveness and ideal dosing, often involving 71 to 143 participants. Conversely, experiments in clinical trial phase 2a 2b necessitate a greater financial investment, with overall expenses ranging from $7 million to $20 million, reflecting their larger patient groups and more complex study frameworks. The typical cost per patient in clinical trial phase 2a 2b studies is approximately $129,777, highlighting the increased resource demands as these studies aim to validate therapeutic outcomes and refine treatment protocols.
Effective resource management is essential in addressing these disparities. Clinical research organizations underscore the importance of adaptive study designs, which can yield cost savings of 10-15% by optimizing patient enrollment and minimizing unnecessary procedures. For example, implementing risk-based monitoring can decrease site visit frequencies by 30-40%, translating to savings of $3,000 to $5,000 per patient without compromising data quality. Additionally, finance teams are encouraged to collaborate closely with clinical teams during protocol development to identify potential cost-saving opportunities, ensuring that both clinical trial phase 2a and 2b studies are adequately funded and supported throughout their duration.
Statistics reveal that patient recruitment can consume 30-40% of Stage 2 budgets, highlighting the need for strategic planning in resource allocation. By understanding the distinct financial requirements of each stage, organizations can more effectively align their resources with scientific priorities, ultimately enhancing the likelihood of successful outcomes.

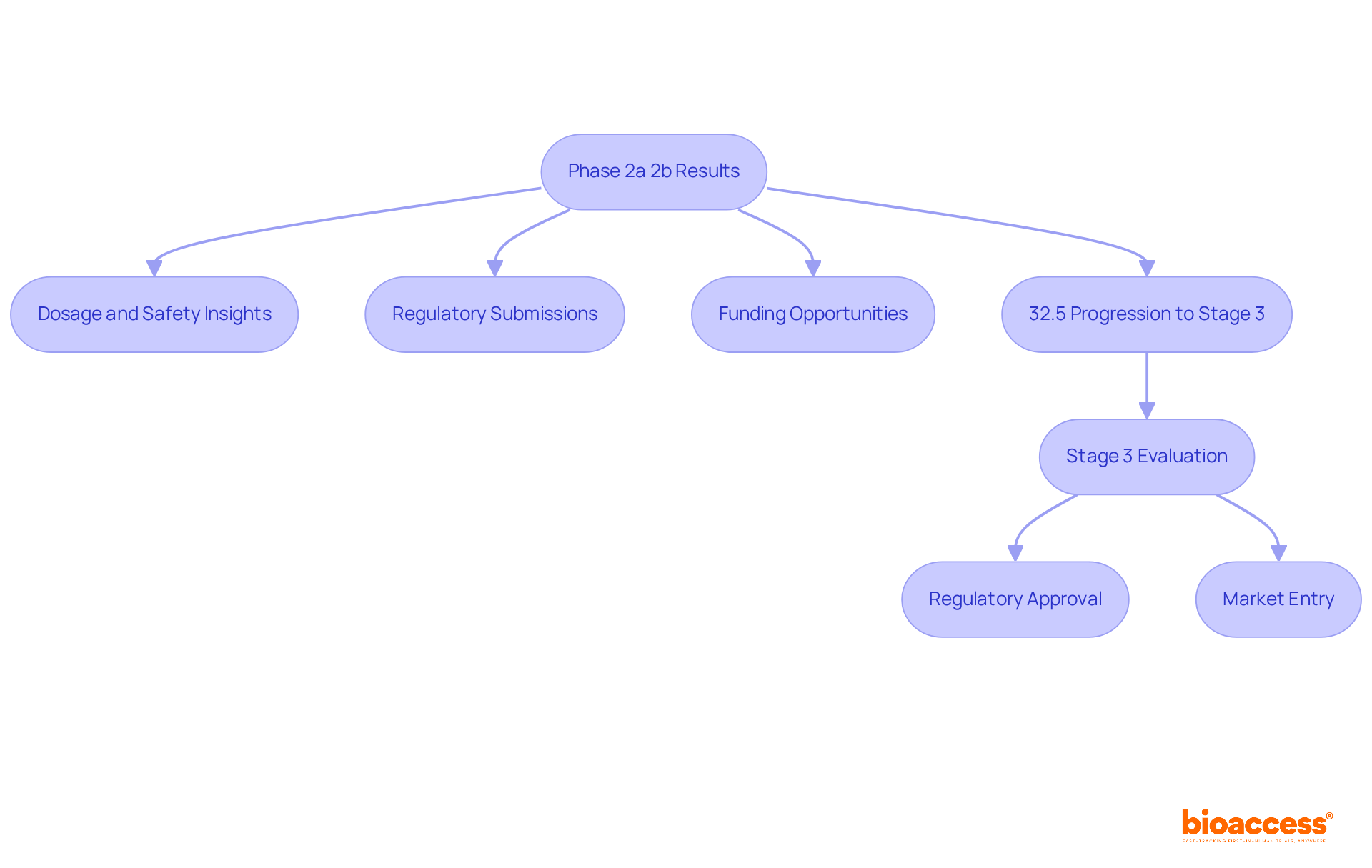
The results from the clinical trial phase 2a 2b are vital in shaping the path of medication advancement. Successful results from the clinical trial phase 2a 2b offer crucial insights into dosage and safety, informing subsequent decisions. In contrast, favorable clinical trial phase 2a 2b results are essential for advancement to Stage 3 assessments, where the stakes are greater. The overall development strategy is shaped by these phases, such as the clinical trial phase 2a 2b, which impact regulatory submissions, funding opportunities, and market entry timelines.
