


This article highlights the essential insights required for achieving success in first-in-human clinical trials. It emphasizes the critical importance of:
By detailing how bioaccess® optimizes trial processes through efficient regulatory approvals and participant recruitment strategies, the article illustrates how these elements ultimately enhance study timelines and improve patient safety.
In the evolving Medtech landscape, the role of bioaccess® in addressing key challenges cannot be overstated. The integration of innovative approaches not only streamlines trial processes but also fosters a culture of collaboration among stakeholders. This synergy is vital for navigating the complexities of clinical research and ensuring that trials are conducted effectively and ethically.
In conclusion, the importance of collaboration in first-in-human clinical trials is paramount. As the industry continues to advance, embracing these insights will be crucial for researchers aiming to achieve successful outcomes. Stakeholders are encouraged to consider the next steps in leveraging these strategies to enhance their clinical trial processes.
The journey from concept to clinical application in medical research is fraught with complexities, particularly for first-in-human trials that pave the way for groundbreaking treatments. As the demand for innovative therapies grows, understanding the pivotal strategies that can enhance trial success becomes essential. This article explores ten key insights that illuminate the path to effective first-in-human trials and address the pressing challenges researchers face in today’s dynamic landscape.
How can stakeholders navigate regulatory hurdles, optimize participant selection, and ensure patient safety while embracing the latest trends in clinical research? The answers lie ahead, promising a roadmap for achieving impactful outcomes in medical advancements.
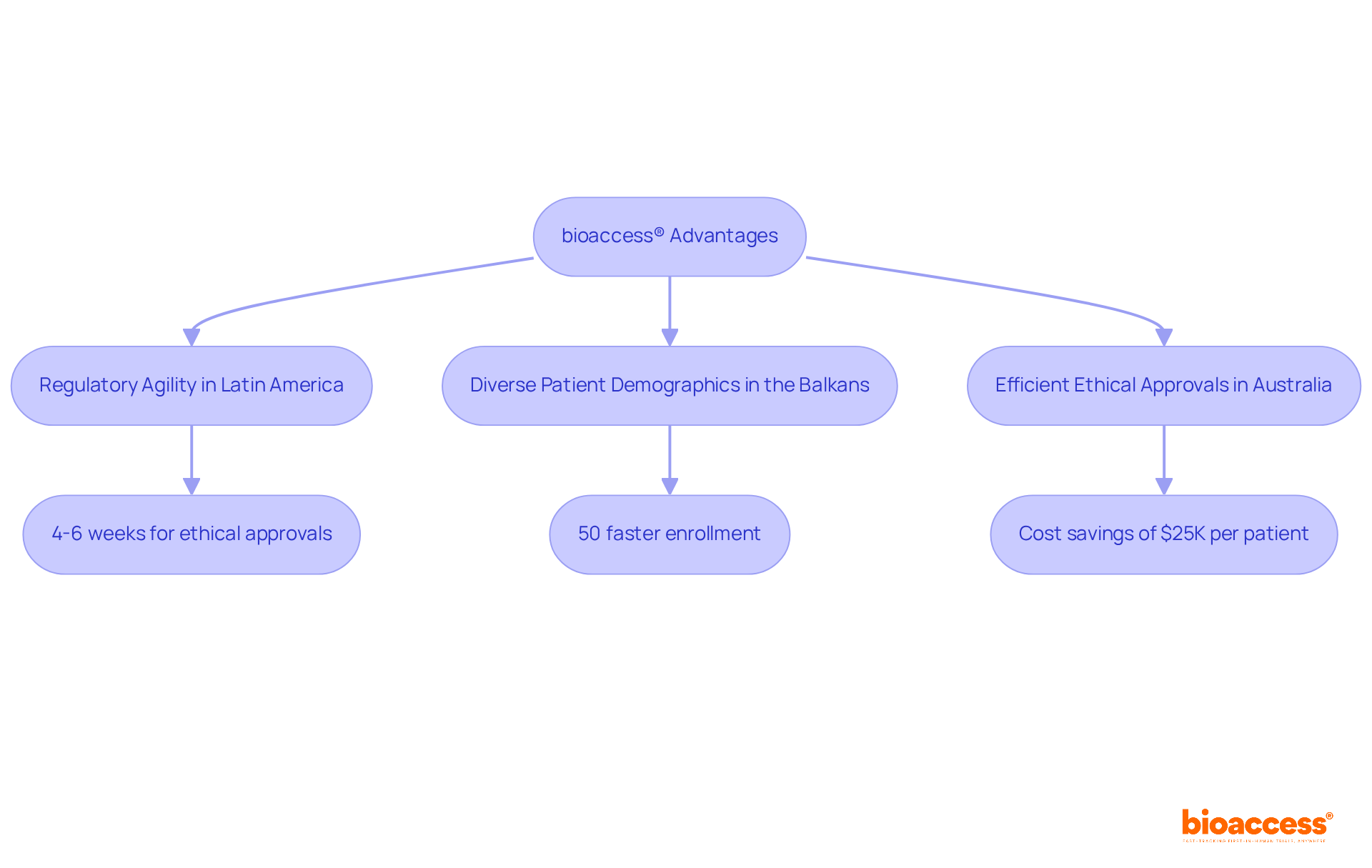
bioaccess® leverages the regulatory agility of Latin America, the diverse patient demographics of the Balkans, and Australia's efficient ethical approval processes to significantly shorten timelines for first in human clinical trials.
With ethical approvals secured in just 4-6 weeks and enrollment rates 50% faster than traditional markets, bioaccess® emerges as a leader in accelerating advancements in medical technology and biopharmaceuticals.
Our team of experts, boasting over 20 years of specialized knowledge in Medtech, empowers innovators to expedite their products' journey to market, swiftly adapting to the evolving healthcare landscape.
By optimizing efficiency, research directors can harness bioaccess®'s capabilities to enhance study timelines and outcomes, achieving substantial cost savings of $25K per patient with FDA-ready data—no rework, no delays.
