


The article titled "10 Key Insights from BIO Europe Spring 2025 for Clinical Research Leaders" presents crucial takeaways for leaders in clinical research from the forthcoming BIO Europe Spring 2025 event. It underscores the significance of:
These elements are vital for navigating the dynamic biopharma industry landscape and enhancing the efficiency and effectiveness of clinical trials. As the industry evolves, understanding these insights will empower leaders to make informed decisions and foster successful collaborations.
The biopharma landscape is evolving at an unprecedented pace, with key events such as BIO Europe Spring 2025 acting as a catalyst for innovation and collaboration. This article explores ten pivotal insights from the conference that clinical research leaders must not overlook. As the industry faces regulatory changes, funding challenges, and the necessity for strategic partnerships, the pressing question arises: how can organizations leverage these insights to navigate the complexities of clinical research and accelerate the development of groundbreaking therapies?
bioaccess® harnesses the regulatory speed of Latin America, the diverse clientele of the Balkans, and the efficient pathways of Australia to secure ethical approvals in merely 4 to 6 weeks. This strategic amalgamation empowers Medtech innovators to markedly accelerate their research timelines, achieving enrollment rates that surpass conventional markets by 50%. By focusing on early-phase studies, bioaccess® enables startups to expedite their innovations to market, ensuring that essential medical advancements reach patients more swiftly.
Looking ahead to 2025, the average duration for ethical approvals is projected to remain competitive, further enhancing the region's appeal for research trials. Insights from industry experts underscore the critical nature of streamlined processes and effective collaboration in reducing delays, which can lead to costs for sponsors ranging from $600,000 to $8 million for each day a trial is postponed.
Committed to innovation, bioaccess® stands at the forefront of transforming medical study environments, establishing itself as an indispensable ally for Medtech firms navigating the complexities of trials.

BIO Europe Spring will provide unparalleled networking opportunities in 2025, bringing together over 3,700 life science professionals from diverse sectors. Attendees will engage in more than 20,000 one-on-one meetings, informal discussions, and evening receptions, all meticulously designed to foster meaningful connections. This event transcends mere attendance; it is an opportunity to cultivate relationships that can evolve into strategic partnerships and investments.
For example, Ramakrishna Guduru from Zasya Life Sciences Pvt Ltd emphasizes their consistent participation in BIO-Europe, citing the heightened quality of attendees as a significant contributor to generating new business leads. Furthermore, the event's inviting atmosphere, praised by Nadine Sommerfield, enhances networking potential, facilitating connections between research professionals and potential collaborators as well as industry experts.
By actively engaging in these networking events, individuals can strengthen their strategic positioning and unlock new opportunities within the life sciences arena. This approach aligns seamlessly with bioaccess's commitment to innovation and quality in healthcare, as nurturing these connections can drive advancements in medical technologies and positively influence local economies.

At BIO Europe Spring, the focus will be on pivotal regulatory developments that are shaping the biopharma sector, particularly the new EU Health Technology Assessment (HTA) process in 2025. This regulation, set to take effect in January 2025, aims to harmonize clinical assessments across EU member states, with an initial emphasis on oncology therapies and advanced therapy medicinal products (ATMPs). It is imperative for clinical research executives to remain vigilant regarding these changes, as they will significantly impact market access strategies.
Engaging with regulatory experts at Bio Europe Spring will be crucial for gaining insights into navigating the complexities of the new HTA process. With 70% of service organizations needing to demonstrate compliance with at least six different frameworks, understanding the implications of the EU HTA is essential for ensuring compliance and capitalizing on innovation opportunities.
Statistics reveal that:
Examples of companies successfully adapting to the new EU HTA processes illustrate the necessity of proactive planning. Organizations that collaborate with experienced partners in health economics and market access, such as bioaccess, are better positioned to navigate regulatory changes. Bioaccess provides extensive trial management services, including:
This collaborative approach not only enhances compliance but also fosters innovation, ultimately facilitating access to new therapies.
The integration of data analytics in biopharma is revolutionizing the industry, empowering companies to make informed decisions through real-time insights. At BIO Europe Spring, discussions will emphasize how analytics can drive growth, improve health outcomes, and simplify research trials in 2025. Organizations leveraging advanced analytics have reported up to a 75% reduction in study execution time, significantly improving trial efficiency.
