


The primary objective of this article is to delineate the essential FDA labeling requirements for medical devices, ensuring compliance and enhancing patient safety. It underscores the imperative of incorporating critical elements such as:
on labels. These components collectively play a vital role in mitigating safety risks and facilitating adherence to regulatory standards.
Navigating the intricate landscape of FDA labeling requirements for medical devices poses a significant challenge for manufacturers. With a multitude of regulations aimed at ensuring safety and efficacy, grasping the essential components of compliance is paramount. This article explores ten critical FDA labeling requirements that not only streamline the approval process but also bolster patient safety.
What common pitfalls do manufacturers face, and how can they adeptly circumvent them to guarantee their products meet regulatory standards?
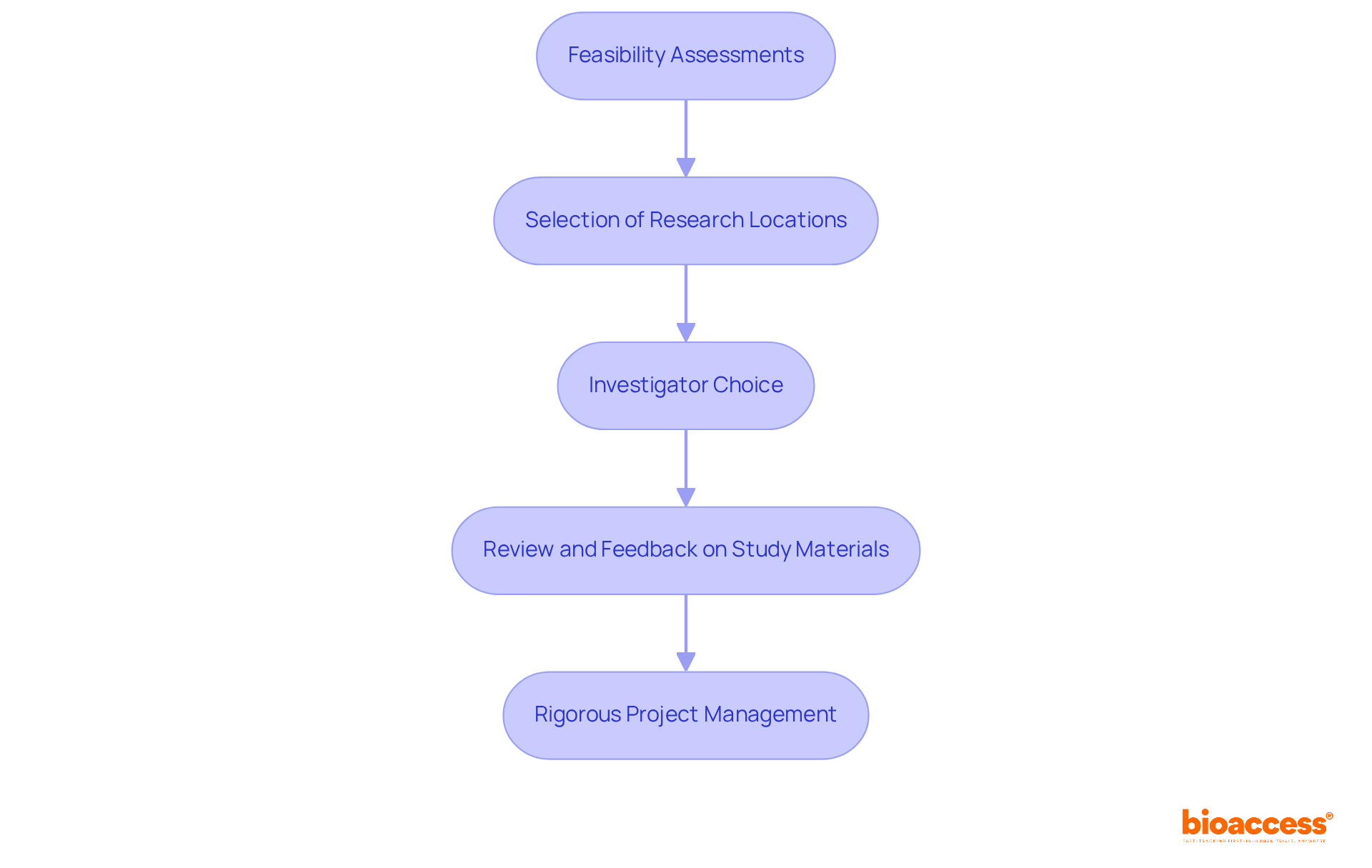
bioaccess® is dedicated to expediting adherence to the FDA labelling requirements for medical devices, leveraging its extensive experience in clinical research and regulatory matters. By concentrating on early-phase studies, bioaccess® empowers medical device manufacturers to meet FDA standards with efficiency, thereby reducing time to market and enhancing patient safety. Our comprehensive process includes:
All designed to ensure regulatory compliance throughout the trial.