For instance, approximately 32.5% of patients in the clinical trial phase 2a 2b progress to Stage 3 evaluation, highlighting the significance of strong Stage 2 outcomes. Industry leaders emphasize that the ultimate objective of the clinical trial phase 2a 2b assessments is to pinpoint the most promising treatment protocols for subsequent stages, underscoring their importance in the wider context of drug development.
Moreover, the typical duration for drug development after Stage 2 evaluations can prolong considerably, frequently requiring several years to progress through Stage 3 and regulatory approval procedures, with expenses approaching around $2.6 billion. This makes the effectiveness of Stage 2 outcomes in the clinical trial phase 2a 2b even more essential.
To support these studies effectively, comprehensive management services for research, such as those provided by bioaccess—including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—play a pivotal role in ensuring that Stage 2 studies are conducted smoothly and yield valuable results.
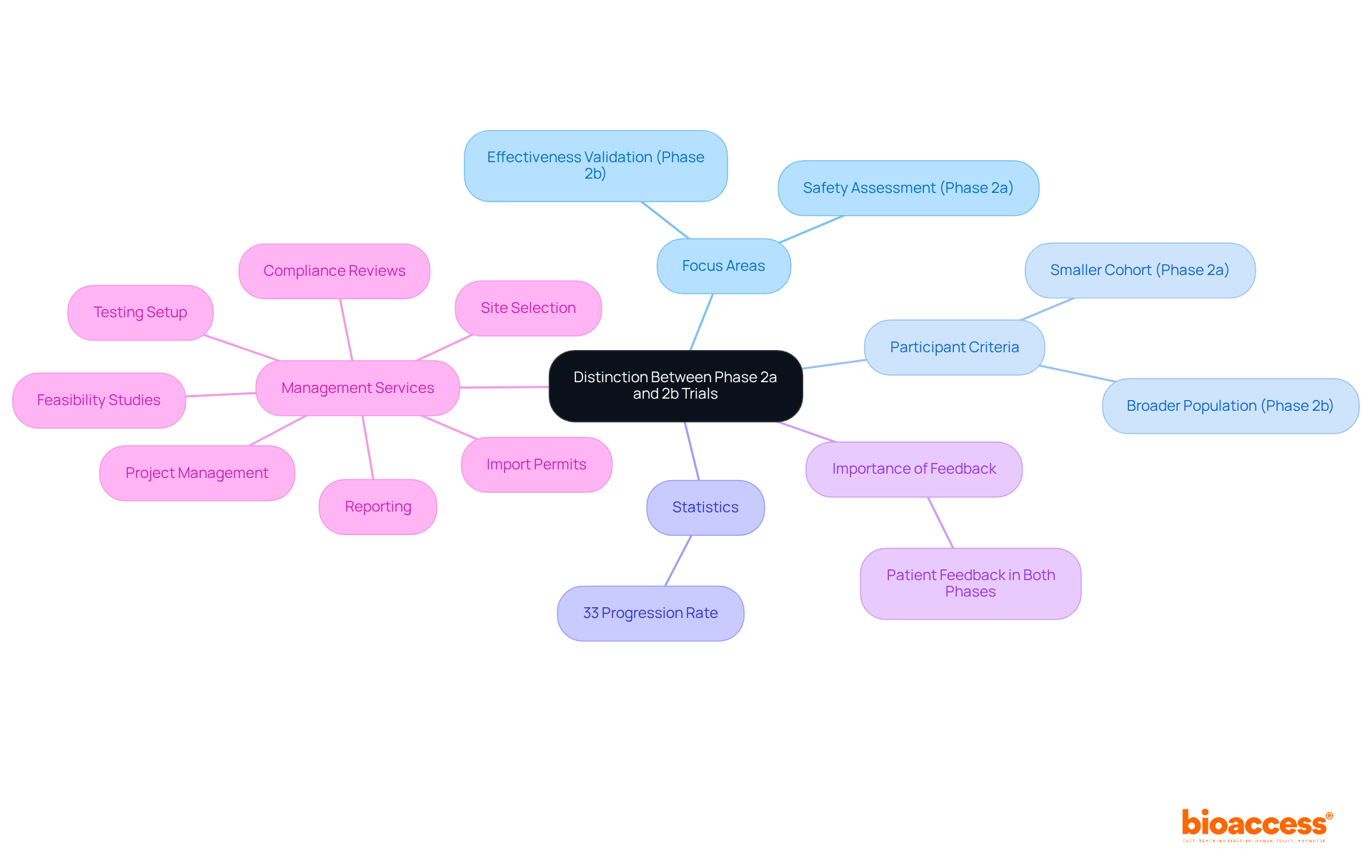
It is crucial for participants in medical research, including sponsors, regulatory agencies, and investigators, to distinguish between studies in clinical trial phase 2a and 2b. Clinical trial phase 2a studies primarily focus on confirming safety and identifying optimal dosages in phase 2a, while phase 2b studies shift the emphasis to evaluating treatment effectiveness. This distinction is vital for informed decision-making, as it significantly influences the planning and execution of clinical studies. For instance, Stage 2a studies typically involve a smaller cohort, facilitating initial safety assessments, whereas Stage 2b studies expand participant criteria to validate effectiveness across a broader population.