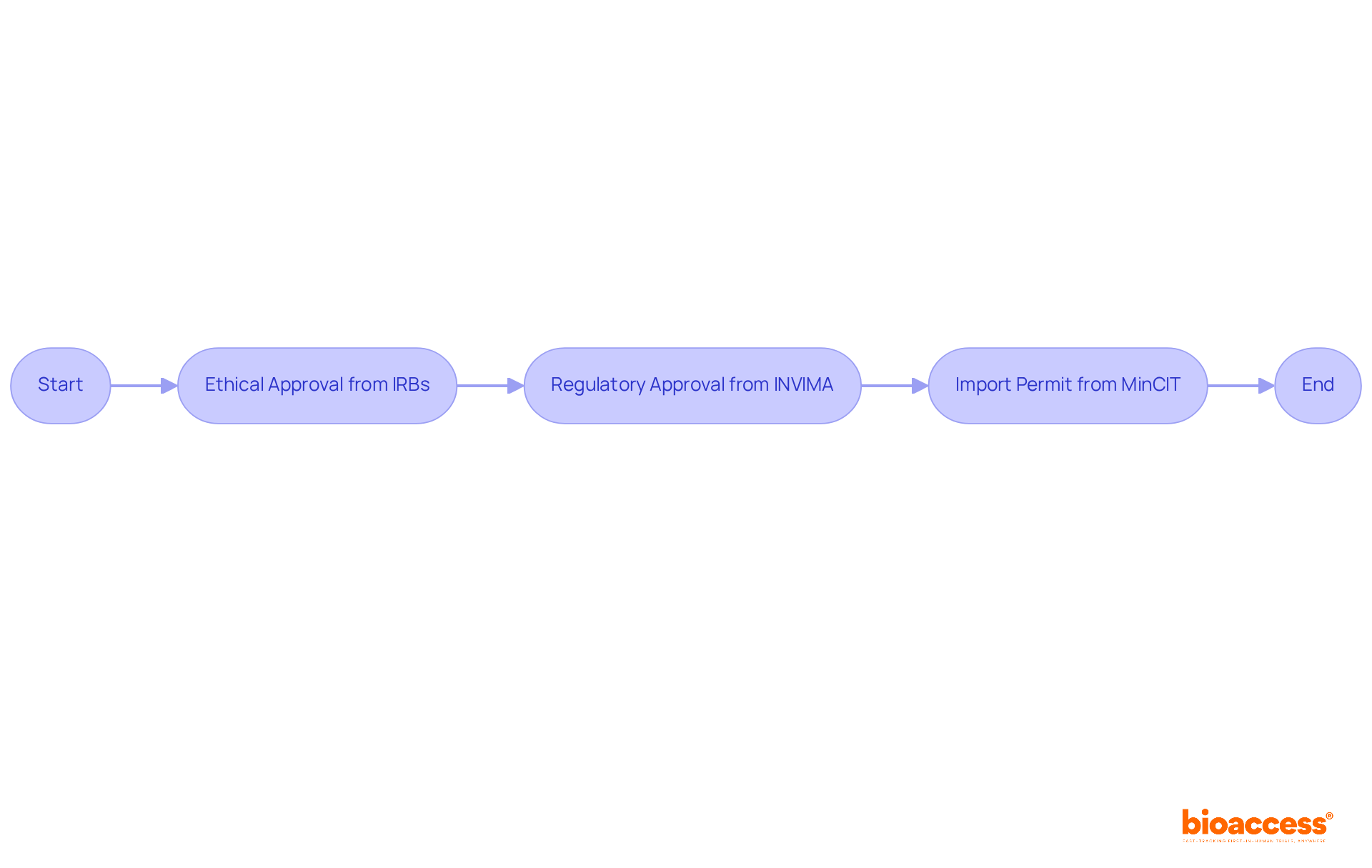
Navigating the regulatory landscape for first in human clinical trials is essential for success, particularly given the rigorous requirements that differ by region. In Colombia, researchers must obtain:
Understanding these specific regulations is vital, as they directly impact study design, participant recruitment, and data management. Notably, a significant percentage of experiments face delays due to regulatory compliance challenges, underscoring the importance of thorough preparation.
Engaging with regulatory bodies early in the trial process can facilitate approvals and mitigate the risk of setbacks. Recent updates to ethical approval processes demonstrate a commitment to enhancing efficiency while ensuring the safety of participants. For instance, streamlined arrangements for informed consent foster clearer communication and trust between researchers and subjects. Furthermore, researchers are now mandated to provide a summary of results to participants in an accessible format, reinforcing ethical compliance and participant engagement.
The new medical research regulations, set to take effect on April 28, 2026, represent a significant transformation of the regulatory environment, the most substantial in two decades. Changes to Research Ethics Committees (RECs) will promote flexibility and align with international good clinical practices, further strengthening the ethical framework of clinical studies. By staying informed about these evolving regulations and proactively collaborating with regulatory bodies, researchers can navigate the ethical approval landscape more effectively, ultimately accelerating the path to successful outcomes.
Bioaccess® offers specialized services, including patient recruitment and study information management, to assist Medtech, Biopharma, and Radiopharma startups in overcoming these hurdles, ensuring rapid site activation and regulatory compliance in Latin America, Eastern Europe, and Australia.