'Bioaccess® exemplifies this with its capability to enroll treatment-naive cardiology or neurology groups 50% faster than Western sites, achieving $25K savings per individual with FDA-ready data—no rework, no delays. By adopting data-driven strategies, healthcare study leaders can optimize operational efficiencies, reduce participant recruitment delays—which account for 80% of trial delays—and better align with stakeholder needs.
Significantly, GlobalCare Clinical Trials has teamed up with bioaccess™ to improve its trial ambulatory services in Colombia, leading to more than a 50% decrease in recruitment time and a retention rate surpassing 95%. Additionally, IDx Technologies has chosen bioaccess™ for data-licensing collaboration in Latin America, enhancing AI-driven disease detection in ophthalmology.
The focus on real-time data analysis not only enables quicker decision-making but also improves the overall quality of clinical research, ensuring that innovative treatments reach individuals more swiftly and effectively.
BIO Europe Spring is set to showcase groundbreaking therapies across diverse therapeutic areas, including oncology, immunology, and rare diseases in 2025. This event promises attendees invaluable insights into the latest advancements, particularly innovative immunotherapies that have demonstrated potential in enhancing patient outcomes. Recent data indicates a significant improvement in the 5-year relative survival rate for melanoma, reflecting the advancements in treatment protocols. Moreover, the event will highlight successful collaborations in oncology, where partnerships between biopharma companies have led to the development of novel therapies addressing unmet medical needs.
Key industry figures will share their viewpoints on advanced solutions, emphasizing the critical role of teamwork in fostering innovation. By engaging with these influential leaders, healthcare study executives can identify potential collaborations and funding opportunities that align with their strategic objectives. Staying informed about these advancements is essential for navigating the evolving landscape of medical studies and ensuring the effective development of new therapies.
BIO Europe Spring will serve as a vital platform in 2025 for startups to engage with potential investors and explore diverse funding avenues. This event features workshops and panels dedicated to strategies for securing investment, offering valuable insights into current venture capital trends and grant application processes. By engaging in these conversations, healthcare development executives can effectively position their organizations to attract essential funding for expansion and innovation.
As the funding landscape evolves, startups must adapt to shifting investor expectations, particularly in the biopharma sector. Investors are increasingly focused on companies that demonstrate clear therapeutic efficacy and robust regulatory progress. This trend underscores the necessity for startups to present compelling preclinical data and articulate a well-defined market strategy.
Moreover, the rise of strategic partnerships with large pharmaceutical companies is becoming a crucial funding strategy. These collaborations not only provide immediate capital but also access to expertise and infrastructure that can accelerate development. Startups that synchronize their fundraising activities with significant development milestones are more likely to obtain investment, making it essential for healthcare innovators to utilize these insights at BIO Europe Spring.
BIO Europe Spring 2025 offers a wealth of opportunities for companies to elevate their brand visibility through strategic sponsorships, presentations, and exhibitions. Organizations that effectively showcase their innovations and expertise can significantly enhance their appeal to potential partners and clients, particularly in the context of advancing medical technologies that transform lives in Latin America. Successful sponsorships from previous events have demonstrated that companies can achieve heightened recognition and engagement by aligning their brand with key industry initiatives that drive global health improvement through international collaboration.
To maximize impact, healthcare study executives should consider customized sponsorship approaches that connect with their target audience. This includes leveraging interactive experiences and immersive presentations that not only highlight their offerings but also foster meaningful connections. By engaging in these visibility-boosting activities, organizations can play a crucial role in promoting Medtech medical studies in Latin America, emphasizing innovation and regulatory excellence. Ultimately, the influence of sponsorships on company recognition in the biopharma sector cannot be overstated, as they serve as a catalyst for establishing a strong industry presence and driving future growth.
BIO Europe Spring will present a distinguished lineup of speakers from the biopharma sector, providing attendees with a unique opportunity to explore the latest trends, challenges, and opportunities that are shaping the industry in 2025. Keynote presentations and panel discussions will deliver valuable insights that research leaders can leverage to enhance their strategic approaches.
