


The article "10 Key Differences in Preclinical vs Clinical Radiopharma Studies" provides a critical examination of the distinct phases and regulatory frameworks that set preclinical studies apart from clinical trials in radiopharmaceutical research. It asserts that preclinical studies primarily focus on evaluating safety and efficacy in animal models, operating under less stringent guidelines. In contrast, clinical trials are subject to rigorous regulatory standards and concentrate on human participants. Understanding these differences is paramount for successful drug development and market entry, highlighting the essential nature of this knowledge in the field of clinical research.
The realm of radiopharmaceutical research is characterized by a crucial distinction between preclinical and clinical studies, each serving a vital function in the advancement of groundbreaking therapies. Grasping these differences not only illuminates the complex processes at play but also underscores the potential to expedite research timelines and improve patient outcomes.
As the landscape of medical research continues to transform, what challenges emerge in the shift from preclinical evaluations to clinical trials? Furthermore, how can researchers adeptly navigate these complexities to ensure the successful development of new drugs?
bioaccess® leverages its strategic presence in Latin America, particularly in Colombia, to accelerate preclinical vs clinical radiopharma studies. This organization capitalizes on the region's regulatory efficiency, achieving ethical approvals in a mere 4-6 weeks—significantly faster than many other areas. Such rapid turnaround is essential for Medtech, Biopharma, and Radiopharma companies aiming to expedite their time to market for innovative therapies, especially when considering preclinical vs clinical radiopharma studies. Moreover, Colombia offers a cost reduction of over 30% compared to experiments conducted in North America or Western Europe, alongside a healthcare system recognized among the best globally, enhancing patient recruitment.
With over 83% of global Phase 3 studies including at least one US site, the ability to streamline processes in these regions not only boosts recruitment but also substantially shortens overall study timelines. Successful examples of accelerated pharmaceutical research in Colombia underscore the effectiveness of bioaccess®'s approach, particularly in terms of preclinical vs clinical radiopharma studies, illustrating how innovative trial designs and real-time performance tracking can optimize patient engagement and site selection. By focusing on regulatory speed and employing diverse patient groups, bioaccess® is transforming the landscape of medical research in radioisotopes, ensuring that companies can launch their products to market more swiftly and effectively.
The regulatory framework overseeing preclinical vs clinical radiopharma studies is notably distinct, and understanding these differences is crucial for clinical research. In preclinical vs clinical radiopharma studies, investigations typically operate under less stringent guidelines, primarily focusing on safety and efficacy evaluations in animal models. In contrast, preclinical vs clinical radiopharma studies are governed by rigorous standards set forth by regulatory authorities such as the FDA and EMA.
These standards require comprehensive documentation, ethical approvals, and strict patient safety protocols. For example:
Grasping these distinctions is essential for researchers, as they significantly influence research design, timelines, and resource allocation. Compliance with these regulatory frameworks not only ensures the integrity of the research but also enhances the likelihood of successful product development and market entry.
Preclinical research is pivotal in advancing radioactive drugs, especially when considering the differences between preclinical vs clinical radiopharma studies, as it primarily focuses on the evaluation of safety and biological activity. These investigations establish a robust foundation for subsequent trials, which focus on assessing the effectiveness and safety of these substances in human participants, particularly in the context of preclinical vs clinical radiopharma studies. This transition is crucial for determining optimal dosages and identifying potential side effects, ultimately facilitating the progression of radioactive drugs from laboratory settings to practical applications.
The duration of preclinical research can vary significantly, often extending over multiple years before entering trial phases. Successful preclinical research has paved the way for numerous radioactive drugs that have advanced to testing, underscoring the importance of comprehensive safety and effectiveness evaluations. As emphasized in industry discussions, ensuring the safety and effectiveness of radioactive medicines is paramount; any miscalculation can lead to adverse reactions and market setbacks.
Thus, the significance of research studies in radiopharmaceutical development cannot be overstated, as they are essential for validating the therapeutic potential of these innovative agents. Furthermore, extensive management services for research projects—including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—are vital for the successful execution of these experiments. These services not only enhance the efficiency of the research process but also bolster local economies through job creation, economic growth, healthcare improvement, and international collaboration.