By capitalizing on Colombia's competitive advantages—such as cost efficiency, regulatory speed, and access to diverse patient populations—bioaccess® facilitates ethical approvals in just 4-6 weeks and achieves enrollment rates that are 50% faster than traditional markets. This unwavering commitment to accelerating clinical trials positions bioaccess® as a premier CRO in Latin America, particularly for Medtech startups navigating the complexities of FDA labelling requirements for medical devices and overall FDA compliance. Collaboration with bioaccess® not only streamlines the research process but also reinforces the importance of adhering to regulatory standards, ultimately enhancing the success of clinical trials.
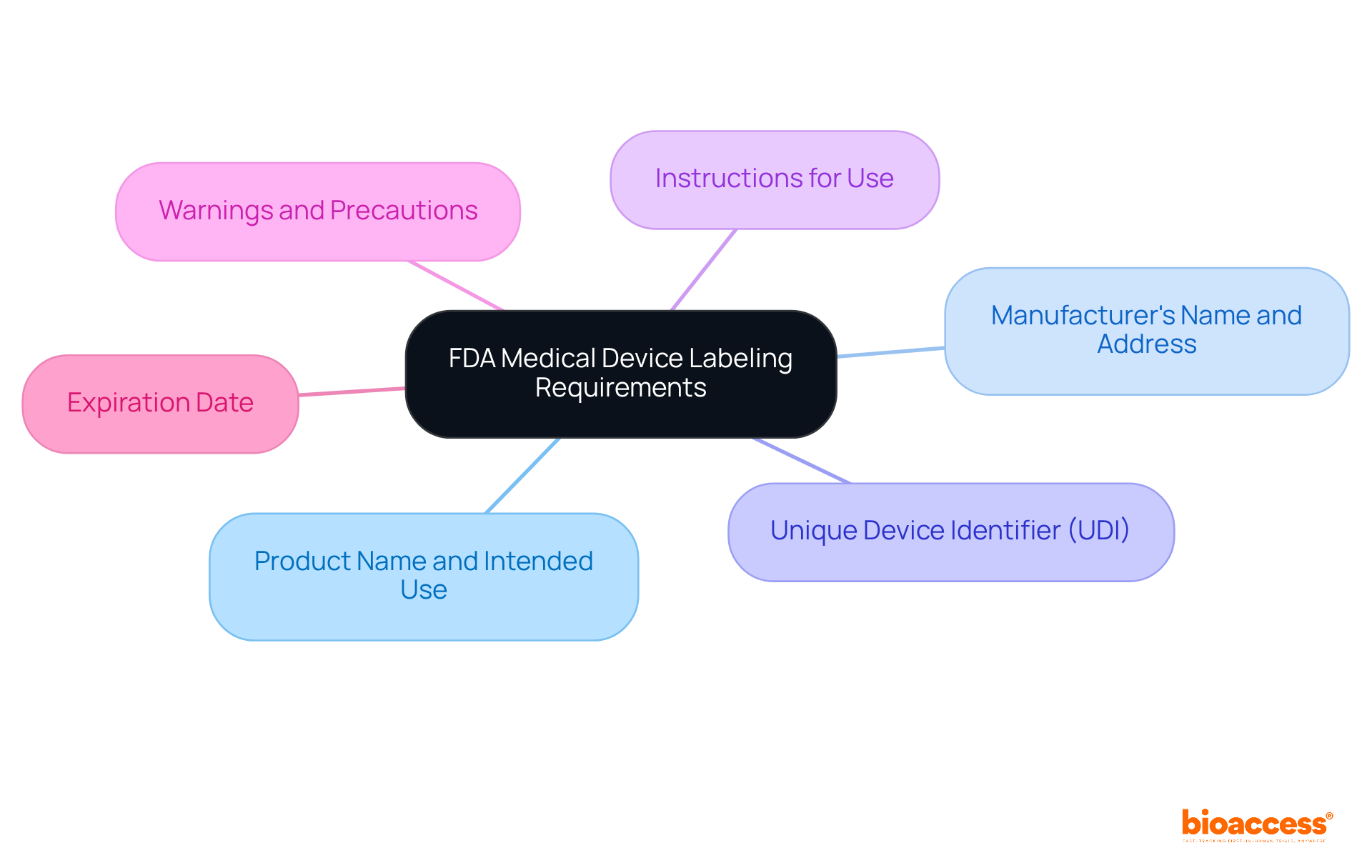
The essential components of FDA labeling requirements for medical devices are critical for ensuring safe and effective use. These components include:
Each element plays a vital role in conveying necessary information to healthcare providers and patients, thereby enhancing the overall safety and efficacy of medical devices.
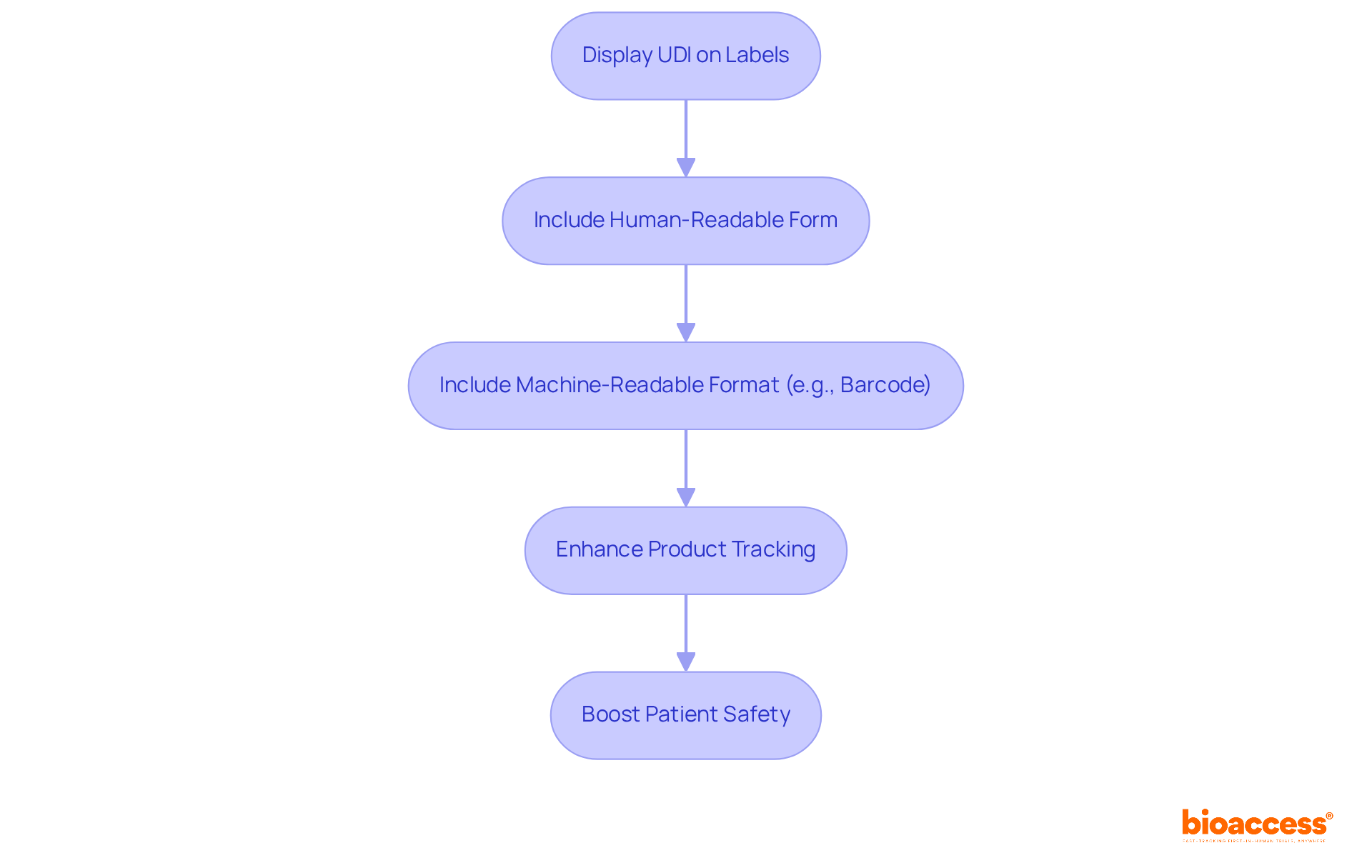
The Unique Identification (UDI) system mandates that all medical instruments display a UDI on their labels. This identifier consists of a unique numeric or alphanumeric code that facilitates the monitoring of equipment throughout its lifecycle. The UDI must be presented in both human-readable form and in a machine-readable format, such as a barcode. Adhering to UDI requirements not only enhances the tracking of products but also significantly boosts patient safety by ensuring that accurate information is readily accessible.
The identification elements required by the FDA labeling requirements for medical devices are critical for ensuring safe utilization of medical devices. These elements include:
Each of these components must be clearly presented on the label to meet the FDA labeling requirements for medical devices, providing users with all necessary information for effective utilization. This clarity not only fosters compliance but also enhances user confidence in the device's safety and efficacy.

Manufacturers must avoid common pitfalls in order to comply with FDA labeling requirements for medical devices. Key issues include:
Manufacturers must prioritize clarity in intended use descriptions, as this is vital for regulatory approval and patient safety. Mislabeling incidents can cost manufacturers an average of $1.3 million annually, with 52% of manufacturers reporting weekly interruptions due to mislabeled products. Furthermore, over 30% of healthcare product recalls are linked to marking issues, emphasizing the necessity for accurate identification practices. By recognizing these pitfalls and implementing effective compliance strategies—such as comprehensive documentation and adherence to specific formatting requirements—manufacturers can enhance their practices and ensure compliance with the FDA labeling requirements for medical devices.