For instance, discussions may address:
Engaging with these industry experts allows attendees to deepen their understanding of the evolving landscape, enabling them to apply these insights to foster innovation and efficiency within their organizations.
BIO Europe Spring highlights the critical importance of collaboration among industry participants. This is exemplified by the alliance between bioaccess™ and Caribbean Health Group, which aims to position Barranquilla as a leading hub for research trials in Latin America. Attendees will have the opportunity to engage in networking events and collaborative workshops designed to connect innovators and explore potential partnerships.
Through collaboration with other organizations, trial leaders can leverage shared knowledge and resources, significantly accelerating their initiatives. Effective partnerships in the biopharma sector, such as those fostered by bioaccess™, demonstrate the potential to enhance health outcomes, illustrating the transformative impact of joint efforts on clinical studies.
This event during bio Europe Spring serves as a vital platform for cultivating these essential connections, ultimately advancing progress in patient care and therapeutic development.
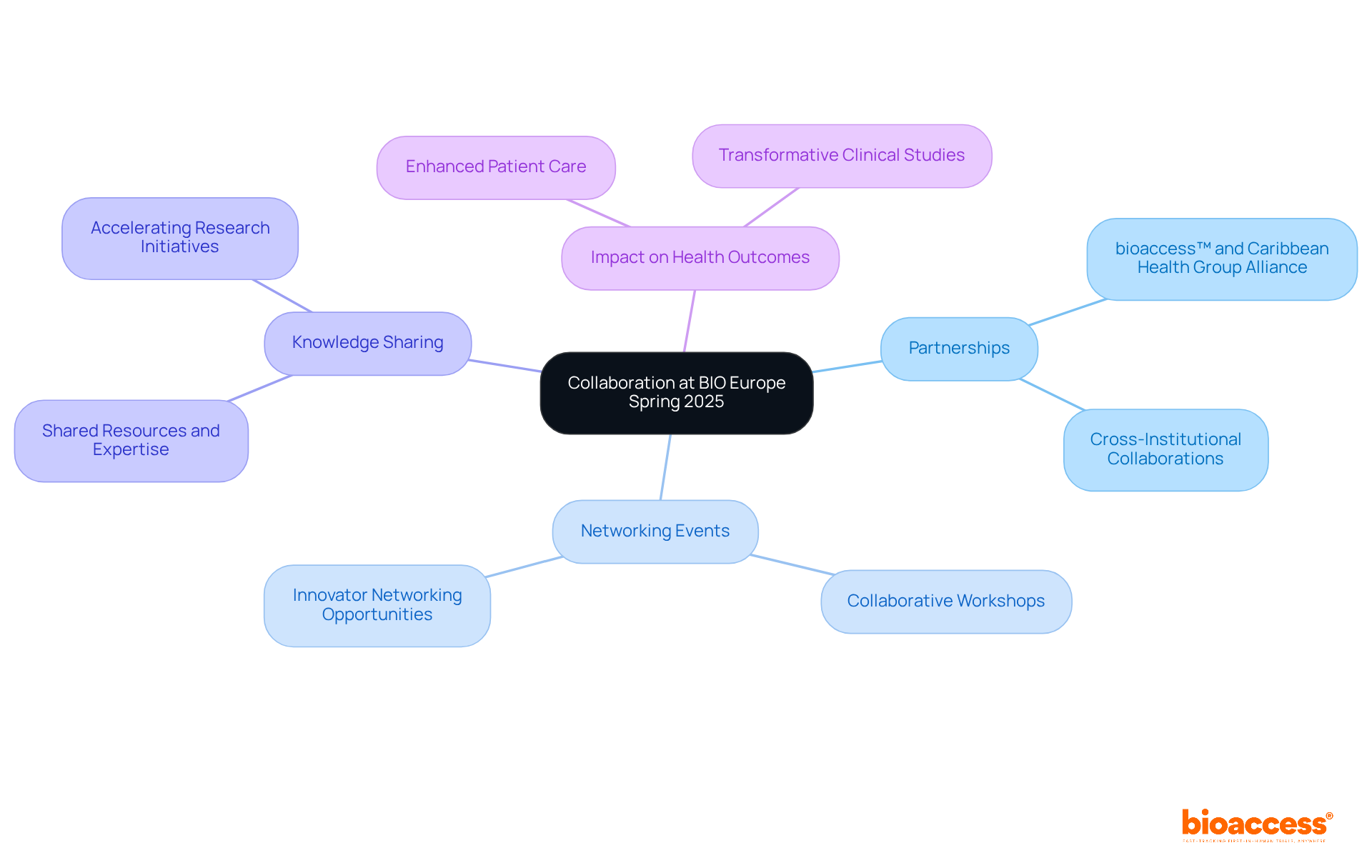
At BIO Europe Spring, the evolving landscape of the biopharma industry will take center stage in 2025, particularly focusing on:
Clinical study heads will delve into pressing issues, such as rising expenses, with 49% of drug creators identifying this as their primary concern for 2024. Additionally, the challenges of patient recruitment are highlighted, with 39% of developers citing this as a significant obstacle.