Methodological strategies in preclinical research encompass both in vitro and in vivo testing, essential for assessing pharmacokinetics and toxicity, thereby establishing a solid foundation for research design. In contrast, medical studies predominantly employ randomized controlled trials (RCTs) to rigorously evaluate the therapeutic efficacy of radiopharmaceuticals across diverse patient populations. RCTs are crucial for generating robust data that not only supports regulatory submissions but also informs medical practice.
For example, the Randomized Aldactone Evaluation Study (RALES) illustrates how RCTs can shape treatment guidelines by demonstrating the efficacy of spironolactone in patients with severe heart failure. Statistics affirm that RCTs represent the gold standard in medical research, providing compelling evidence for the effectiveness of medical interventions. Their structured design minimizes bias and ensures comparability among treatment groups, which is vital for achieving reliable outcomes.
As the landscape of radiopharmaceutical research continues to evolve, the integration of RCTs remains indispensable for advancing therapeutic innovations and enhancing patient care.
The goal of data generation in preclinical vs clinical radiopharma studies is primarily to establish safety profiles and biological activity, typically utilizing animal models to predict human responses. This foundational work is crucial, as it sets the stage for subsequent research focused on assessing patient outcomes, side effects, and overall effectiveness. Such a shift is essential; it not only informs future research directions but also plays a pivotal role in regulatory decisions.