Optimal methods for ensuring compliance with FDA labelling requirements for medical devices encompass the following key strategies:
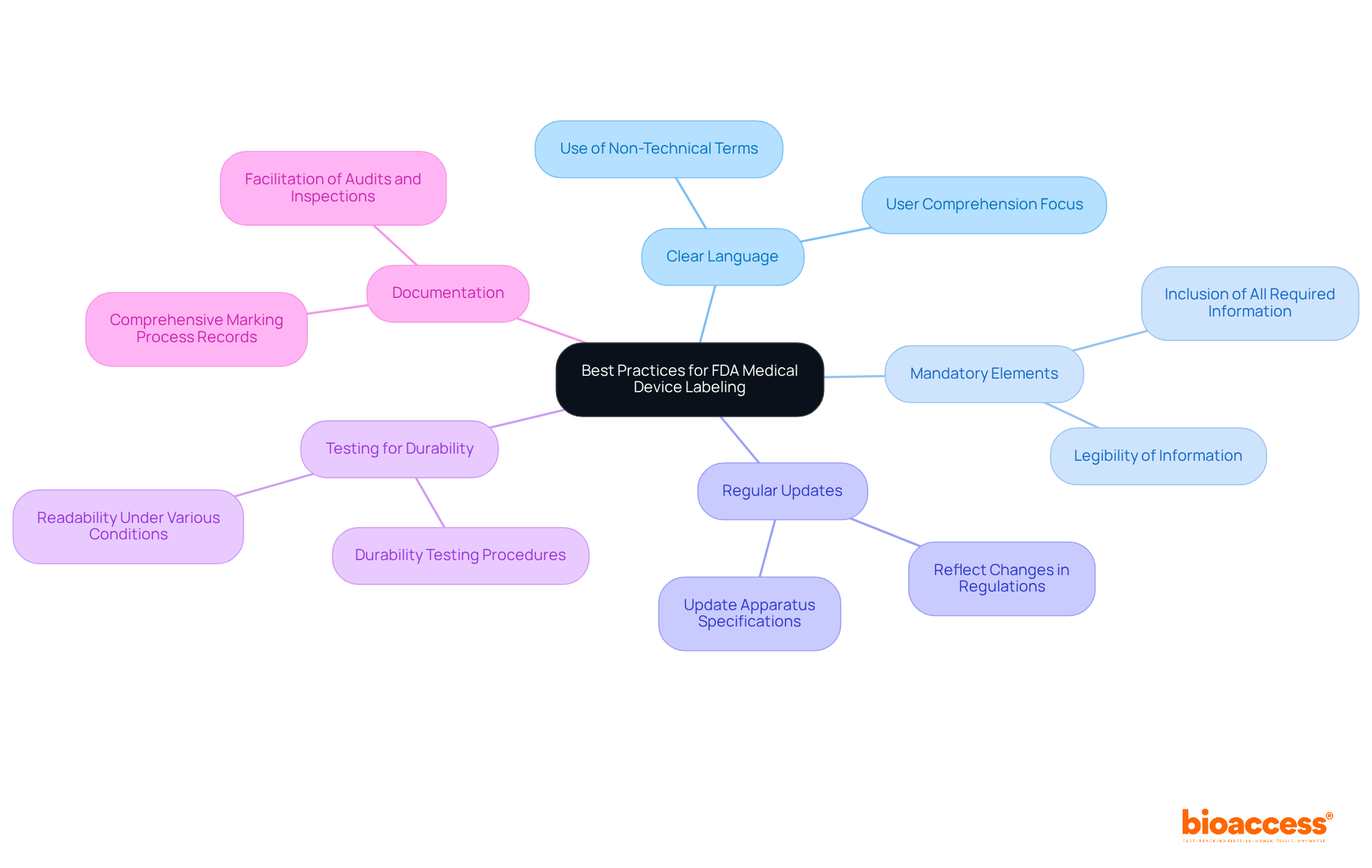
Ensuring label integrity is crucial throughout the lifecycle of medical devices, as it guarantees that labels remain legible and securely attached during production, storage, and distribution. Manufacturers must implement robust quality control measures to verify that labels are intact and compliant with the FDA labelling requirements for medical devices, specifically under 21 CFR 801.1 and 21 CFR 801.4.
Compliance inspections carried out by the FDA are essential not only for evaluating conformity to packaging requirements but also for protecting patient safety and guaranteeing market access. Any discrepancies identified during these inspections can lead to substantial penalties, including fines and product recalls. In fact, over 30% of healthcare product recalls are associated with packaging issues, underscoring the importance of precision in this area.
As pointed out by Ana Criado, Director of Regulatory Affairs and a specialist in biomedical engineering, 'It is essential for manufacturers to meticulously examine their marking practices to comply with FDA labelling requirements for medical devices, ensuring that all statements are truthful and backed by strong evidence.'
Regular internal audits are crucial for manufacturers, assisting in recognizing potential marking issues before they develop into regulatory concerns. By emphasizing regulatory inspections and keeping current product information practices, manufacturers can boost consumer confidence and guarantee the safety and effectiveness of their goods.