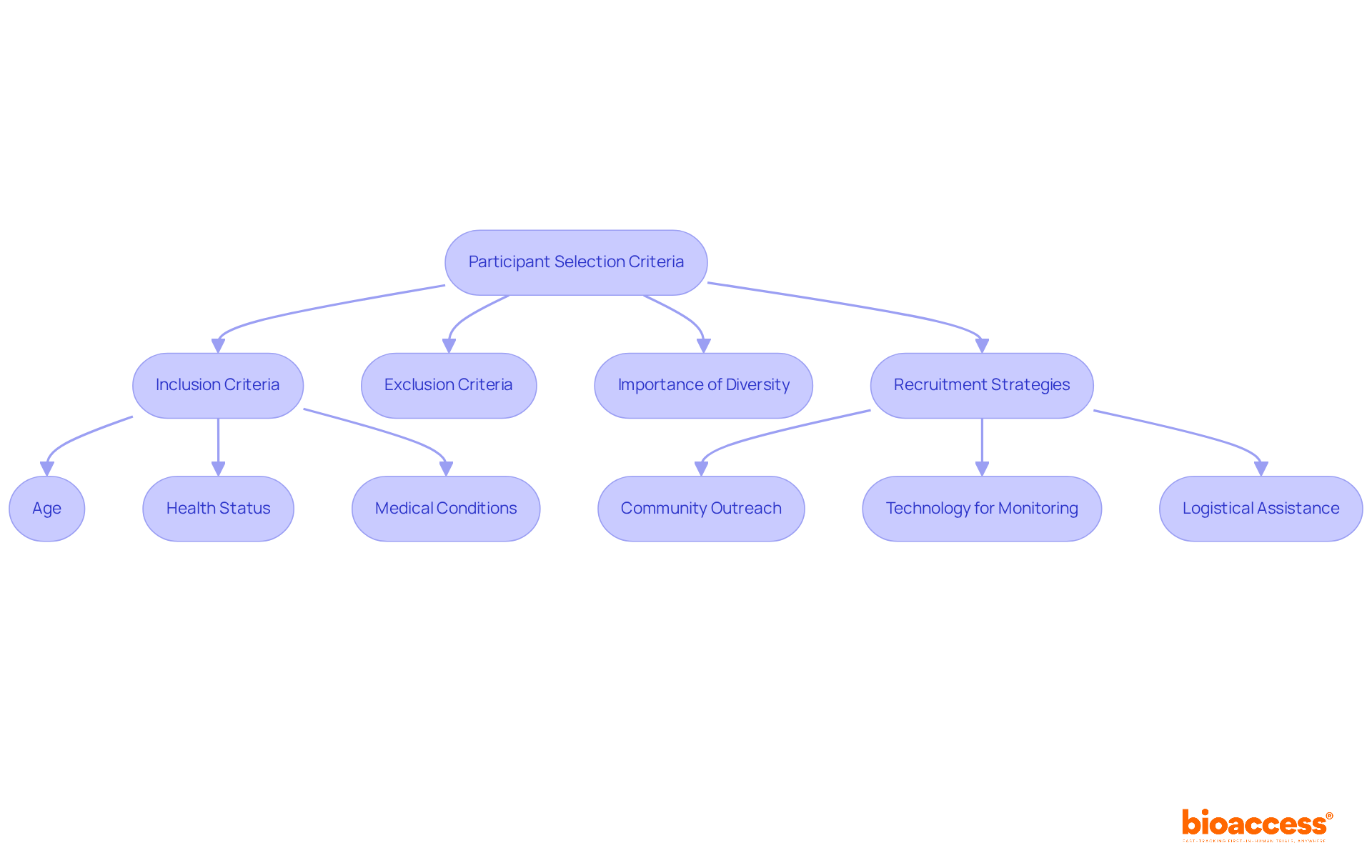
Choosing suitable individuals for first in human clinical trials is crucial for ensuring safety and securing reliable information. Inclusion and exclusion criteria typically encompass age, health status, and specific medical conditions pertinent to the study. The importance of variety in clinical study subjects cannot be overstated; it improves the external validity of outcomes and ensures that the results are relevant to a wider population. For instance, in ReGelTec's Early Feasibility Study on HYDRAFIL™ for treating chronic low back pain in Colombia, eleven individuals with degenerative disc disease were enrolled, which is significant as it marks the treatment's first in human clinical trials and demonstrates the importance of selecting subjects who meet specific health criteria.
Researchers must navigate the delicate balance between fostering a diverse group of individuals and ensuring that candidates meet the trial's specific objectives. Clear and well-defined criteria streamline recruitment processes and improve the quality of data collected. Effective approaches for attracting a varied group of individuals involve:
By applying these strategies, researchers can establish trust and promote a more representative group in medical studies, ultimately resulting in safer and more effective healthcare advancements.
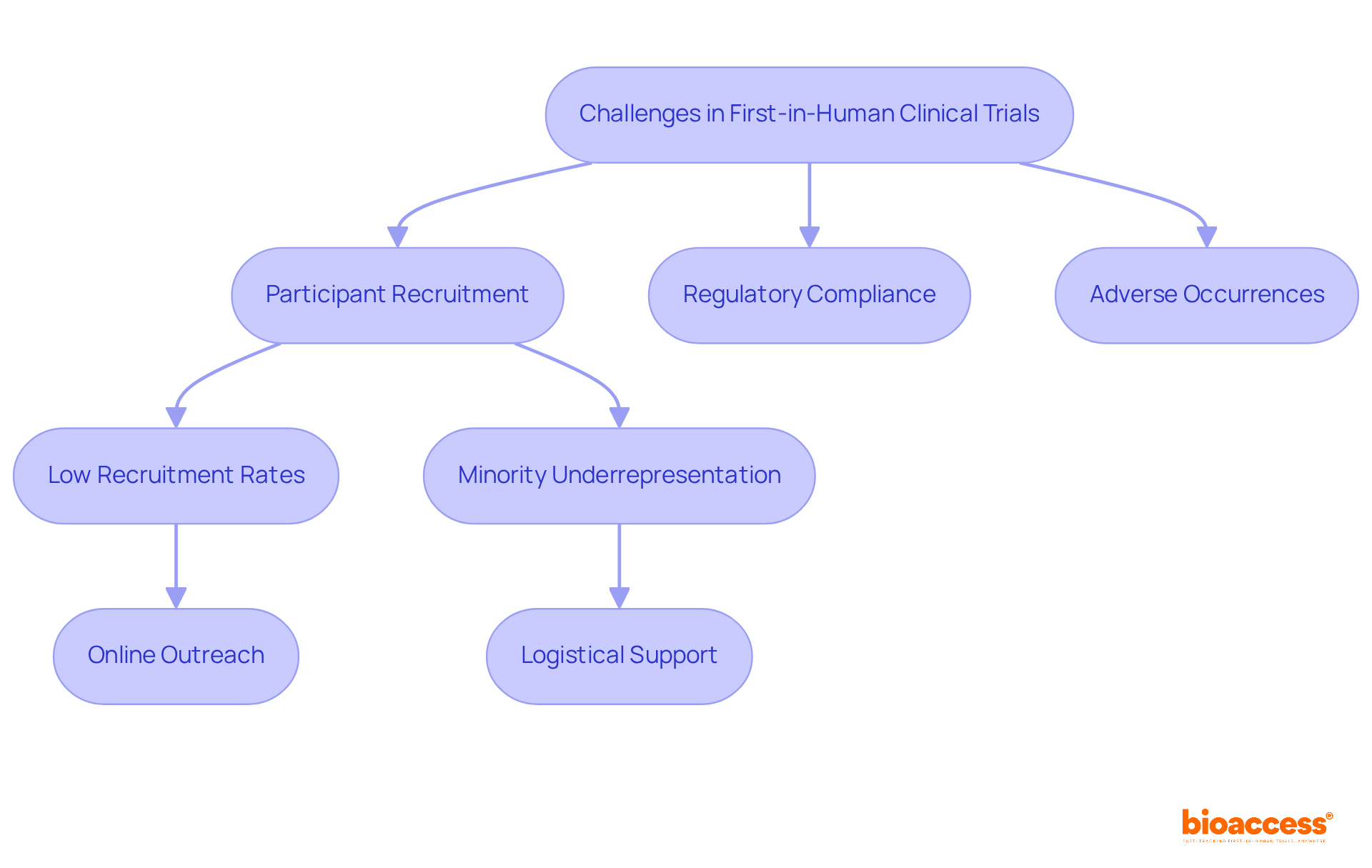
Conducting first in human clinical trials presents a complex array of challenges, particularly in participant recruitment, regulatory compliance, and the management of unexpected adverse occurrences. Recruitment remains a significant hurdle, with fewer than 4% of adults in the U.S. participating in research studies, and as many as 85% of studies failing to meet their recruitment targets. This underrepresentation is especially pronounced among minorities, who historically engage less in clinical research, resulting in skewed data and potential safety concerns.
