


The landscape of clinical research is rapidly evolving, with randomized controlled trials (RCTs) leading the charge in delivering reliable medical insights. As the demand for swift and effective trial designs grows, grasping the key elements that underpin successful RCTs is essential for researchers and innovators alike.
How can strategic approaches to trial design - such as randomization, control groups, and ethical considerations - transform the outcomes of medical studies? This article explores ten critical components that can enhance the efficacy of randomized controlled trials, paving the way for groundbreaking advancements in healthcare.
bioaccess® leverages the regulatory speed of Latin America, the diverse patient populations in the Balkans, and Australia's efficient ethical approval processes to dramatically shorten timelines for randomized controlled studies. With ethical approvals secured in just 4-6 weeks and enrollment rates that are 50% faster than traditional markets, bioaccess® empowers Medtech, Biopharma, and Radiopharma innovators to expedite their studies effectively. Our expertise spans:
This agility is essential in today’s medical landscape, where swift access to timely data can significantly accelerate the introduction of new therapies to the market.
Recent advancements in randomized controlled studies underscore the necessity for such efficiency, as companies increasingly focus on participant experiences and outcomes to boost recruitment and retention rates. Have you considered how navigating regulatory complexities swiftly could be a game-changer for your research? As industry experts emphasize, this capability is a crucial determinant of success in medical research.
In summary, collaboration and strategic partnerships are vital for overcoming the challenges in clinical research. By choosing bioaccess®, you position yourself at the forefront of innovation, ready to tackle the evolving demands of the Medtech landscape.
Randomization stands as a cornerstone of randomised controlled trial design, playing a crucial role in ensuring that participants are assigned to treatment or control categories by chance. This essential process significantly reduces selection bias, allowing for a more precise comparison of outcomes between these categories. Techniques such as simple randomization, block randomization, and stratified randomization are employed to achieve this vital goal. By guaranteeing that each participant has an equal opportunity of being allocated to any category, researchers can draw more trustworthy conclusions about the effectiveness of the intervention being tested.
Consider the implications: without a randomised controlled trial design, the integrity of clinical trials could be compromised, leading to unreliable results. The Medtech landscape increasingly recognizes this necessity, as it addresses key challenges in clinical research. Collaboration among researchers, regulatory bodies, and industry leaders is paramount to enhance the reliability of trial outcomes. As we move forward, it is essential to prioritize these methodologies to ensure the advancement of medical science.
Control sets are crucial for establishing a benchmark against which the effects of experimental treatments can be accurately measured. In randomised controlled trial design, participants in the control set do not receive the treatment being evaluated; instead, they may receive a placebo or standard therapy. This comparison is vital for determining whether observed effects stem from the treatment itself or are influenced by other factors. The selection of the control group - whether it be active, placebo, or historical - can significantly impact the trial's outcomes and interpretations.
Current methods in control selection underscore the importance of reducing biases and ensuring that the cohorts are comparable. Random assignment is a key technique employed to minimize differences between treatment and control sets, thereby enhancing the validity of the results. Notably, almost 60% of surgical inquiries cannot be effectively resolved by randomised controlled trial design alone, which highlights the necessity of careful control design to address complex medical situations.
Recent studies have explored various control strategies in medical investigations, including the implementation of synthetic control arms, particularly in studies for rare diseases. These innovative approaches can reduce development time and costs while maintaining scientific rigor. For instance, synthetic arms have been successfully utilized in assessments evaluating therapies for rare types of lung cancer and pediatric illnesses, demonstrating their capacity to enhance the research process. As Sammi Tang, former Vice-President Global Head of Biometrics at Servier, stated, 'Randomization remains the gold standard for clinical studies,' underscoring the essential role of randomised controlled trial design in ensuring robust study outcomes.
Experts assert that the influence of control selection on trial outcomes cannot be overstated. The integrity of control sets is vital for ensuring the internal validity of research findings. Without a well-defined control set, it becomes challenging to ascertain whether changes in the treatment cohort are due to the intervention or simply natural progression over time. Therefore, meticulous planning and the choice of control sets remain foundational to effective comparison in studies.
Blinding, or masking, stands as a pivotal technique in randomized controlled studies (RCTs), essential for minimizing bias in treatment administration and outcome evaluation. In single-blind studies, participants are kept unaware of their group assignments, while double-blind studies extend this concealment to both participants and researchers. This dual approach significantly mitigates the risk of performance and detection bias, thereby enhancing the credibility of study results. For instance, a systematic review revealed that studies employing double-blinding reported significantly different treatment effect sizes compared to those that did not, underscoring the necessity of this methodological feature.
