


This article outlines ten key elements that are essential for a successful pharmaceutical market access strategy. These elements include:
Each component is supported by insights demonstrating how effective execution in these areas can significantly enhance a company's ability to navigate market challenges. By optimizing product launches, these strategies ultimately improve patient access to new therapies.
Understanding the Medtech landscape is crucial, as it presents unique challenges that require tailored solutions. Bioaccess plays a pivotal role in addressing these challenges, ensuring that companies can effectively implement their market access strategies. Collaboration among stakeholders is vital, as it fosters a comprehensive approach to overcoming barriers and enhancing patient outcomes.
In conclusion, the importance of these ten elements cannot be overstated. Companies must prioritize these strategies to ensure successful market access and improve patient access to innovative therapies. The next steps involve assessing current practices and identifying areas for improvement, ultimately leading to better health outcomes.
The pharmaceutical landscape presents a complex web of challenges and opportunities, where the speed and strategy of market access can significantly influence the success of innovative therapies. As companies work to navigate this competitive arena, grasping the essential components of a successful market access strategy is crucial.
What key elements can propel a product from concept to patient? How can organizations effectively tackle the complexities of regulatory approvals, reimbursement, and stakeholder engagement? This article explores ten critical aspects that not only facilitate market entry but also secure long-term success in the dynamic world of pharmaceuticals.
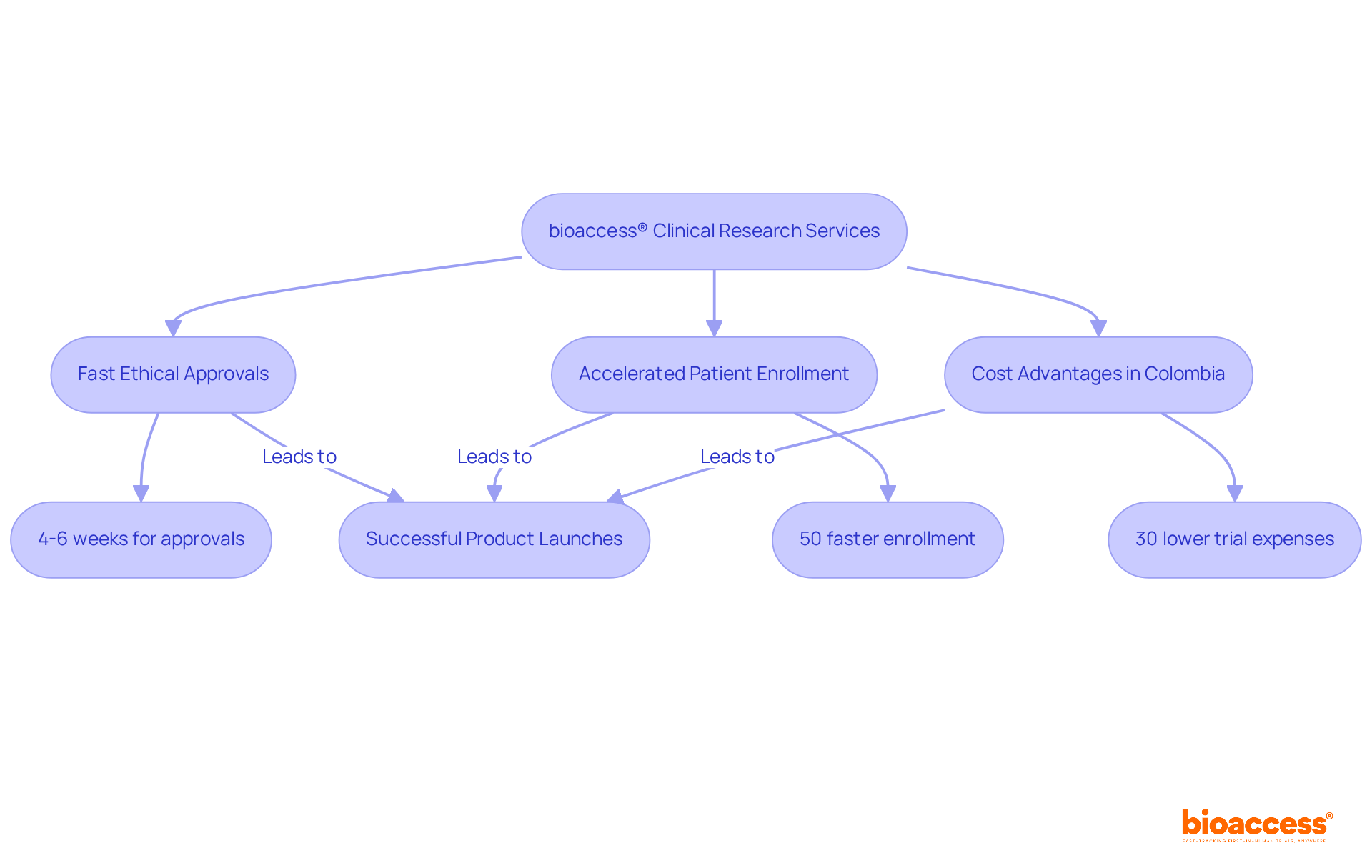
bioaccess® harnesses its extensive experience and regional advantages to enhance entry for Medtech, Biopharma, and Radiopharma innovators. With ethical approvals secured in just 4-6 weeks and patient enrollment occurring at a rate 50% faster than traditional markets, bioaccess® significantly reduces time-to-market. This agility is crucial in a competitive landscape where timely availability can dictate commercial success.
Colombia offers a distinct advantage, with trial expenses over 30% lower than in North America or Western Europe, complemented by a healthcare system recognized among the best globally. Aligning medical research timelines with a pharmaceutical market access strategy is vital for optimizing outcomes, as effective market access can influence over 70% of revenue outcomes in the pharmaceutical sector. Moreover, understanding the evolving regulatory landscape and engaging with regulatory bodies early can mitigate risks and streamline processes.