Statistics indicate that approximately 33% of medications in Stage 2 progress to the next stage, underscoring the significance of these evaluations in the drug development continuum. Stakeholders often assert that a clear understanding of these differences enhances their strategic capabilities. As one expert noted, "The logistics of executing a fully sequential dose-finding study can be daunting," highlighting the complexities inherent in study design.
Moreover, the incorporation of patient feedback in both phases is increasingly acknowledged as essential for refining therapeutic strategies. This feedback not only enriches the understanding of patient experiences but also informs the creation of more effective treatments. By understanding the nuances of the clinical trial phase 2a and 2b, stakeholders can optimize their research strategies, ultimately leading to improved patient outcomes and higher rates of drug approvals.
Furthermore, comprehensive clinical study management services, such as those offered by bioaccess, play a pivotal role in facilitating these phases. Essential services include:
These services are critical for ensuring that assessments are conducted efficiently and effectively. The impact of Medtech clinical studies extends beyond the trials themselves, contributing to local economies through job creation, economic growth, and healthcare enhancement, while fostering international collaboration in research initiatives.
Understanding the distinctions between clinical trial phases 2a and 2b is essential for stakeholders in the medical research field. Phase 2a primarily focuses on safety and dosage assessment, while Phase 2b shifts the emphasis to evaluating the efficacy of treatments across a broader patient demographic. This clarity not only aids in informed decision-making but also shapes the strategic planning and execution of clinical studies, ultimately influencing the trajectory of drug development.
Throughout this discussion, key points have been highlighted, such as:
These points further underscore the complexities involved in these stages of clinical research. By recognizing the differences in objectives and methodologies, stakeholders can enhance their research strategies, leading to improved patient outcomes and higher rates of successful drug approvals.
In conclusion, the significance of distinguishing between Phase 2a and 2b trials cannot be overstated. Emphasizing safety in Phase 2a and efficacy in Phase 2b is vital for the advancement of innovative therapies. As the landscape of clinical research continues to evolve, leveraging comprehensive management services and embracing adaptive study designs will be crucial for overcoming challenges and optimizing resource allocation. This proactive approach not only fosters advancements in medical science but also ensures that groundbreaking therapies reach the market more efficiently, ultimately benefiting patients worldwide.
What is bioaccess® and what role does it play in clinical trials?
bioaccess® is a company that accelerates Phase 2a and 2b clinical trials by leveraging its extensive knowledge and strategic positioning in Latin America, particularly in Colombia, to expedite studies and reduce costs.
Why is Colombia considered a premier destination for clinical trials?
Colombia is considered a premier destination for first-in-human studies due to cost savings exceeding 30% compared to North America and Western Europe, along with rapid regulatory processes that yield ethical approvals in just 4-6 weeks.
How does bioaccess® enhance the efficiency of clinical trials?
bioaccess® enhances efficiency by integrating swift regulatory frameworks with access to a diverse patient population, achieving enrollment rates that are 50% faster than traditional markets, leading to quicker and more reliable outcomes.
What advancements have been made in clinical trial procedures by bioaccess®?
Recent advancements include the launch of virtual research platforms for remote patient monitoring and the implementation of patient-focused designs, which streamline operations and ensure studies are conducted efficiently.
What is the significance of Phase 2a trials in clinical research?
Phase 2a trials focus on evaluating the safety and optimal dosage of new therapies, typically involving a smaller cohort of participants. They are critical for understanding drug interactions and ensuring participant safety before advancing to larger efficacy studies.
What are the common challenges faced during Phase 2a trials?
Common challenges include recruitment difficulties and budget limitations, which bioaccess® addresses through extensive management services such as feasibility assessments, site selection, and project oversight.
What is the focus of Phase 2b trials in the clinical trial process?
Phase 2b trials focus on assessing the efficacy of treatments across a broader patient demographic, providing essential evidence to determine whether a treatment can advance to Stage 3 studies.
How do successful Phase 2a and 2b trials impact drug development?
Successful Phase 2a and 2b trials can lead to significant advancements in treatment options, with a notable transition rate from these phases to Stage 3 studies, influencing the future of medication development.
What services does bioaccess® offer to ensure successful clinical trials?
bioaccess® offers a range of services including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting to ensure the successful execution of clinical trials.