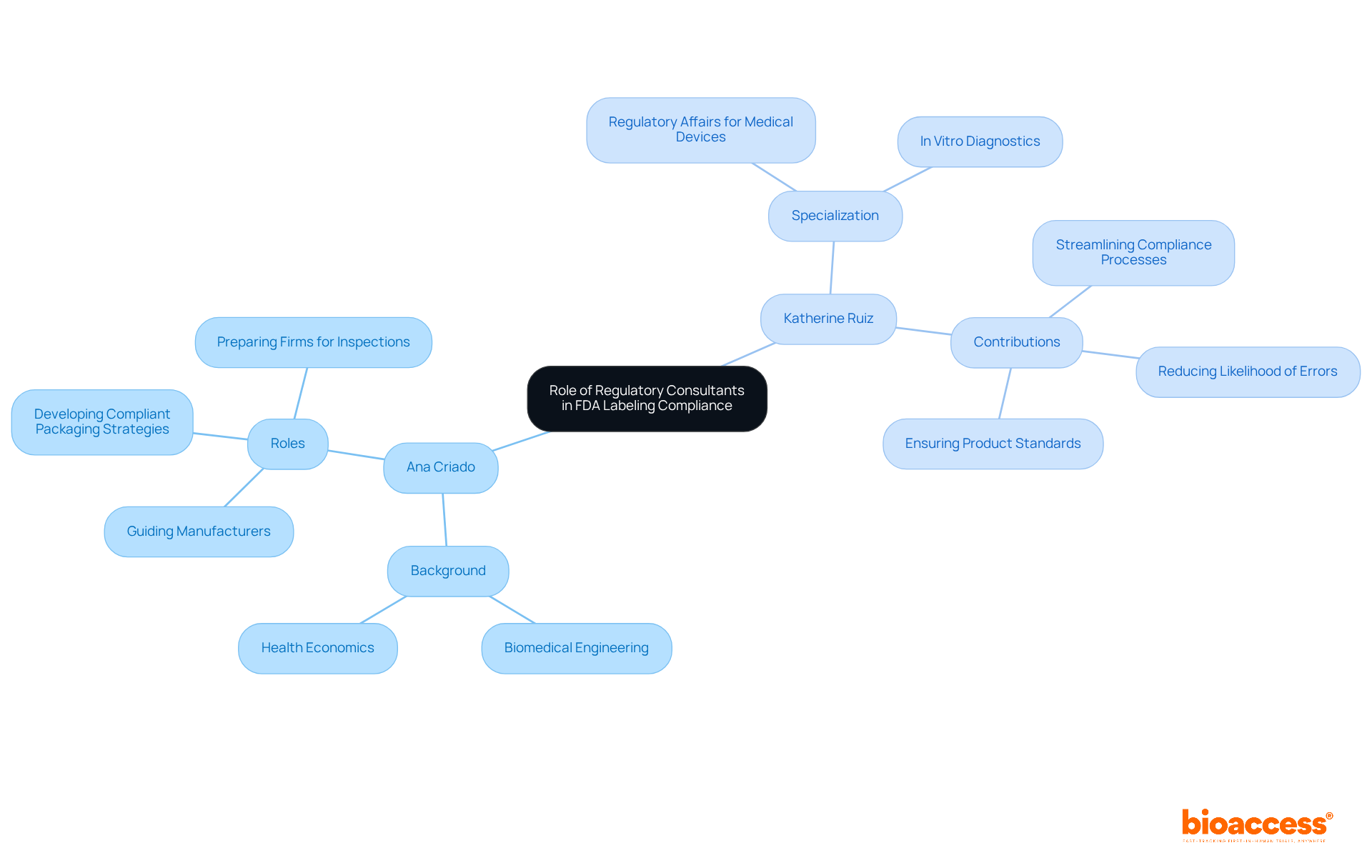
Regulatory advisors, such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, are pivotal in guiding manufacturers through the complexities of the FDA labelling requirements for medical devices. With her extensive background in biomedical engineering and health economics, Ana offers expert counsel on regulatory mandates, aids in the development of compliant packaging strategies, and prepares firms for inspections.