The emphasis on rapid medical research services not only accelerates product launches but also fosters trust among stakeholders, ensuring that innovations reach the patients who need them most. With R&D tax incentives and a robust patient recruitment framework, bioaccess® is well-positioned to assist startups in navigating the complexities of trials in Latin America. Additionally, the 'Patient Velocity' feature enables patient enrollment that is 50% faster than Western sites, translating to substantial savings of $25K per patient with FDA-ready data, ensuring both efficiency and effectiveness in clinical trials.
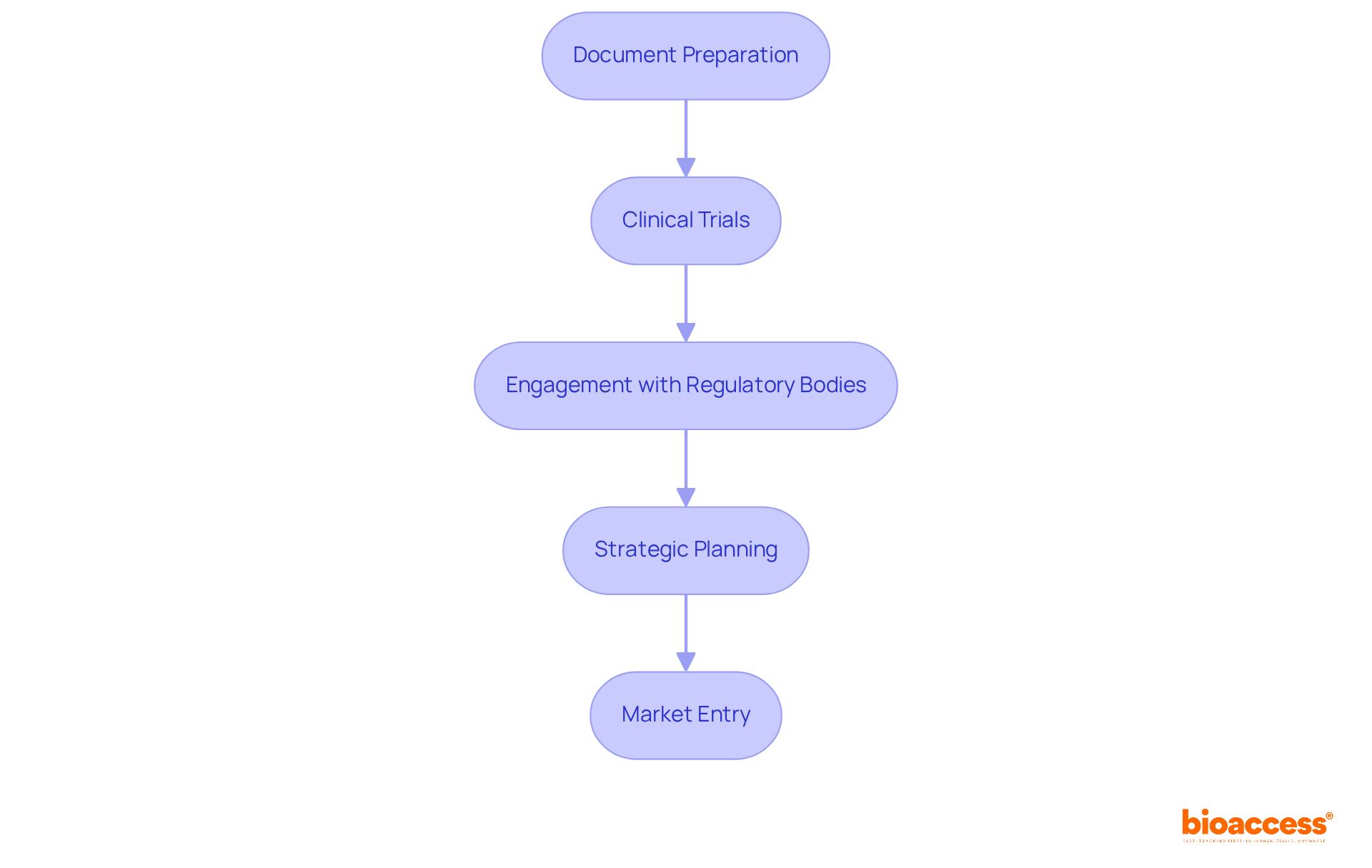
Regulatory approval stands as a pivotal element in gaining access to the market, demanding a thorough grasp of complex guidelines and strict adherence to both local and international standards. Insights from regulatory affairs specialists underscore the necessity of understanding the specific regulatory landscape of each target area. This process involves:
For example, the European Medicines Agency (EMA) typically mandates a pre-authorization evaluation that can last up to 210 days, highlighting the critical need for strategic planning throughout the approval journey.
By focusing on regulatory strategies, including trial organization and project oversight, businesses can significantly reduce delays and strengthen their pharmaceutical market access strategy to improve their chances of successful market entry. Moreover, cultivating robust relationships with regulatory authorities is essential, as evidenced by bioaccess's adept navigation of these intricate guidelines, which has led to effective product commercialization. Staying abreast of evolving regulations is crucial for maintaining compliance and ensuring a seamless path to market entry.