The importance of blinding in RCTs cannot be overstated, especially in 2025, as it is recognized as a crucial strategy for maintaining the integrity of clinical research. Effective blinding ensures that outcomes are attributable solely to the intervention being tested, rather than external influences or biases introduced by participants or researchers. In complex intervention studies, where multifaceted treatments are evaluated, the challenges of implementing blinding become pronounced. A survey of UK researchers indicated that 91% acknowledged significant difficulties in achieving adequate blinding, particularly in complex interventions. Notably, only 22% of open studies applied outcome assessment blinding, in contrast to 66% where it was feasible, emphasizing the underutilization of this essential methodological aspect.
Successful double-blind studies in biopharma research illustrate the effectiveness of this approach. For example, studies evaluating the safety of high-visibility bicycle apparel demonstrated that blinding significantly decreased the incidence of reporting biases, resulting in more trustworthy outcomes. As emphasized by experts, "Blinding is an important methodological feature of RCTs to minimize bias and maximize the validity of the results." This highlights the ongoing need for researchers to prioritize blinding in their study designs to ensure robust and credible findings.
Establishing an appropriate sample size is crucial in study design, as it significantly impacts the research's power - the probability of detecting a true effect when it exists. Sample size calculations take into account several factors, including:
Studies that are underpowered risk overlooking significant differences, while excessively large samples can waste resources and expose more participants than necessary to potential risks. Researchers can utilize various tools and formulas to ensure these calculations are performed accurately, reinforcing the integrity of their findings.
Ethical aspects in medical studies are crucial for safeguarding participant rights and upholding the integrity of the investigative process. Key ethical principles, such as:
are foundational to this effort. Researchers must guarantee that participants fully understand the study's purpose, procedures, potential risks, and benefits before they agree to take part.
At bioaccess, we offer comprehensive research study management services that include:
Each of these services is meticulously designed to uphold these ethical standards. By adhering to these principles, we not only protect participants but also enhance the reliability of our findings, reinforcing our commitment to ethical research practices.
In the ever-evolving Medtech landscape, collaboration is key to addressing the challenges faced in clinical research. By partnering with us, you can ensure that your studies are conducted with the highest ethical standards, ultimately leading to more trustworthy outcomes. Let's work together to navigate these complexities and drive innovation in healthcare.

A clinical study protocol serves as a vital roadmap, outlining the study's objectives, randomised controlled trial design, methodology, and statistical considerations. A meticulously crafted protocol clearly delineates the roles and responsibilities of the study team, specifies inclusion and exclusion criteria for participants, and details the procedures for data collection and analysis. This document is crucial for ensuring compliance with regulatory requirements and fostering effective communication among stakeholders. By providing a clear framework, a well-defined protocol minimizes deviations from the study plan, thereby enhancing the reliability and validity of research outcomes.
In 2025, the emphasis on clear protocols in medical studies has never been more pronounced. They guide the study process and ensure alignment among all parties in achieving the study's objectives. Successful examples of protocol designs in Medtech studies illustrate how clarity and precision can lead to more efficient processes and improved outcomes. Notably, bioaccess® facilitates accelerated regulatory approval in just 6-8 weeks-significantly faster than the typical 6-12 months seen in the US and EU. This swift approval procedure, along with the ability to enroll treatment-naive cardiology or neurology groups 50% quicker than Western locations, underscores the importance of a clear strategy for success.
Efficient planning is essential for attaining desired outcomes in medical studies that employ a randomised controlled trial design. As we navigate the complexities of clinical research, collaboration becomes paramount. By leveraging the strengths of all stakeholders, we can enhance the effectiveness of our protocols and ultimately improve patient outcomes.
Data management stands as a cornerstone of research studies, encompassing the organized procedures of gathering, verifying, and analyzing information. Ensuring data accuracy, completeness, and security is crucial for drawing valid conclusions from findings. This necessitates the establishment of clear data collection protocols, the implementation of rigorous quality control measures, and the employment of appropriate statistical methods for analysis.
Current trends underscore the adoption of automated data validation tools and Electronic Data Capture (EDC) systems equipped with built-in validation checks. These advancements significantly enhance data integrity and facilitate regulatory compliance. For instance, a large-scale medical study that utilized automated validation tools reported a notable reduction in data entry errors, leading to improved data quality and expedited regulatory approvals.
Moreover, the establishment of Data Monitoring Committees (DMCs) is vital in overseeing data validation processes, ensuring adherence to protocols, and promptly addressing discrepancies. As we approach 2025, the emphasis on data precision in medical investigations intensifies, with studies revealing that nearly 67% of analytics leaders view organizational culture as a barrier to becoming data-driven.
Robust data management practices not only bolster the reliability of study outcomes but also aid in navigating evolving regulatory requirements, ultimately fostering trust in the research process. At bioaccess, our comprehensive research study management services include:
Each of these elements is essential to ensuring the success of research studies.