For instance, the success rate of transitioning from Phase II to Phase III trials in U.S. drug development was only 28.9% from 2011 to 2020, underscoring the importance of robust patient outcome measurements. Furthermore, with over 158,000 registered medical studies in the U.S. as of mid-2025, the emphasis on patient outcomes has never been more pronounced.
Evaluating these outcomes in preclinical vs clinical radiopharma studies ensures that radiopharmaceuticals meet the stringent safety and efficacy standards required for market approval, ultimately enhancing patient care and treatment effectiveness.
In the context of Latin America, comprehending the INVIMA regulatory framework becomes essential, as it oversees the management of studies and medical devices, ensuring adherence to local regulations.
With bioaccess®'s comprehensive trial management services—including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies—the process is streamlined, enhancing data generation reliability and adherence to regulatory standards.
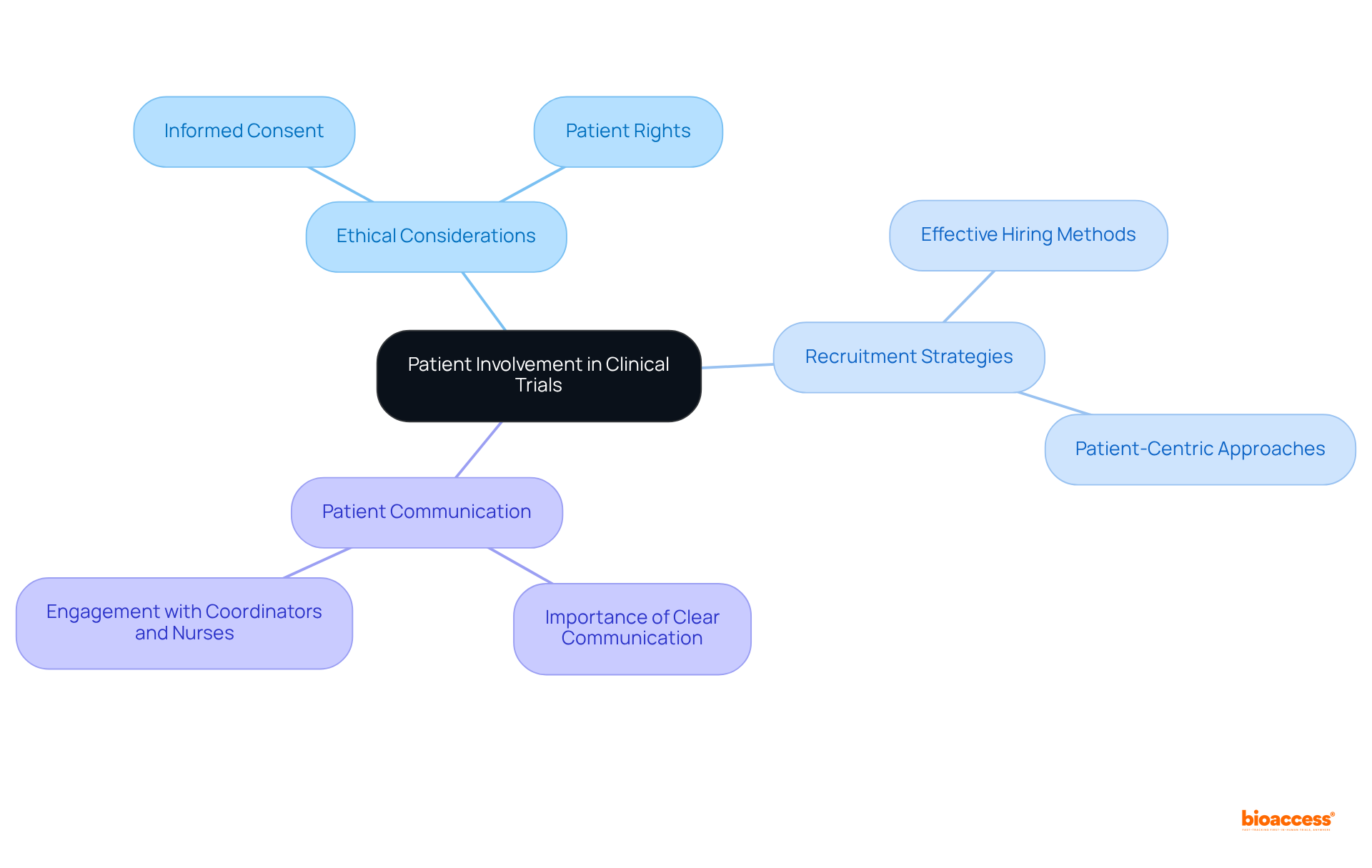
In preclinical vs clinical radiopharma studies, ethical factors are paramount in clinical trials, particularly regarding patient participation, which is often limited in research focused on animal models. Guaranteeing informed consent is essential; it not only safeguards patient rights but also promotes transparency throughout the research process.