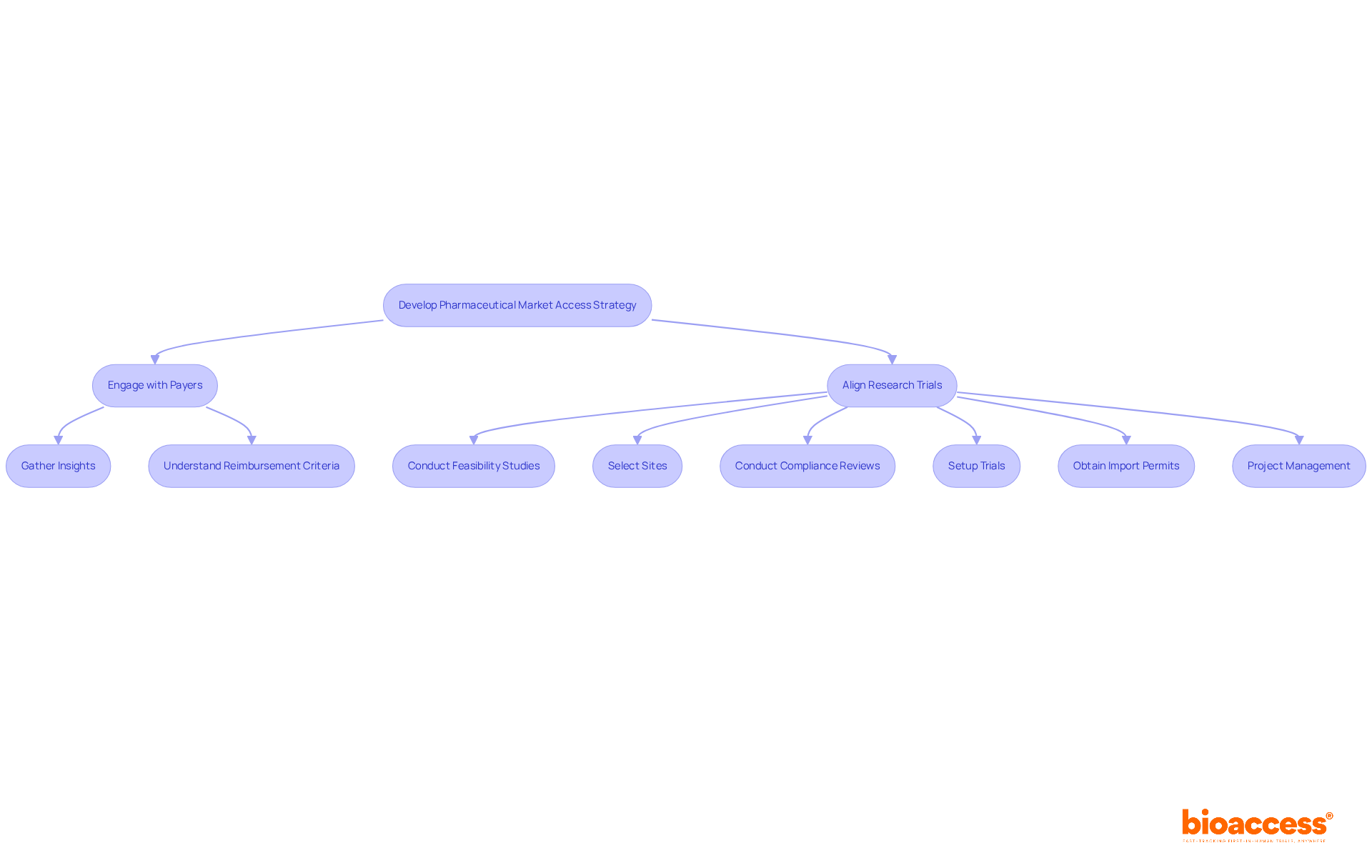
Developing a pharmaceutical market access strategy is essential for ensuring the financial viability of new drugs. This process, as part of the pharmaceutical market access strategy, requires a deep understanding of payer requirements and a clear demonstration of the product's economic value. At bioaccess®, we recognize the critical importance of engaging early with payers as it is essential for our pharmaceutical market access strategy to gather valuable insights into their expectations and reimbursement criteria. Our strategic reimbursement and health economics solutions are meticulously crafted to maximize pricing and coverage across both public and private systems throughout Latin America.
By aligning research trial results with payer requirements, companies can significantly enhance their pharmaceutical market access strategy, which is vital for securing favorable reimbursement terms and achieving commercial success. Furthermore, our comprehensive trial management services—including:
ensure that every aspect of the research process is streamlined and compliant. This thorough approach not only supports effective entry into the market but also reinforces the importance of collaboration in navigating the complexities of clinical research.
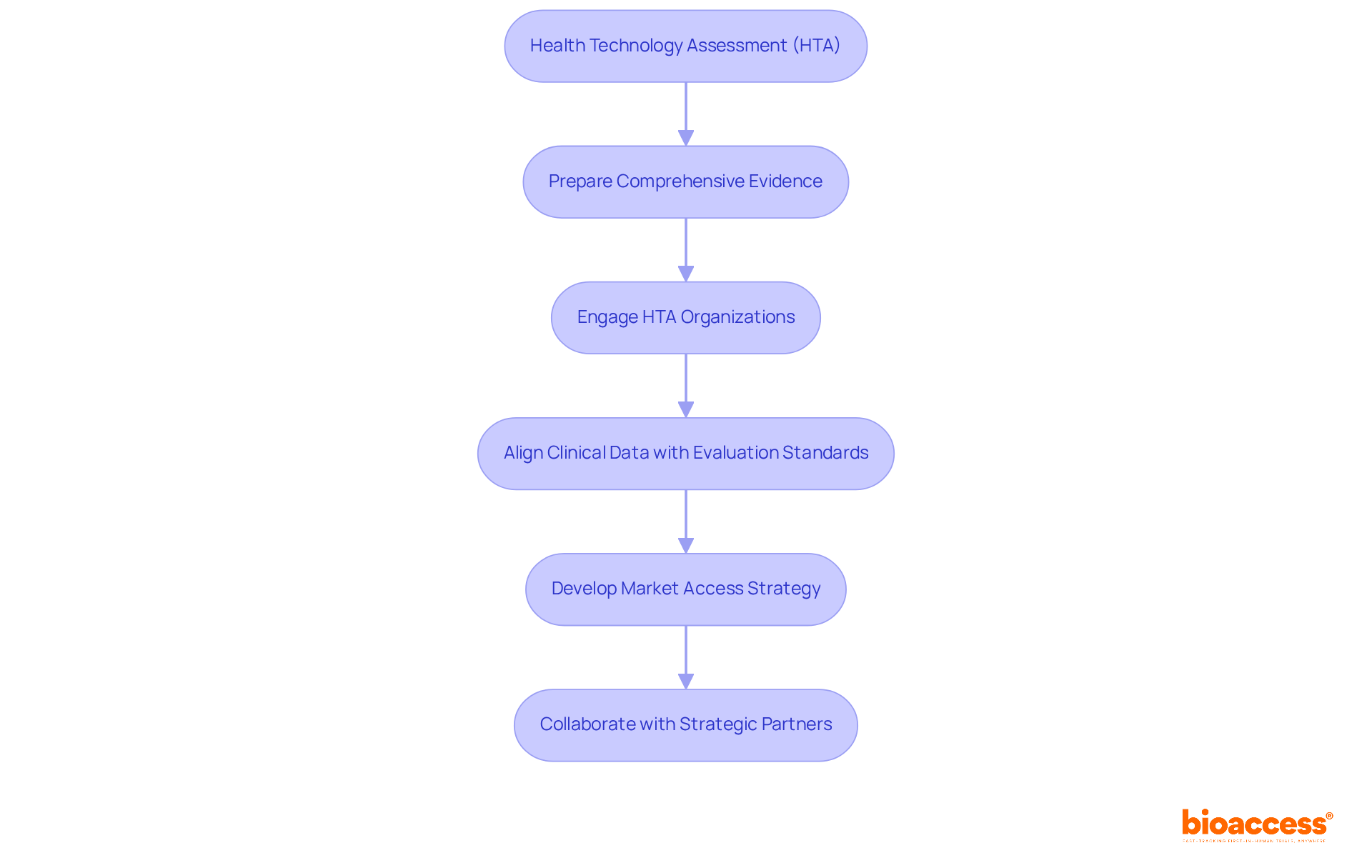
Health Technology Assessment (HTA) plays a pivotal role in the systematic evaluation of health technologies, guiding decision-making on healthcare resource allocation. Understanding HTA processes is essential for companies aiming to develop a pharmaceutical market access strategy, as emphasized by the Clinical Research Management Specialist. To succeed, businesses must prepare comprehensive evidence that showcases the medical and economic value of their products. Engaging HTA organizations early in the process can help align clinical data with their evaluation standards, which is a crucial component of an effective pharmaceutical market access strategy, facilitating a smoother market entry.