Regulatory compliance is essential in clinical studies, ensuring adherence to established laws, guidelines, and ethical standards. This involves securing necessary approvals from regulatory authorities, such as ethics committees and health ministries, while following Good Clinical Practice (GCP) guidelines and maintaining detailed documentation throughout the study process. Compliance not only safeguards participant rights and welfare but also enhances the credibility of research findings.
As we look ahead to 2025, medical researchers face evolving regulatory challenges that demand a proactive strategy to stay informed about updates and ensure studies meet all legal requirements. For instance, bioaccess provides a comprehensive approach to improving medical device evaluations, encompassing feasibility studies, site selection, compliance reviews, setup, and obtaining import permits. Implementing robust data management protocols and utilizing Electronic Data Capture (EDC) systems can significantly boost compliance, reducing data errors by up to 90%. Moreover, risk-based monitoring (RBM) can potentially cut on-site visits by 50%, optimizing resource allocation.
Establishing dedicated compliance task forces can enhance communication and resource allocation, ultimately improving operational efficiency. As the landscape of medical studies continues to evolve, a commitment to adherence not only protects participants but also upholds the integrity of medical science. Collaboration between operational teams and compliance groups is crucial for the successful application of regulations, ensuring that every aspect of the study aligns with compliance standards.
Continuous training and education for research personnel are vital for maintaining regulatory compliance and adapting to new challenges. The financial repercussions of non-compliance can be substantial, highlighting the necessity of adhering to regulations. As noted by Infonetica, "Clinical research compliance is not just about following rules - it's about fostering a culture of integrity that advances medical science while protecting participants.
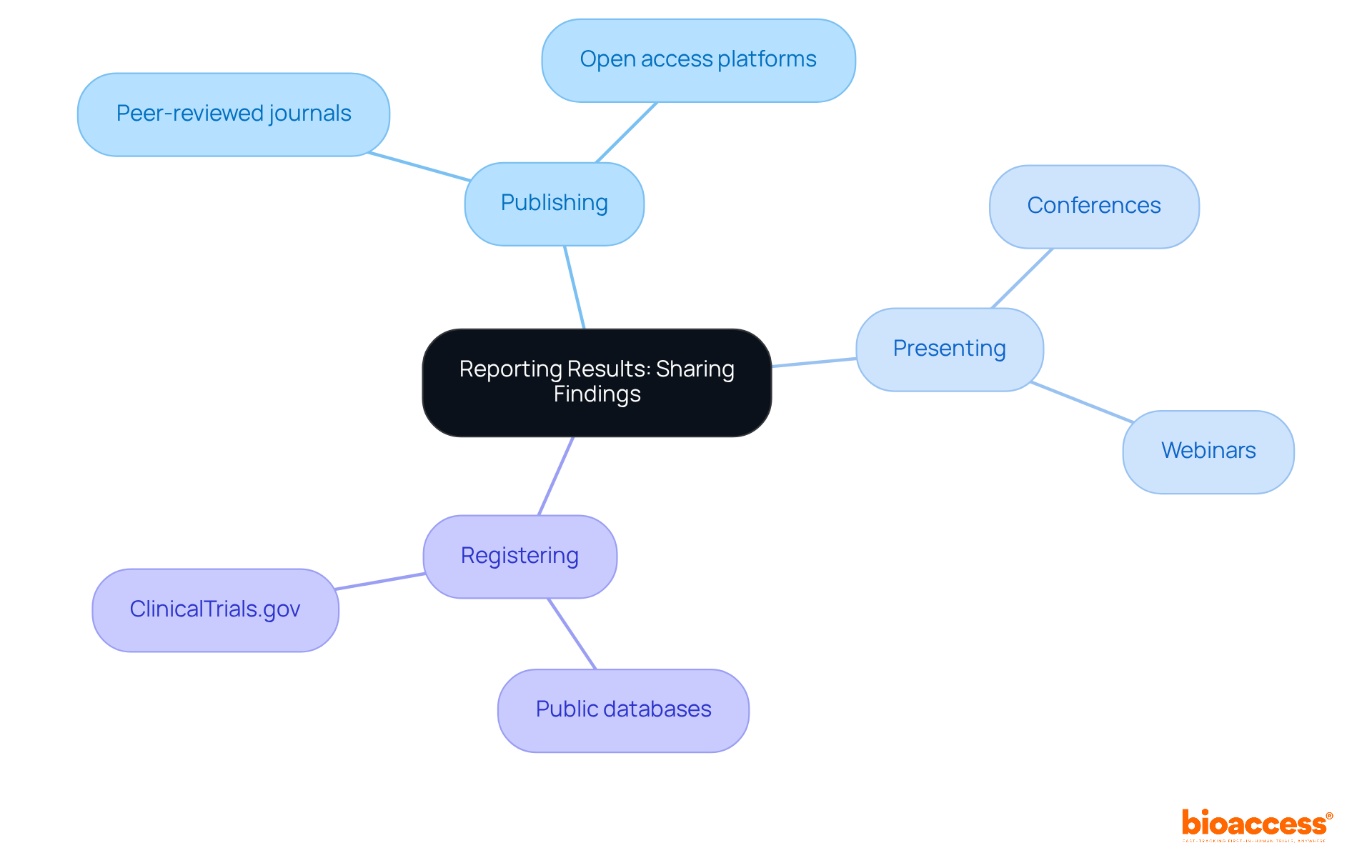
Reporting clinical study results is essential for advancing medical knowledge and enhancing clinical practice. Researchers have a fundamental obligation to share their findings-whether outcomes are positive or negative-to contribute meaningfully to the scientific community. This responsibility encompasses:
Recent trends highlight a growing emphasis on transparency, with 63% of results now accessible in registry records, showcasing a commitment to open science. Such transparent reporting cultivates trust in the inquiry process and enables scholars to build upon existing knowledge, ultimately leading to improved patient care and more effective treatment options. As the FDA continues to advocate for clinical trial transparency, the integration of standardized reporting formats and enhanced registry functionalities is expected to facilitate the sharing of findings further. This ensures that insights gained from clinical trials are readily available for future research and application.
The design of randomized controlled trials (RCTs) is crucial for advancing medical research and ensuring reliable results. Understanding and implementing key elements - such as randomization, control groups, blinding, sample size determination, ethical considerations, protocol development, data management, and regulatory compliance - enables researchers to create robust studies that significantly contribute to medical knowledge.