Effective hiring methods that prioritize patient safety and adhere to ethical standards are crucial for establishing trust and achieving successful research outcomes. Notably, over 90% of chronic illness patients emphasize the importance of discussing research studies with coordinators and nurses, highlighting the need for clear communication.
Furthermore, ethical guidelines established in 2025 underscore the necessity of patient-centric approaches, ensuring that participants are fully informed and engaged in their treatment decisions. By adhering to these principles, researchers can enhance the integrity of medical experiments and improve recruitment and retention rates, ultimately leading to more reliable and representative results.
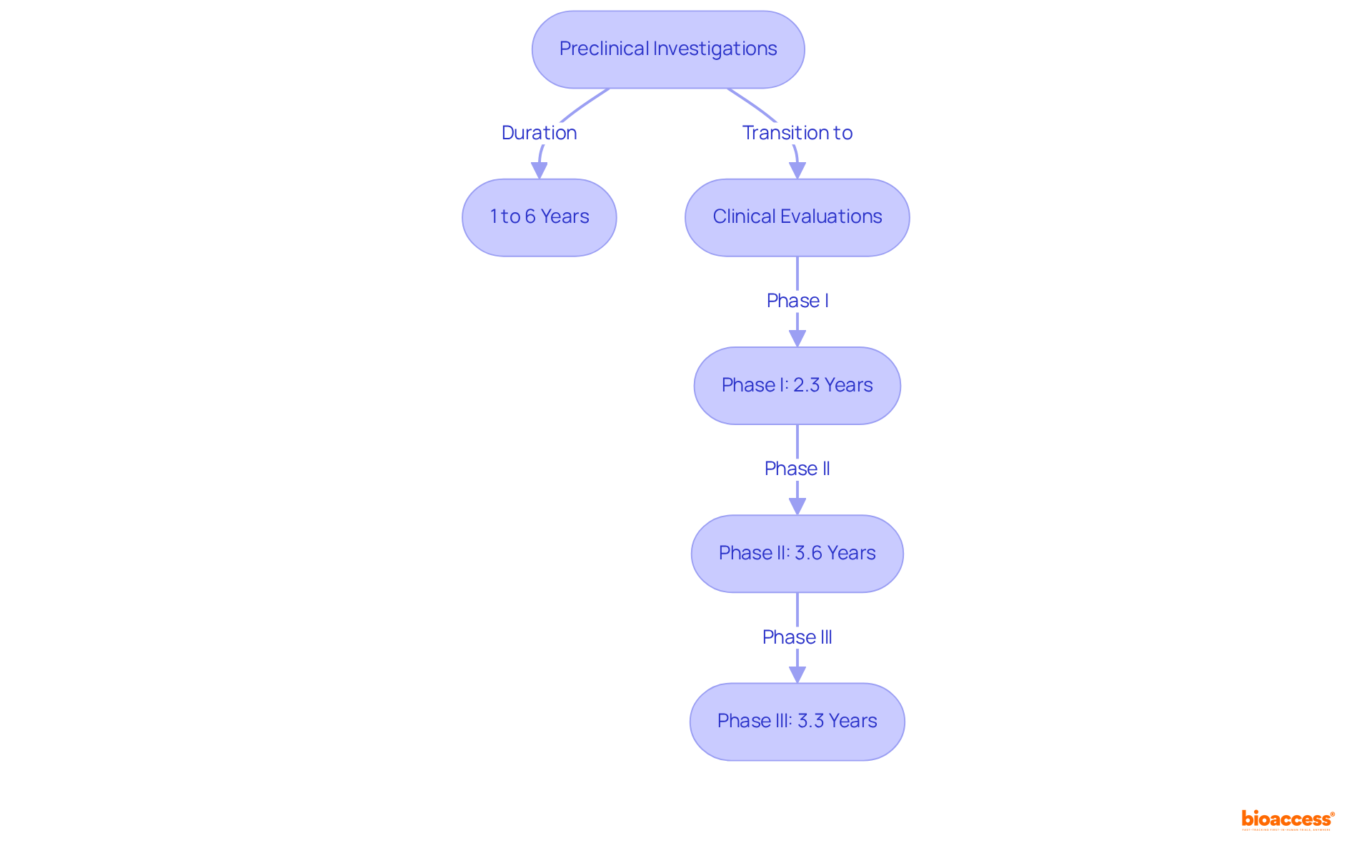
Preclinical investigations are a critical component of preclinical vs clinical radiopharma studies and often extend over several years, typically ranging from 1 to 6 years. This duration is influenced by the complexity of the research and the regulatory requirements involved. In contrast, medical examinations adhere to a more established timeline:
This structured approach emphasizes the necessity for meticulous planning and resource allocation, which are essential for facilitating timely advancement through each phase. Efficient oversight of these timelines is vital; it was reported that the typical length for FDA-approved medications in trials was 89.8 months between 2014 and 2018. This statistic underscores the significance of strategic foresight in the execution of clinical trials.