In the dynamic Medtech landscape, bioaccess® stands out by offering strategic reimbursement and health economics solutions that optimize pricing and coverage across public and private systems in Latin America. By leveraging pre-validated templates, interactive pricing calculators, and reimbursement rate databases, companies can effectively navigate the complex reimbursement environments in Brazil, Colombia, and Chile. A compelling case study illustrates bioaccess®'s impact: it successfully reduced a digital health startup’s FONASA reimbursement timeline by nine months through GRD alignment, significantly accelerating their entry efforts.
Collaboration with HTA organizations and strategic partners is crucial for overcoming the challenges in clinical research. As companies seek to enhance their market presence, understanding the intricacies of HTA can be crucial for formulating a successful pharmaceutical market access strategy that provides a competitive edge. The next steps involve actively engaging with bioaccess® to explore tailored solutions that meet specific needs in the ever-evolving healthcare landscape.
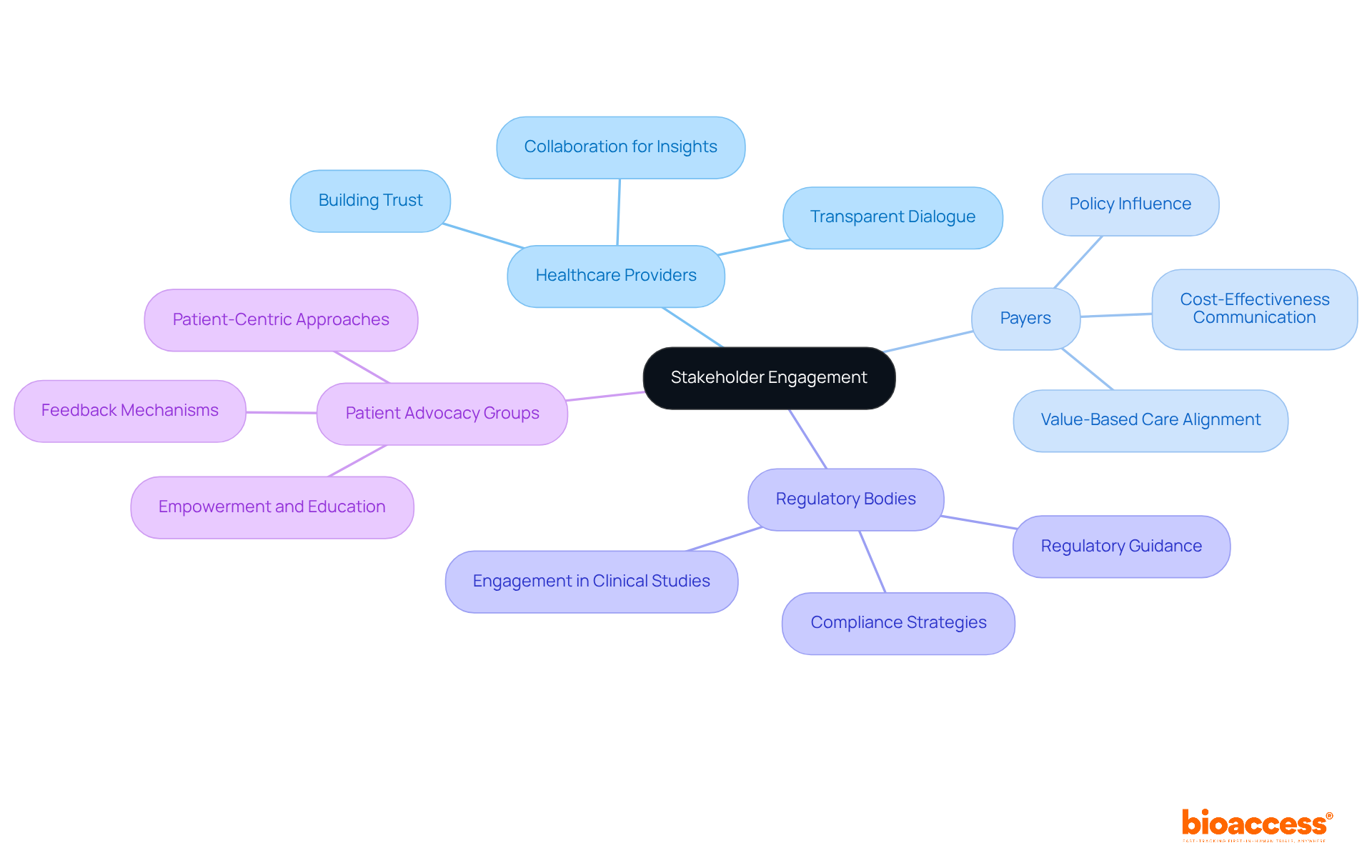
Effective stakeholder involvement is essential for a successful pharmaceutical market access strategy in the sector. Cultivating strong relationships with healthcare providers, payers, regulatory bodies, and patient advocacy groups is key to developing an effective pharmaceutical market access strategy. Authorities in clinical studies emphasize that fostering transparent dialogue and teamwork can yield valuable insights and support throughout the journey into the industry. By nurturing these relationships, companies can navigate challenges more effectively, enhance their product's acceptance, and ultimately improve patient outcomes.