To combat these recruitment challenges, researchers are increasingly implementing innovative strategies. For example, leveraging online platforms and social media can enhance outreach, given that 80% of internet users seek health information online. Furthermore, providing logistical support, such as home visits, has proven to significantly boost participation rates, particularly among individuals with chronic conditions who may encounter mobility issues.
Data indicates that government-funded experiments typically achieve higher accrual rates compared to their industry-funded counterparts, underscoring the importance of funding type in recruitment success. Moreover, effective communication regarding the study process, including the informed consent procedure, can foster trust and encourage participation. As one researcher noted, "It is crucial to create approaches to enhance study design for improved feasibility, inclusiveness, and recruitment efficiency to boost the sustainability and impact of research in healthcare."
In conclusion, while first in human clinical trials present unique challenges, adopting proactive strategies and focusing on engaging individuals can substantially enhance recruitment success, ultimately leading to more robust and reliable clinical outcomes.
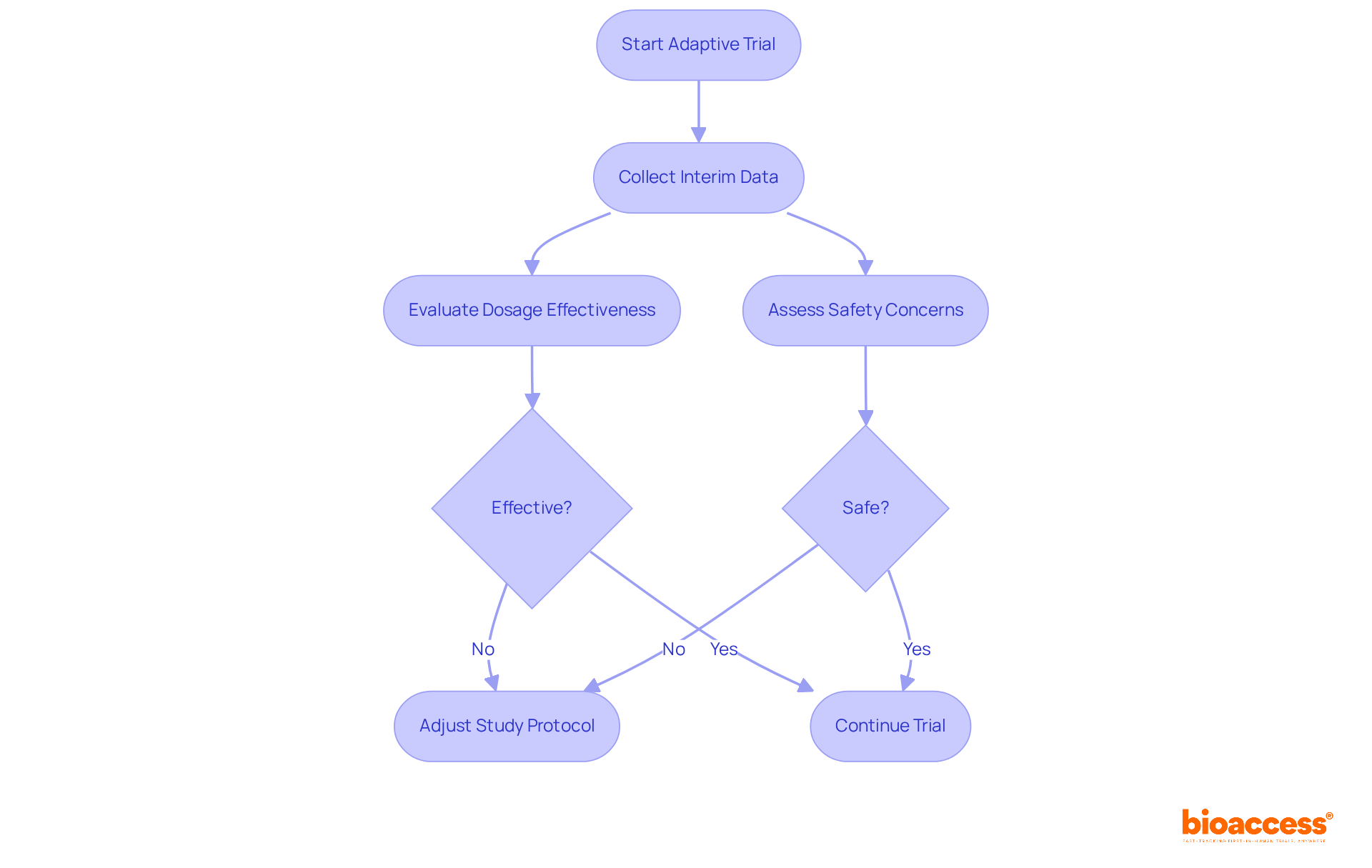
Adaptive study designs empower researchers to modify study parameters based on interim results, facilitating more efficient resource utilization and enhancing participant safety. For example, should initial data reveal that a specific dosage is ineffective or poses safety concerns, researchers can swiftly adjust the study protocol. This inherent flexibility significantly boosts the success rates of first in human clinical trials by enabling real-time decision-making and refining study designs.