The integration of digital health technologies is becoming increasingly crucial; 63% of respondents foresee outsourcing AI-driven monitoring activities to enhance precision medicine. Moreover, the industry is experiencing a shift towards patient-centric approaches, with 51% of sponsors recognizing personalized medicine as a transformative trend.
By keeping abreast of these developments, clinical research leaders can strategically navigate the challenges ahead, ensuring their organizations remain competitive in a rapidly changing environment.
BIO Europe Spring 2025 serves as a pivotal platform for clinical research leaders, underscoring the critical interplay between innovation, collaboration, and regulatory progress within the biopharma sector. The insights shared during this event illuminate the necessity for stakeholders to adapt to an evolving landscape, where accelerated timelines and strategic partnerships are essential for success.
Key takeaways from the event emphasize the importance of:
Additionally, the event showcases groundbreaking therapies and funding opportunities that can significantly influence the trajectory of Medtech innovations and startups.
As the biopharma industry continues to evolve, it is crucial for leaders to engage actively in these discussions and collaborations. Embracing the insights and opportunities presented at BIO Europe Spring 2025 will not only drive advancements in clinical research but also ensure that innovative therapies reach patients more effectively. The future of healthcare hinges on the collective efforts of industry players, making participation in such events not merely beneficial but essential for sustained growth and impact.
What is bioaccess and how does it benefit Medtech innovators?
bioaccess® is a service that accelerates clinical research for Medtech innovations by securing ethical approvals in 4 to 6 weeks across regions like Latin America, the Balkans, and Australia. This speed allows Medtech startups to expedite their research timelines and achieve enrollment rates that exceed conventional markets by 50%.
What is the projected duration for ethical approvals in 2025?
The average duration for ethical approvals is expected to remain competitive in 2025, further enhancing the appeal of the region for research trials.
What are the potential costs associated with delays in clinical trials?
Delays in clinical trials can cost sponsors between $600,000 to $8 million for each day a trial is postponed.
What networking opportunities will be available at BIO Europe Spring 2025?
BIO Europe Spring 2025 will offer networking opportunities for over 3,700 life science professionals, including more than 20,000 one-on-one meetings and informal discussions designed to foster meaningful connections and potential partnerships.
How does participation in BIO Europe benefit companies like Zasya Life Sciences?
Companies like Zasya Life Sciences report that participation in BIO Europe helps generate new business leads due to the high quality of attendees and the inviting atmosphere that facilitates networking.
What regulatory developments will be discussed at BIO Europe Spring 2025?
The event will focus on the new EU Health Technology Assessment (HTA) process set to take effect in January 2025, which aims to harmonize clinical assessments across EU member states, particularly for oncology therapies and advanced therapy medicinal products (ATMPs).
Why is understanding the new EU HTA process important for clinical research executives?
Understanding the new EU HTA process is crucial for clinical research executives as it will significantly impact market access strategies and compliance requirements, with 70% of service organizations needing to demonstrate compliance with multiple frameworks.
What services does bioaccess provide to help navigate regulatory changes?
bioaccess offers trial management services including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, which enhance compliance and foster innovation.