Successful examples illustrate that organizations prioritizing these connections are better positioned to achieve their objectives related to pharmaceutical market access strategy. This ensures that innovative therapies reach those who need them most. As you consider your own challenges in clinical research, reflect on how strengthening stakeholder relationships could pave the way for your success.
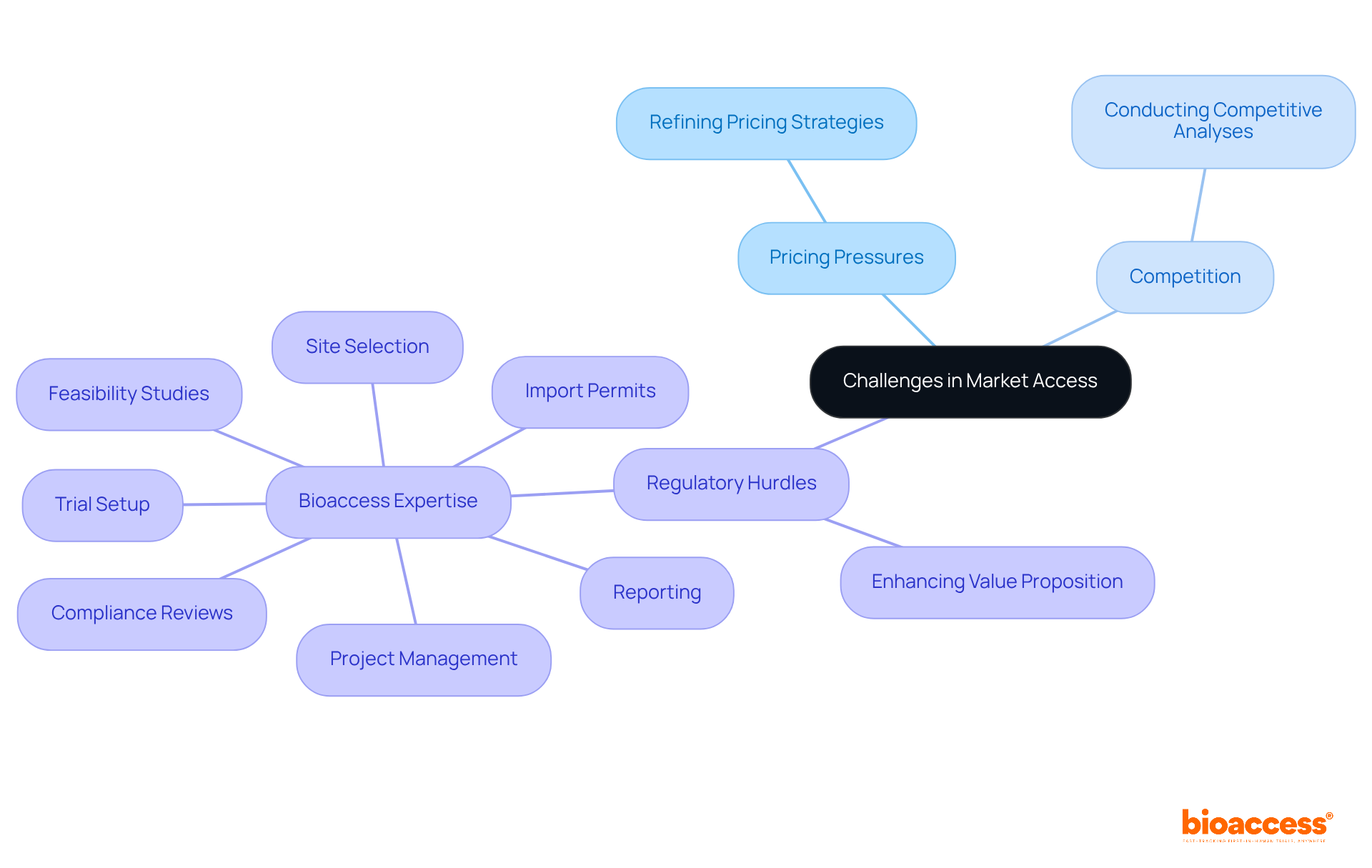
Market access presents numerous challenges, such as pricing pressures, competition, and regulatory hurdles. Organizations must be prepared to adapt their strategies in response to these obstacles. This could mean:
Moreover, leveraging comprehensive trial management services, like those offered by bioaccess, can significantly streamline the process.
With expertise in:
bioaccess accelerates regulatory approval, achieving it in just 6-8 weeks compared to the typical 6-12 months in the US/EU. By proactively addressing these challenges and implementing a pharmaceutical market access strategy, organizations can significantly improve their chances of successful entry into the industry.
In the ever-evolving Medtech landscape, collaboration is key. Organizations that partner with experienced service providers can navigate the complexities of clinical research more effectively. Are you ready to enhance your pharmaceutical market access strategy? Consider how bioaccess can support your journey toward regulatory success.