Notably, adaptive designs are increasingly adopted, with 41.1% of phase 2 studies utilizing such methodologies, underscoring their growing importance in medical research. Experts emphasize that the adaptability of these designs allows for continuous adjustments, ultimately improving study outcomes and reducing the number of patients exposed to ineffective treatments. By integrating these dynamic approaches, researchers can adeptly navigate the complexities of early-phase studies, ensuring that the most promising treatment options are prioritized and evaluated effectively.
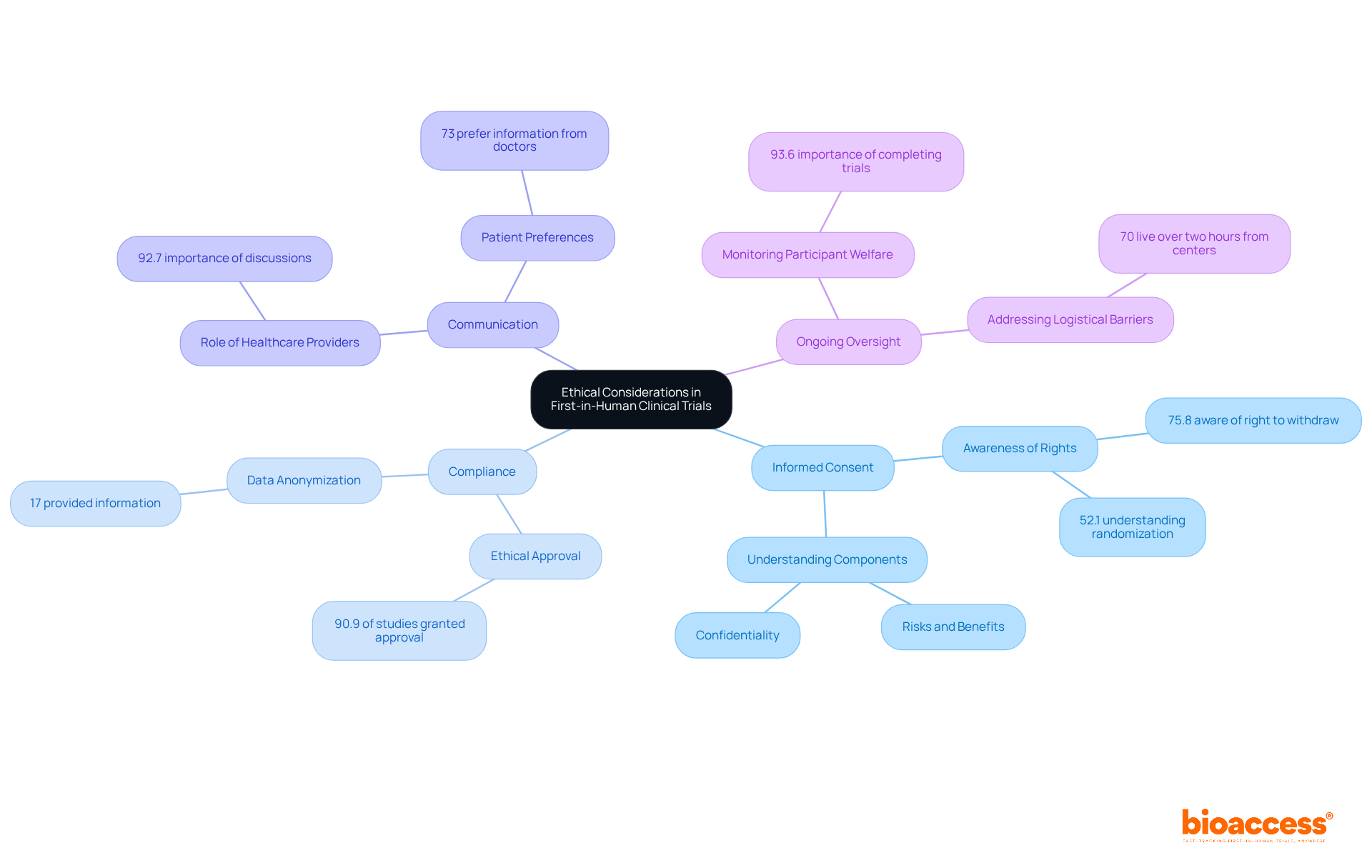
Informed consent remains a cornerstone of ethical considerations in first in human clinical trials, which is essential for safeguarding individuals and maintaining public trust in medical research. As we approach 2025, updates indicate that understanding of informed consent components has stagnated, with only 75.8% of individuals aware of their right to withdraw and just 52.1% grasping concepts like randomization. Significantly, the comprehension of informed consent elements has not notably evolved over the past thirty years, emphasizing the persistent challenge of ensuring that individuals fully grasp the risks and benefits related to their involvement.
Effective informed consent processes are characterized by clear communication and transparency. Researchers must articulate the potential risks and benefits of participation in a manner that is accessible and understandable. A recent meta-analysis underscores the necessity of enhancing consent forms and engaging in extended discussions to improve participant comprehension. As Ken Getz pointed out, clinical care providers are the most reliable sources for health, medical, and clinical research information, highlighting their role in promoting patient engagement.
Moreover, compliance with current ethical guidelines in first in human clinical trials is paramount. Reports indicate that ethical approval was granted in 90.9% of studies, yet only 17% provided information on data anonymization, revealing gaps in adherence to best practices. This compliance not only fulfills regulatory requirements but also fosters a culture of responsibility within medical research.