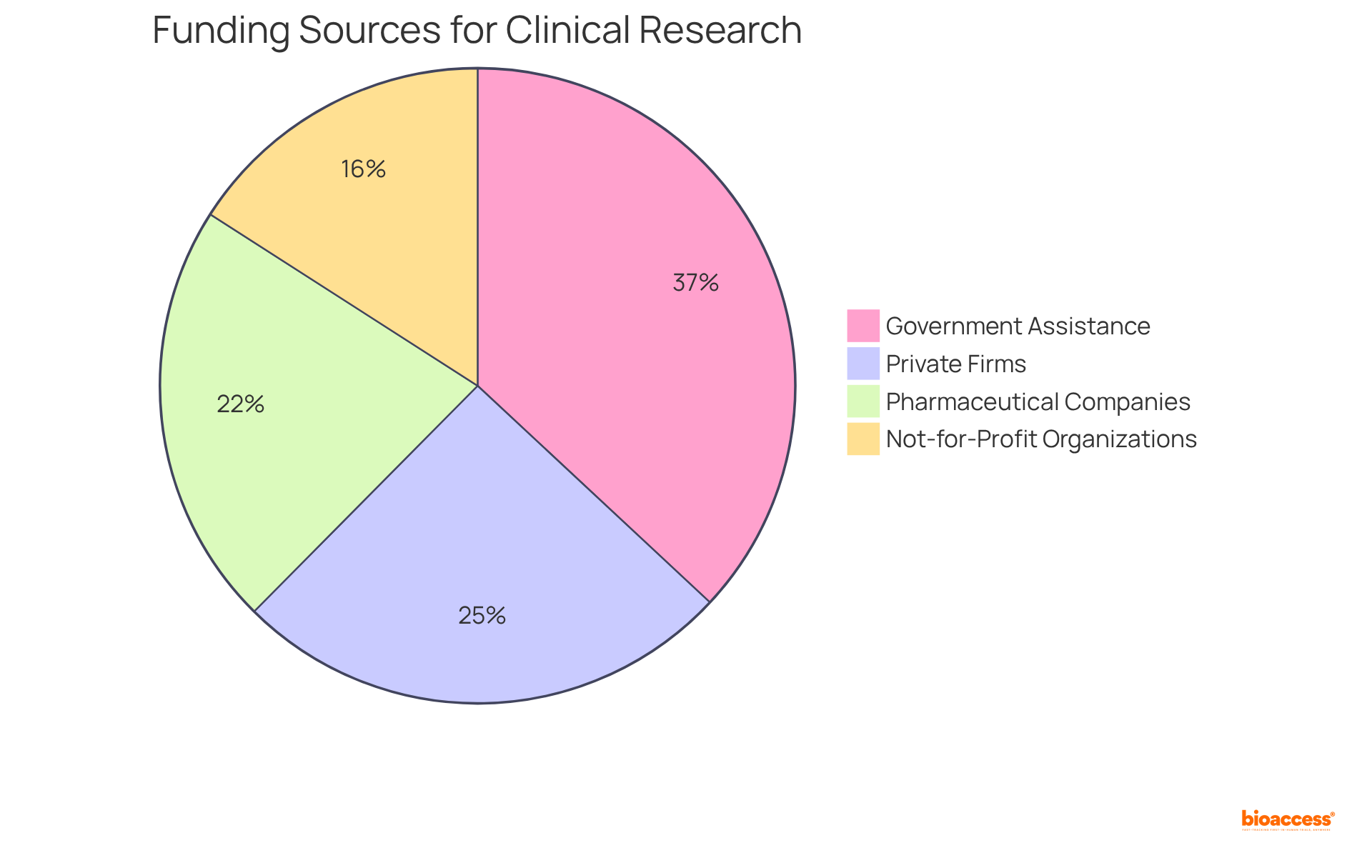
Funding for preclinical research predominantly relies on grants, private investors, and institutional resources, primarily aimed at early-stage investigations. Conversely, research trials necessitate substantially larger budgets, typically sourced from pharmaceutical firms, venture capital, and government funding. Notably, approximately 40% of research projects receive financing from private firms, while 58% secure governmental assistance. This financial landscape underscores the importance of understanding funding dynamics; a 2017 meta-analysis revealed that research backed by pharmaceutical companies presented a 1.34 risk ratio for favorable conclusions.
Effective financing strategies in research often encompass diverse funding sources, including not-for-profit organizations, which contribute nearly 25% of trial funding. Moreover, comprehensive clinical trial management services—such as feasibility assessments, compliance evaluations, trial preparation, import permits, project oversight, and reporting—are crucial for the successful execution of these evaluations. By adeptly navigating these financial channels, researchers can obtain the essential resources needed for successful project implementation, thereby advancing innovative therapies in the medical imaging sector.