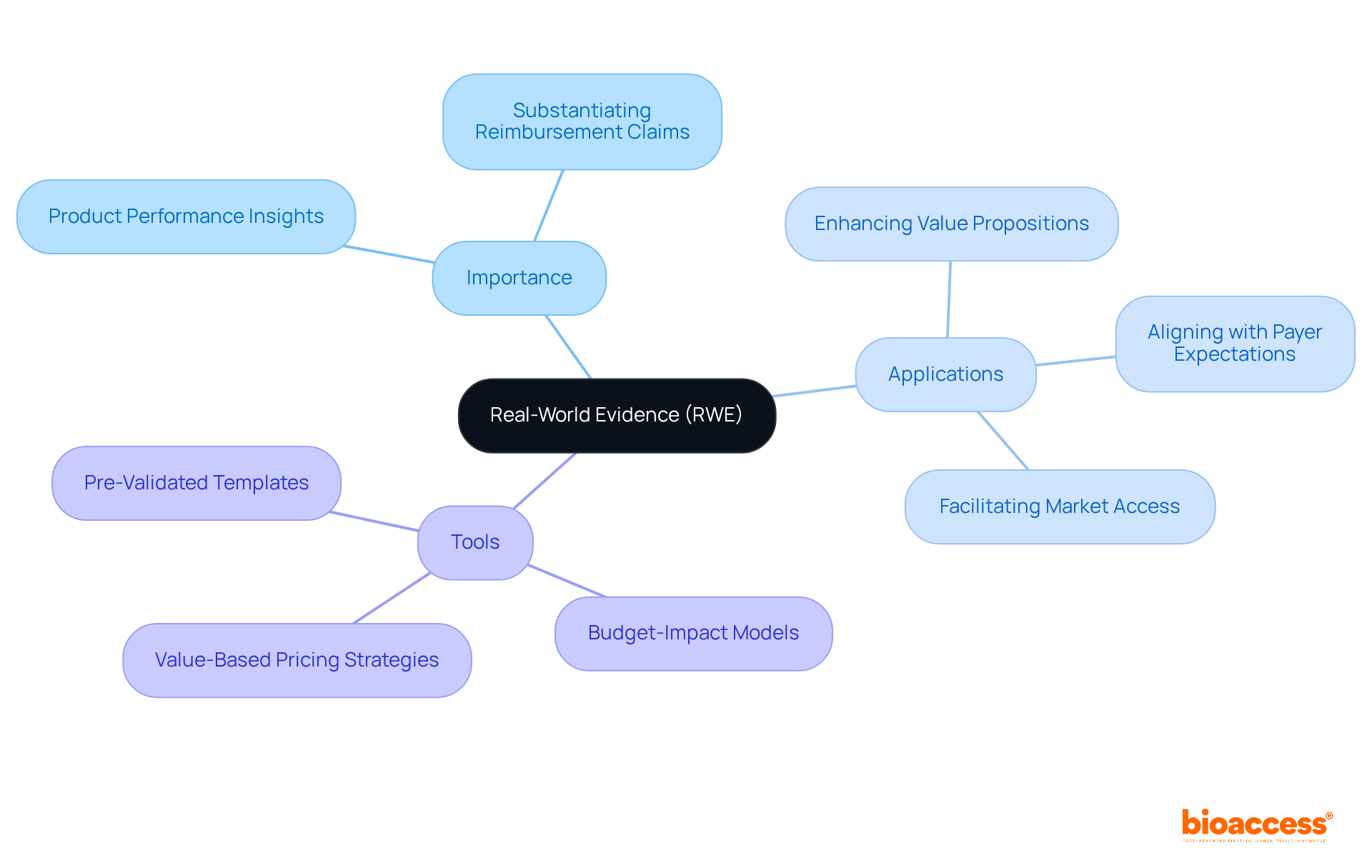
Real-World Evidence (RWE) encompasses data gathered outside traditional clinical trials, offering valuable insights into product performance in everyday settings. This data serves as a crucial asset for the pharmaceutical market access strategy, allowing companies to effectively substantiate their reimbursement claims. By demonstrating the safety and efficacy of products among various populations, RWE strengthens the argument for entry into the industry as part of the pharmaceutical market access strategy and reimbursement approval. Notably, the global RWE sector is projected to reach $48 billion by 2032, underscoring its growing significance in the pharmaceutical landscape.
Incorporating RWE into clinical development plans not only enhances the value proposition of new therapies but also aligns with payer expectations. The EU Joint Clinical Assessment (JCA) aims to streamline evaluations across member states, emphasizing the need for robust evidence to support reimbursement decisions. As pharmaceutical firms increasingly depend on RWE, those that invest in real-time data infrastructure will be better positioned to effectively implement their pharmaceutical market access strategy in 2025.
Clinical researchers recognize the importance of RWE in reimbursement discussions, noting that it shifts the narrative from merely hoping payers see value to actively proving it. By utilizing RWE, organizations can develop a pharmaceutical market access strategy that includes persuasive payer-specific evidence packages to engage stakeholders, ultimately facilitating easier entry to therapies and enhancing patient outcomes. To remain competitive, companies should prioritize investments in RWD infrastructure.
Additionally, bioaccess® offers strategic reimbursement and health economics solutions tailored for Latin America, including:
These tools can significantly enhance the effectiveness of RWE in supporting the pharmaceutical market access strategy for decision-making.