Bioethicists stress that informed consent is not just a formality but an essential process that enhances the well-being of those involved. As one observed, 'Obtaining informed consent from individuals in clinical research is essential because it promotes their welfare and ensures their rights.' Ongoing oversight for negative occurrences is equally vital, guaranteeing that the safety of individuals is prioritized throughout the study. In fact, 93.6% of patients with chronic/acute conditions stated it was 'important' or 'very important' to know they would be able to complete the entire study.
In summary, the ethical environment of first in human clinical trials necessitates rigorous attention to informed consent procedures, ongoing participant welfare monitoring, and strict adherence to ethical guidelines to foster trust and integrity in medical research.
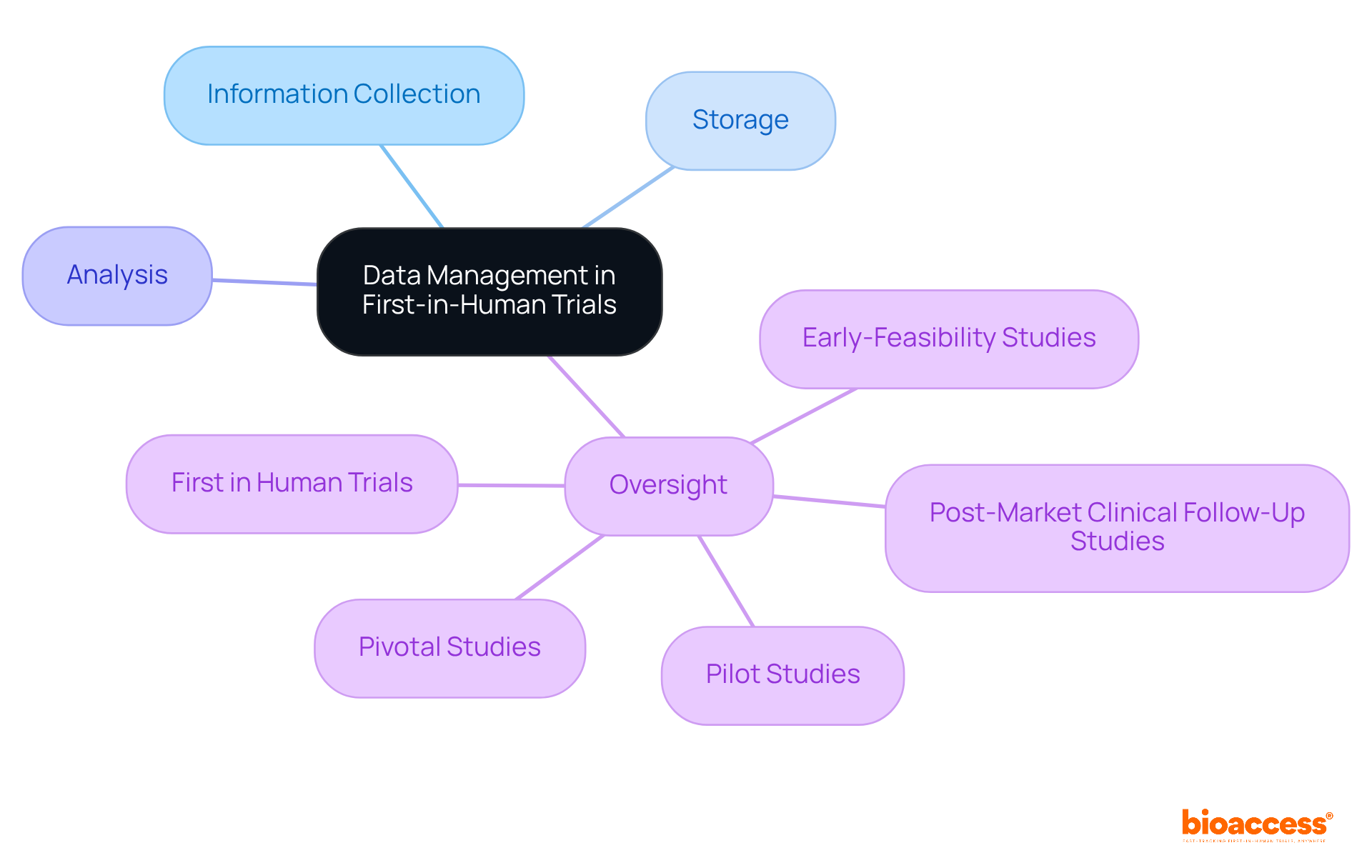
Efficient information management and oversight are paramount for the success of first in human clinical trials. Researchers must establish robust systems for precise information collection, storage, and analysis to ensure regulatory compliance. Consistent oversight of experimental information is essential, as it facilitates the prompt recognition of patterns and potential concerns, enabling timely actions.
The adoption of electronic data capture (EDC) systems is increasingly prevalent, significantly enhancing accuracy and streamlining reporting processes. These systems not only bolster the effectiveness of information management but also play a vital role in the overall success of medical studies.
As bioaccess® demonstrates with over 20 years of experience in overseeing:
Strong data management is indispensable for navigating the complexities of clinical research.