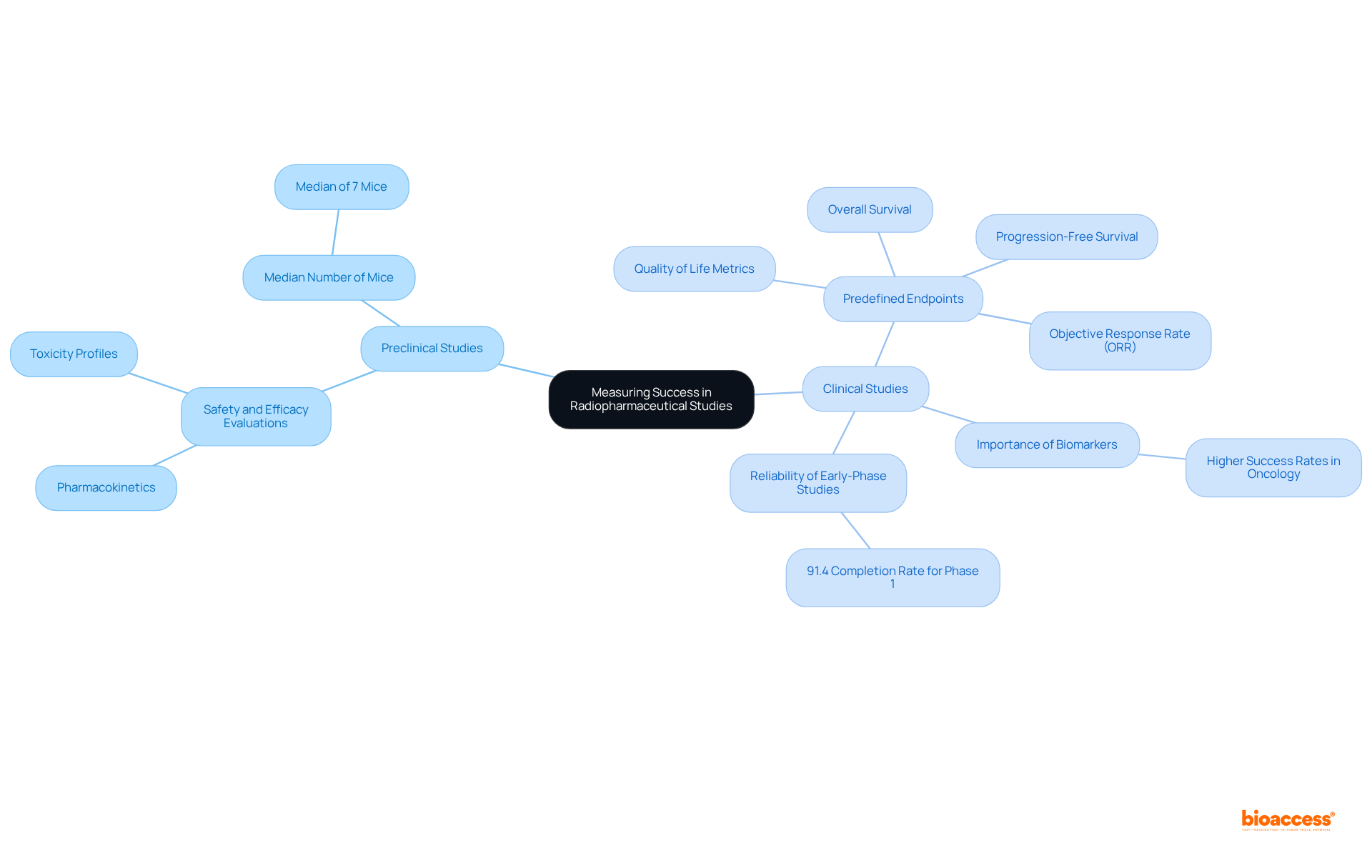
In preclinical vs clinical radiopharma studies, the success of radiopharmaceuticals is primarily measured through safety and efficacy evaluations in animal models, emphasizing pharmacokinetics and toxicity profiles. Investigations frequently involve a median of seven mice administered the drug, underscoring the extensive nature of preclinical research.
In contrast, preclinical vs clinical radiopharma studies utilize predefined endpoints to evaluate outcomes, such as:
These endpoints are essential for determining the therapeutic benefits of radiopharmaceuticals and play a pivotal role in shaping future research directions. Recent evaluations suggest that studies employing biomarkers for patient categorization yield higher success rates, particularly in oncology, highlighting the importance of customized strategies in healthcare environments.