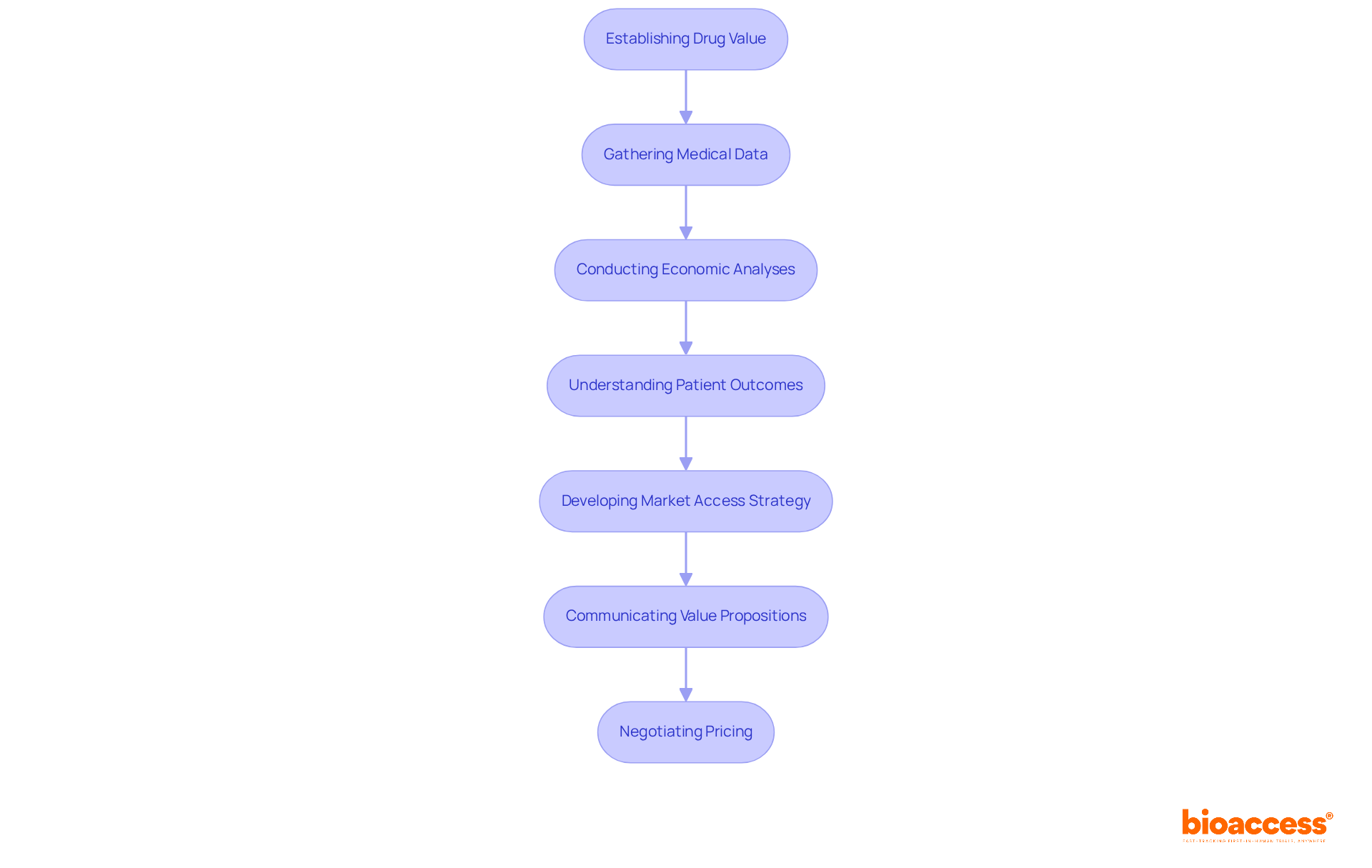
Establishing the value of a new drug is essential for justifying its price. This process requires strong medical data, comprehensive economic analyses, and insights from patient-reported outcomes. Research management specialists emphasize the necessity for organizations to develop a pharmaceutical market access strategy that includes clear value propositions that resonate with payers and other stakeholders. By effectively communicating the benefits of their offerings, businesses can significantly improve their pharmaceutical market access strategy, which is vital for securing favorable pricing and reimbursement conditions essential for success in the industry.
For example, bioaccess® provides strategic reimbursement and health economics solutions, including budget-impact models for integration into public health systems like Brazil's SUS and value-based pricing strategies to address low reimbursement rates in Colombia and Peru. The incorporation of cost-effectiveness analyses, such as the incremental cost-effectiveness ratio (ICER) used for pricing in many countries, alongside real-world evidence, can bolster the credibility of pricing arguments. This makes it easier for payers to grasp the drug's value in enhancing patient outcomes.
Moreover, effective communication strategies that highlight health benefits and financial advantages can facilitate negotiations, ultimately leading to improved patient access and a more sustainable position for pharmaceutical firms through their pharmaceutical market access strategy. However, the varying definitions of 'value' among different stakeholders complicate pricing discussions, underscoring the need for transparency in drug pricing determination processes, as emphasized by the European Parliament's call for full transparency.
By leveraging bioaccess®'s expertise in patient entry mapping, competitor analysis, and utilizing tools like interactive pricing calculators and reimbursement rate databases, companies can further refine their value propositions to align with industry expectations.
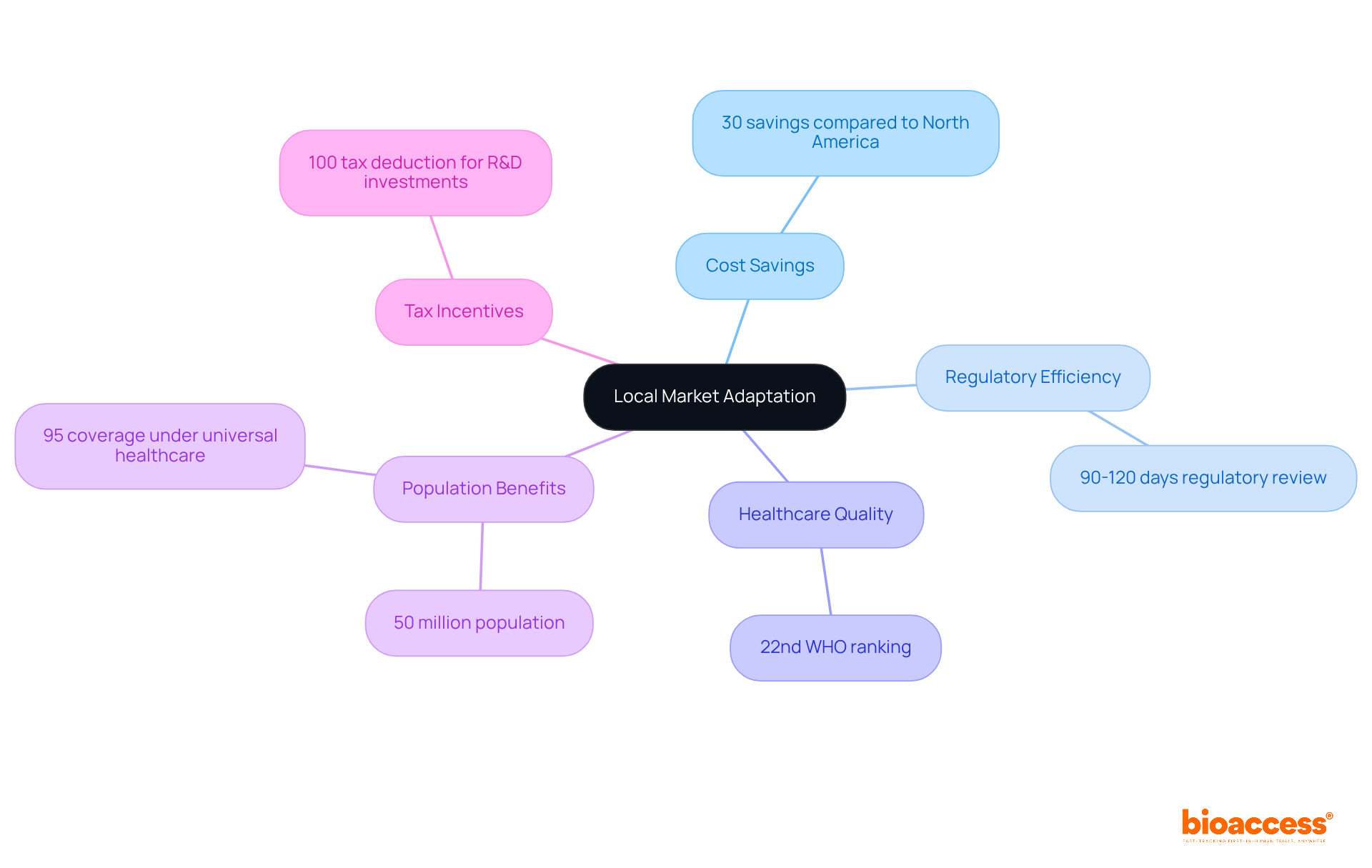
Local adaptation is crucial for successful access, especially in regions like Colombia, which offers significant competitive advantages for first-in-human trials. With cost savings exceeding 30% compared to North America and Western Europe, and a regulatory review process that typically spans just 90 to 120 days, Colombia emerges as a prime destination for clinical research. The country's high-quality healthcare system, ranked 22nd by the World Health Organization, coupled with a rigorous ICH/GCP certification process for hospitals, guarantees that studies are conducted with the highest integrity.
Moreover, with a population exceeding 50 million and 95% coverage under universal healthcare, patient recruitment is robust. Companies can also take advantage of R&D tax incentives, including a 100% tax deduction for investments in science and technology. By tailoring their pharmaceutical market access strategy to leverage these local benefits, organizations can significantly enhance their impact in Colombia. To maximize success, consider partnering with local experts who understand the regulatory landscape and can navigate the complexities of entering this promising market.
Emerging trends in the pharmaceutical market access strategy, particularly the growing significance of digital health solutions and personalized medicine, are fundamentally reshaping the clinical research landscape. The Clinical Research Management Expert emphasizes that organizations must proactively stay ahead of these trends to maintain a competitive edge. This proactive approach involves:
bioaccess® stands at the forefront, offering expedited clinical trial services that empower organizations to navigate these challenges with efficiency. With a proven track record spanning over 15 years, bioaccess® enables organizations to achieve regulatory approval in as little as 6-8 weeks—significantly faster than the typical 6-12 months required in the US and EU. By leveraging bioaccess®'s expertise in early-phase studies and strategic reimbursement solutions, companies can effectively create a pharmaceutical market access strategy for sustained success in the market.