This article highlights the critical components of successful RCT design. Randomization plays a vital role in reducing bias, while well-defined control groups are necessary for effective comparisons. Blinding enhances the validity of outcomes, and considerations around sample size, ethical practices, meticulous protocol development, and stringent data management are indispensable for achieving credible results. Furthermore, the emphasis on regulatory compliance underscores the importance of adhering to established guidelines to protect participant rights and maintain research integrity.
In summary, the significance of well-structured randomized controlled trials cannot be overstated. As the medical landscape evolves, prioritizing these elements will streamline the research process and enhance the reliability of findings. Researchers are encouraged to embrace collaboration and innovation to navigate the complexities of clinical trials effectively. By doing so, they can contribute to the advancement of healthcare and ensure that new therapies reach those in need more swiftly.
What is bioaccess® and how does it benefit randomized controlled trials?
bioaccess® accelerates randomized controlled trials by leveraging the regulatory speed of Latin America, diverse patient populations in the Balkans, and efficient ethical approval processes in Australia. It significantly shortens study timelines, with ethical approvals secured in 4-6 weeks and enrollment rates 50% faster than traditional markets.
What types of studies does bioaccess® support?
bioaccess® supports various types of studies including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies.
Why is agility important in the current medical landscape?
Agility is essential because swift access to timely data can significantly accelerate the introduction of new therapies to the market, which is increasingly important as companies focus on participant experiences and outcomes to enhance recruitment and retention rates.
What role does randomization play in clinical trials?
Randomization is crucial in randomized controlled trial design as it ensures participants are assigned to treatment or control categories by chance, reducing selection bias and allowing for more precise comparisons of outcomes.
What techniques are used for randomization in clinical trials?
Techniques used for randomization include simple randomization, block randomization, and stratified randomization.
Why are control groups important in clinical trials?
Control groups are vital for establishing a benchmark to accurately measure the effects of experimental treatments, helping to determine whether observed effects are due to the treatment or influenced by other factors.
What types of control groups can be used in trials?
Control groups can be active, placebo, or historical, and their selection can significantly impact the trial's outcomes and interpretations.
What challenges exist in the design of control groups for clinical trials?
Almost 60% of surgical inquiries cannot be effectively resolved by randomized controlled trial design alone, highlighting the need for careful control design to address complex medical situations.
What are synthetic control arms and how are they used?
Synthetic control arms are innovative approaches used in medical investigations, particularly for rare diseases, that can reduce development time and costs while maintaining scientific rigor. They have been successfully utilized in studies evaluating therapies for rare types of lung cancer and pediatric illnesses.
What is the significance of meticulous planning in control group selection?
Meticulous planning and the choice of control sets are foundational to effective comparison in studies, as they ensure the internal validity of research findings and help ascertain whether changes in treatment cohorts are due to the intervention or natural progression over time.