Furthermore, the objective response rate (ORR) serves as a crucial endpoint in research studies, indicating the percentage of patients exhibiting a substantial decrease in tumor size, which is vital for assessing the effectiveness of new treatments. The reliability of early-phase studies is further emphasized by the 91.4% completion rate for Phase 1 studies.
By focusing on these key metrics, researchers can navigate the complexities of drug development more effectively and enhance the likelihood of successful outcomes.
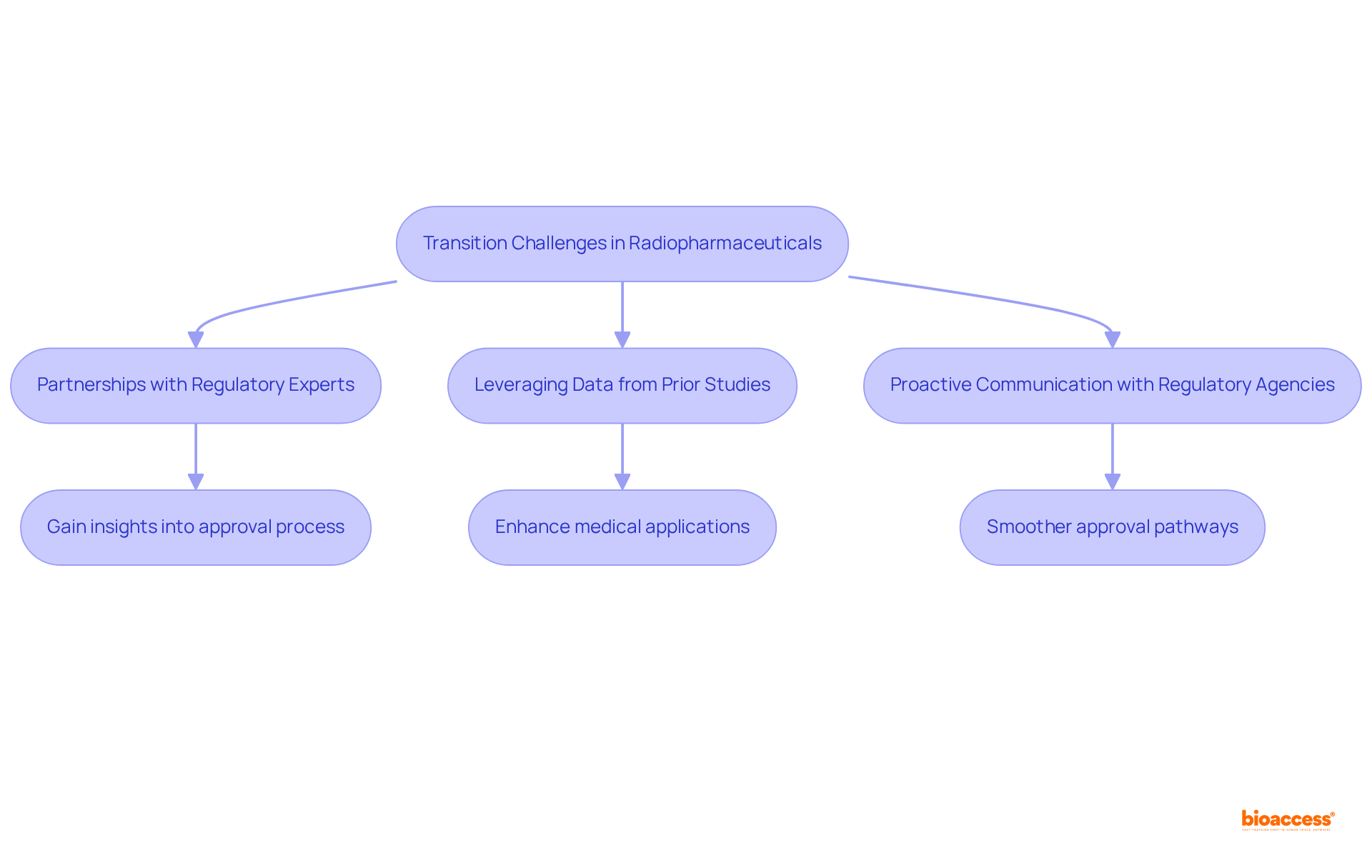
The transition from preclinical vs clinical radiopharma studies to human trials in radiopharmaceuticals presents significant regulatory challenges that can impede progress and escalate costs. As we look toward 2025, the landscape remains daunting, with numerous applicants experiencing delays due to rigorous regulatory requirements. Notably, the average cost of drug development, including unsuccessful attempts, is estimated to range from $1.5 to $2.6 billion, emphasizing the financial stakes at play.