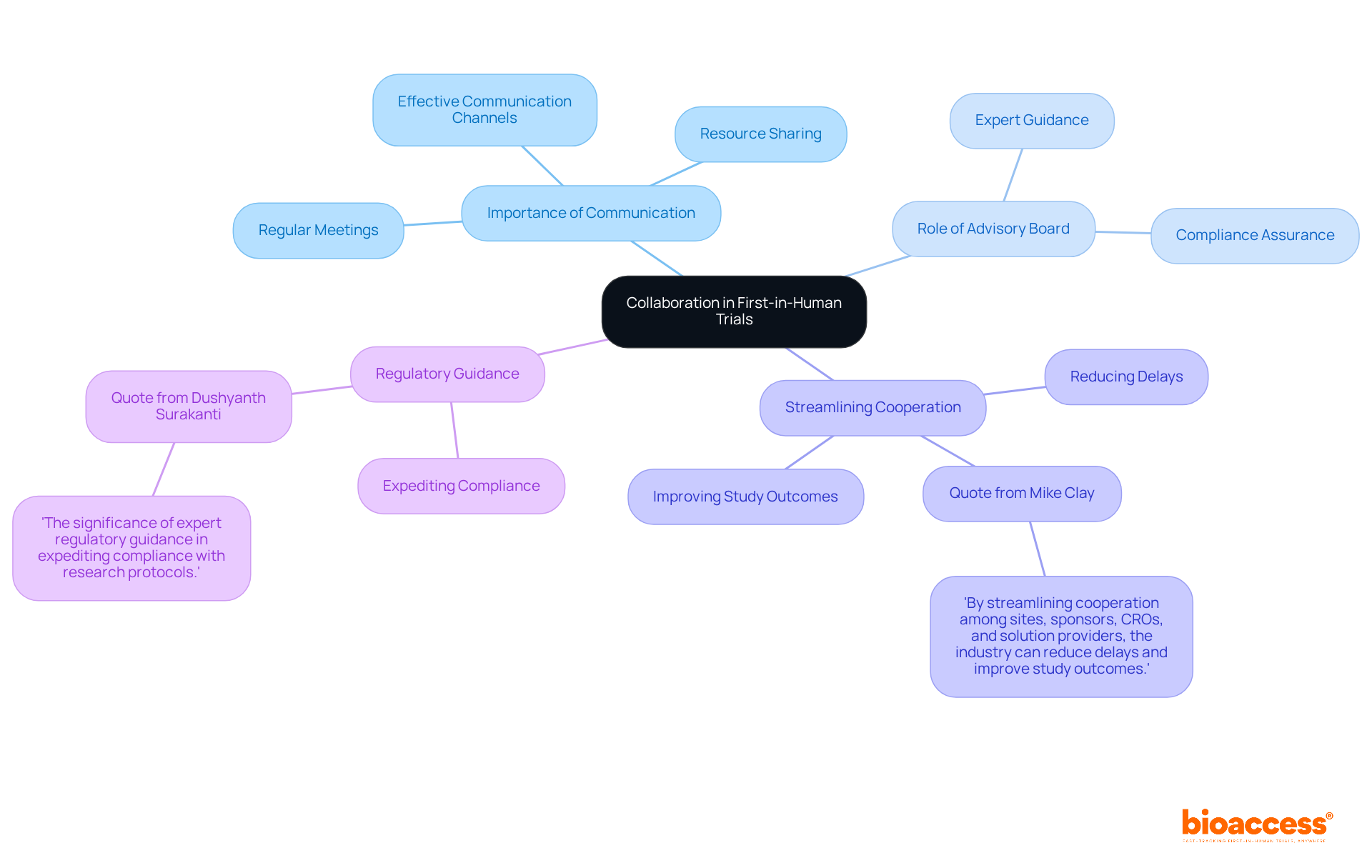
Cooperation among research groups, sponsors, and regulatory agencies is essential for the success of first in human clinical trials. Effective communication channels foster a collaborative environment, leading to efficient problem-solving and resource sharing. Regular meetings and updates are crucial to keep all stakeholders aligned on project objectives and progress, significantly enhancing the overall effectiveness of the research effort. Research shows that effective communication practices can enhance results, with groups that emphasize clear dialogue attaining greater levels of productivity and innovation.
Key Insights:
To further enhance communication strategies, conducting regular assessments of these practices can help identify areas for improvement and ensure that all team members are effectively engaged.
Patient safety is paramount in first in human clinical trials; therefore, it is essential to implement robust monitoring protocols for swiftly identifying and managing adverse events. Researchers must establish transparent reporting mechanisms that ensure immediate access to medical care for individuals when necessary.
In 2023, serious events accounted for 4.1% of total reports, marking the highest percentage since reporting began. This statistic underscores the urgent need for vigilant oversight. By prioritizing patient safety, researchers not only adhere to ethical standards but also enhance the credibility and integrity of the research process.
Effective monitoring protocols—such as real-time safety evaluations and comprehensive risk management strategies—are crucial for protecting participants and ensuring the dependability of study results.
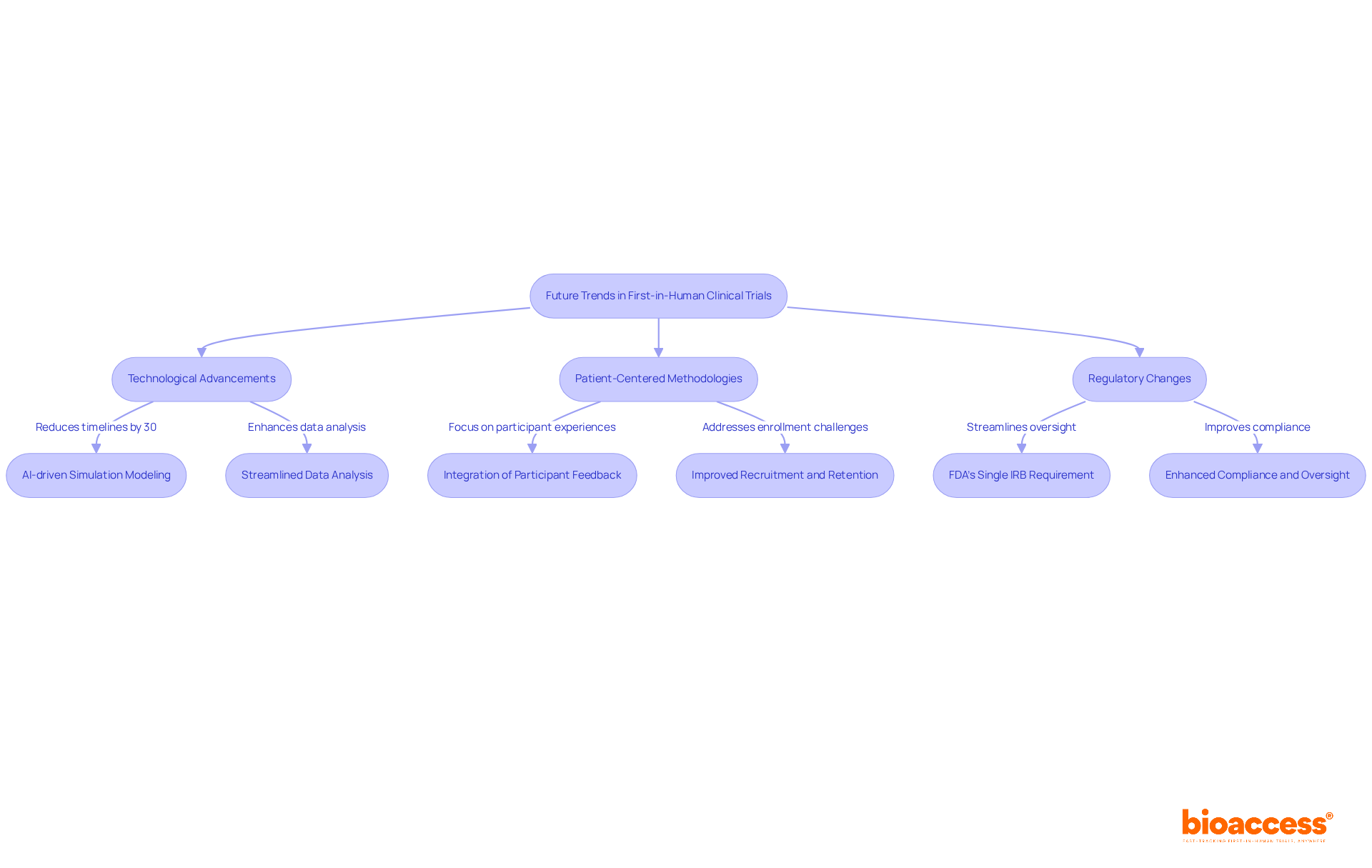
The landscape of first in human clinical trials is on the brink of significant transformation, driven by technological advancements, particularly in artificial intelligence (AI) and machine learning. These innovations are enhancing study design and streamlining data analysis, empowering researchers to make more informed decisions. For instance, AI-driven simulation modeling can project success rates prior to trials, potentially reducing timelines by as much as 30% and costs by up to 20%.
Moreover, the shift towards patient-centered methodologies is reshaping study designs to prioritize participant experiences and outcomes. This focus on inclusivity is expected to improve recruitment and retention rates, addressing the alarming statistic that nearly 80% of clinical studies fail to achieve their initial enrollment targets. By integrating participant feedback and leveraging technologies such as wearables, studies can become increasingly adaptable and responsive to patient needs.
As regulatory frameworks evolve, researchers must remain vigilant to changes that may impact study execution and compliance. The forthcoming implementation of the FDA's single IRB requirement is designed to streamline oversight and diminish administrative burdens, further fostering innovation in clinical research. In this rapidly changing environment, staying informed and flexible will be essential for success in first in human clinical trials.
The success of first-in-human clinical trials relies on a multifaceted approach that incorporates regulatory agility, effective participant selection, robust data management, and ethical considerations. By leveraging the strengths of regions like Latin America, the Balkans, and Australia, organizations such as bioaccess® are redefining the timelines for these critical studies, ensuring that innovations in medical technology reach the market more swiftly and efficiently.
Key insights from the article underscore the necessity of navigating complex regulatory landscapes, fostering diversity in participant recruitment, and implementing adaptive trial designs that enhance safety and efficacy. Moreover, ethical considerations, particularly regarding informed consent, remain paramount in maintaining public trust and ensuring participant welfare throughout the research process.