Complementing this expertise is Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia. By leveraging the knowledge of regulatory consultants like Ana and Katherine, manufacturers can streamline their compliance processes, reduce the likelihood of errors, and ensure their products meet all necessary standards.
Labeling considerations for patient use and safety are paramount. First, providing clear instructions for use that are easy to follow is essential. Second, including warnings and precautions that are prominently displayed is crucial. Third, utilizing symbols and pictograms allows for the quick conveyance of critical information. Finally, ensuring that all information is presented in a format accessible to diverse patient populations is vital. By prioritizing patient safety in product identification, manufacturers enhance user experience and significantly reduce the likelihood of misuse.

Maintaining compliance with continuous FDA requirements is crucial for producers in the clinical research landscape. To effectively navigate regulatory updates and shifts in industry standards, manufacturers must take proactive steps. This can be achieved by:
By adopting such measures, manufacturers not only safeguard their products but also enhance patient safety.
Ensuring compliance with FDA labeling requirements for medical devices is essential for manufacturers aiming to maintain safety and efficacy in their products. This article outlines the critical components of labeling, emphasizing the necessity for clear instructions, accurate product identification, and adherence to Unique Device Identification (UDI) standards. By understanding and implementing these requirements, manufacturers fulfill regulatory obligations while enhancing user trust and safety.
Key insights discussed include the mandatory labeling components that must be clearly presented, alongside common pitfalls to avoid, such as omitting critical information or using overly complex language. Best practices for labeling—such as employing clear communication and maintaining label integrity throughout the product lifecycle—are vital for successful compliance. Furthermore, collaboration with regulatory consultants can significantly streamline the process, equipping manufacturers to navigate the complexities of FDA regulations effectively.
In conclusion, prioritizing FDA labeling compliance transcends mere regulatory obligation; it represents a commitment to patient safety and product reliability. By actively engaging in continuous education, conducting regular audits, and leveraging expert guidance, manufacturers can safeguard their products and contribute positively to the healthcare landscape. The significance of accurate and compliant labeling cannot be overstated, as it directly impacts patient outcomes and the overall success of medical devices in the market.
What is bioaccess® and what role does it play in FDA medical device labeling compliance?
bioaccess® is a company dedicated to expediting adherence to FDA labeling requirements for medical devices. It leverages its extensive experience in clinical research and regulatory matters to help medical device manufacturers meet FDA standards efficiently, thereby reducing time to market and enhancing patient safety.
What services does bioaccess® provide to facilitate compliance with FDA requirements?
bioaccess® offers a comprehensive process that includes feasibility assessments, careful selection of research locations, investigator choice, thorough review and feedback on study materials, and rigorous project management, all designed to ensure regulatory compliance throughout clinical trials.
How does bioaccess® leverage Colombia's advantages for clinical trials?
bioaccess® capitalizes on Colombia's competitive advantages, such as cost efficiency, regulatory speed, and access to diverse patient populations, facilitating ethical approvals in just 4-6 weeks and achieving enrollment rates that are 50% faster than traditional markets.
What are the core elements of FDA medical device labeling requirements?
The essential components of FDA labeling requirements for medical devices include the product name and intended use, manufacturer's name and address, Unique Device Identifier (UDI), instructions for use, warnings and precautions, and expiration date.
What is the Unique Device Identifier (UDI) system and why is it important?
The Unique Device Identifier (UDI) system mandates that all medical instruments display a UDI on their labels, consisting of a unique numeric or alphanumeric code. This identifier facilitates the monitoring of equipment throughout its lifecycle and must be presented in both human-readable and machine-readable formats, such as a barcode. Adhering to UDI requirements enhances product tracking and significantly boosts patient safety by ensuring accurate information is readily accessible.