To navigate these challenges, researchers can implement several strategic approaches:
The significance of robust data cannot be overstated. Regulatory authorities demand comprehensive evidence to support research applications, and those who invest in high-quality preclinical studies are more equipped to meet these expectations. In fact, approximately 70% of drugs granted FDA Priority Review receive approval, underscoring the advantages of thorough preparation.
Successful navigation of regulatory challenges is exemplified by companies that have adopted adaptive trial designs, allowing for adjustments based on interim results, thereby increasing the chances of approval. By concentrating on these strategies, researchers can alleviate the difficulties associated with moving from preclinical vs clinical radiopharma studies, ultimately accelerating the development of groundbreaking radiopharmaceuticals.
The distinctions between preclinical and clinical radiopharmaceutical studies are pivotal for comprehending the progression of drug development. By scrutinizing these differences, researchers can adeptly navigate the complexities inherent in each stage, thereby ensuring that innovative therapies are brought to market efficiently and safely.
Throughout this article, we have underscored key points related to the regulatory frameworks, study objectives, methodological approaches, and financial dynamics that define both preclinical and clinical phases. From the expedited regulatory processes in regions such as Colombia to the stringent standards imposed by authorities like the FDA and EMA, these insights highlight the necessity for strategic planning and compliance in research endeavors. Moreover, the significance of patient involvement and ethical considerations is paramount, as they are instrumental in shaping successful clinical outcomes.
Ultimately, grasping the nuances between preclinical and clinical studies is essential for advancing the field of radiopharmaceuticals. As the landscape of medical research continues to evolve, embracing these distinctions will not only enhance the efficiency of drug development but also improve patient care. Researchers and organizations are urged to utilize these insights to foster collaboration, optimize study designs, and ensure that the transition from preclinical to clinical phases is as seamless as possible, paving the way for groundbreaking advancements in healthcare.
What is bioaccess and what role does it play in radiopharma studies?
bioaccess® is an organization that accelerates preclinical and clinical radiopharma studies, leveraging its strategic presence in Latin America, particularly Colombia. It capitalizes on the region's regulatory efficiency to achieve ethical approvals in 4-6 weeks, significantly faster than other areas.
How does Colombia's healthcare system benefit radiopharma studies?
Colombia's healthcare system is recognized among the best globally, which enhances patient recruitment for studies. Additionally, conducting experiments in Colombia can reduce costs by over 30% compared to North America or Western Europe.
What are the key differences between preclinical and clinical radiopharma studies?
Preclinical studies focus on safety and efficacy evaluations in animal models under less stringent guidelines, while clinical studies are governed by rigorous standards from regulatory authorities like the FDA and EMA, requiring detailed documentation and strict patient safety protocols.
What regulatory requirements must be met for clinical radiopharma studies?
Clinical studies must comply with regulations such as the FDA's Investigational New Drug (IND) application and the EMA's Clinical Trials Regulation, which includes robust risk assessments and informed consent processes.
What are the main objectives of preclinical research in radiopharma?
Preclinical research aims to evaluate the safety and biological activity of radioactive drugs, establishing a foundation for subsequent clinical trials that assess effectiveness and safety in human participants.
How long does preclinical research typically take before entering trial phases?
The duration of preclinical research can vary significantly, often extending over multiple years before transitioning to clinical trial phases.
Why is ensuring safety and effectiveness in radioactive medicines important?
Ensuring safety and effectiveness is crucial because any miscalculation can lead to adverse reactions and setbacks in bringing products to market.
What additional services are important for the successful execution of radiopharma research projects?
Important services include feasibility assessments, site selection, compliance evaluations, project oversight, and reporting, which enhance research efficiency and contribute to local economies through job creation and healthcare improvements.