As the field evolves, embracing technological advancements and patient-centered methodologies will be essential for overcoming existing challenges and achieving successful outcomes in first-in-human trials. Stakeholders are encouraged to stay informed about regulatory changes and adopt best practices that prioritize collaboration, safety, and ethical integrity. By doing so, the future of clinical research can be both innovative and inclusive, ultimately leading to improved healthcare solutions for all.
What is bioaccess® and how does it facilitate first-in-human clinical trials?
bioaccess® leverages regulatory agility in Latin America, diverse patient demographics in the Balkans, and efficient ethical approval processes in Australia to significantly shorten timelines for first-in-human clinical trials. It secures ethical approvals in 4-6 weeks and achieves enrollment rates 50% faster than traditional markets.
What expertise does the bioaccess® team bring to clinical trials?
The bioaccess® team has over 20 years of specialized knowledge in Medtech, which empowers innovators to expedite their products' journey to market while adapting to the evolving healthcare landscape.
How does bioaccess® help improve study timelines and outcomes?
By optimizing efficiency, bioaccess® allows research directors to enhance study timelines and outcomes, achieving substantial cost savings of $25K per patient with FDA-ready data, without the need for rework or delays.
What are the regulatory requirements for conducting first-in-human clinical trials in Colombia?
In Colombia, researchers must obtain ethical approval from institutional review boards (IRBs), regulatory approval from INVIMA, and an import permit from the Ministry of Industry and Commerce (MinCIT) for investigational devices.
Why is it important to understand regulatory requirements for clinical trials?
Understanding regulatory requirements is vital as they directly impact study design, participant recruitment, and data management. Many experiments face delays due to regulatory compliance challenges, highlighting the need for thorough preparation.
What recent updates have been made to ethical approval processes for clinical trials?
Recent updates include streamlined arrangements for informed consent, clearer communication, and a requirement for researchers to provide a summary of results to participants in an accessible format, promoting ethical compliance and participant engagement.
What changes are expected in medical research regulations in 2026?
New medical research regulations set to take effect on April 28, 2026, will transform the regulatory environment, promoting flexibility and aligning with international good clinical practices to strengthen the ethical framework of clinical studies.
How does bioaccess® assist startups in overcoming regulatory hurdles?
bioaccess® offers specialized services such as patient recruitment and study information management to help Medtech, Biopharma, and Radiopharma startups ensure rapid site activation and regulatory compliance in Latin America, Eastern Europe, and Australia.
What criteria are important for participant selection in first-in-human trials?
Inclusion and exclusion criteria typically encompass age, health status, and specific medical conditions relevant to the study. A diverse group of participants improves external validity and ensures results are applicable to a wider population.
What strategies can researchers use to attract a diverse group of participants?
Effective strategies include leveraging community outreach, utilizing technology for remote monitoring, and offering logistical support such as transportation and home visits to establish trust and promote a representative group in medical studies.