In this dynamic environment, collaboration is essential. Organizations that embrace these emerging trends and partner with experts like bioaccess® will not only enhance their operational efficiency but also secure their place in the competitive landscape of clinical research.
The success of a pharmaceutical market access strategy relies on a multifaceted approach that integrates various essential elements. Understanding the regulatory landscape, developing reimbursement strategies, engaging stakeholders, and adapting to local markets are all vital components that ensure new therapies reach patients efficiently and effectively. By prioritizing these key elements, organizations can significantly enhance their chances of successful market entry and commercial viability.
Throughout this article, we have emphasized the importance of:
These factors collectively contribute to a robust market access strategy, enabling companies to navigate challenges such as pricing pressures and competition while demonstrating the value of their products. By leveraging local advantages and emerging trends, organizations can position themselves for success in a dynamic healthcare landscape.
As the pharmaceutical industry continues to evolve, embracing innovative strategies and fostering collaboration will be crucial for achieving sustained success. Companies are encouraged to actively engage with experts and adapt their approaches to meet the changing demands of the market. By doing so, they not only enhance their market access capabilities but also ensure that patients gain timely access to the therapies they need.
What services does bioaccess® provide for Medtech, Biopharma, and Radiopharma innovators?
bioaccess® offers fast clinical research services that enhance market entry by securing ethical approvals in 4-6 weeks and enrolling patients 50% faster than traditional markets.
How does bioaccess® help reduce time-to-market for clinical trials?
By providing rapid medical research services and a robust patient recruitment framework, bioaccess® significantly accelerates product launches, which is crucial for success in a competitive environment.
What are the cost advantages of conducting clinical trials in Colombia?
Trial expenses in Colombia are over 30% lower than in North America or Western Europe, making it a cost-effective location for clinical research.
Why is aligning medical research timelines with market access strategy important?
Aligning these timelines is vital because effective market access can influence over 70% of revenue outcomes in the pharmaceutical sector.
What role does regulatory approval play in market access?
Regulatory approval is essential for market access, requiring a thorough understanding of complex guidelines and adherence to local and international standards.
What steps are involved in the regulatory approval process?
The regulatory approval process involves meticulous documentation preparation, essential clinical trials, and proactive engagement with regulatory bodies.
How does bioaccess® navigate regulatory challenges?
bioaccess® cultivates robust relationships with regulatory authorities and stays updated on evolving regulations to ensure compliance and effective product commercialization.
What is the significance of a reimbursement strategy in pharmaceutical market access?
A reimbursement strategy is crucial for ensuring the financial viability of new drugs by demonstrating economic value and aligning research trial results with payer requirements.
How does bioaccess® approach payer engagement?
bioaccess® emphasizes early engagement with payers to gather insights on their expectations and reimbursement criteria, which is essential for maximizing pricing and coverage.
What services are included in bioaccess®'s trial management?
bioaccess® offers comprehensive trial management services including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.