


This article delves into the critical insights surrounding the 510(k) premarket notification process, particularly for leaders in the medical technology field. It underscores the necessity of thorough preparation and strategic planning to secure successful submissions. Notably, meticulous documentation, the appropriate selection of predicate devices, and proactive engagement with regulatory consultants are highlighted as factors that can significantly enhance the likelihood of timely FDA approvals. This assertion is supported by compelling case studies and statistical data reflecting submission outcomes, prompting readers to consider their own challenges in navigating the clinical research landscape.
The 510(k) premarket notification process serves as a critical pathway for Medtech innovators who seek to bring their products to market swiftly and efficiently. As the landscape of medical device regulation continues to evolve, grasping the nuances of this process can provide significant advantages. This article explores ten key insights that illuminate the complexities of 510(k) submissions, offering invaluable guidance on navigating potential pitfalls and maximizing the chances of success. With so much at stake, Medtech leaders must consider how to ensure their submissions not only meet regulatory standards but also distinguish themselves in a competitive marketplace.
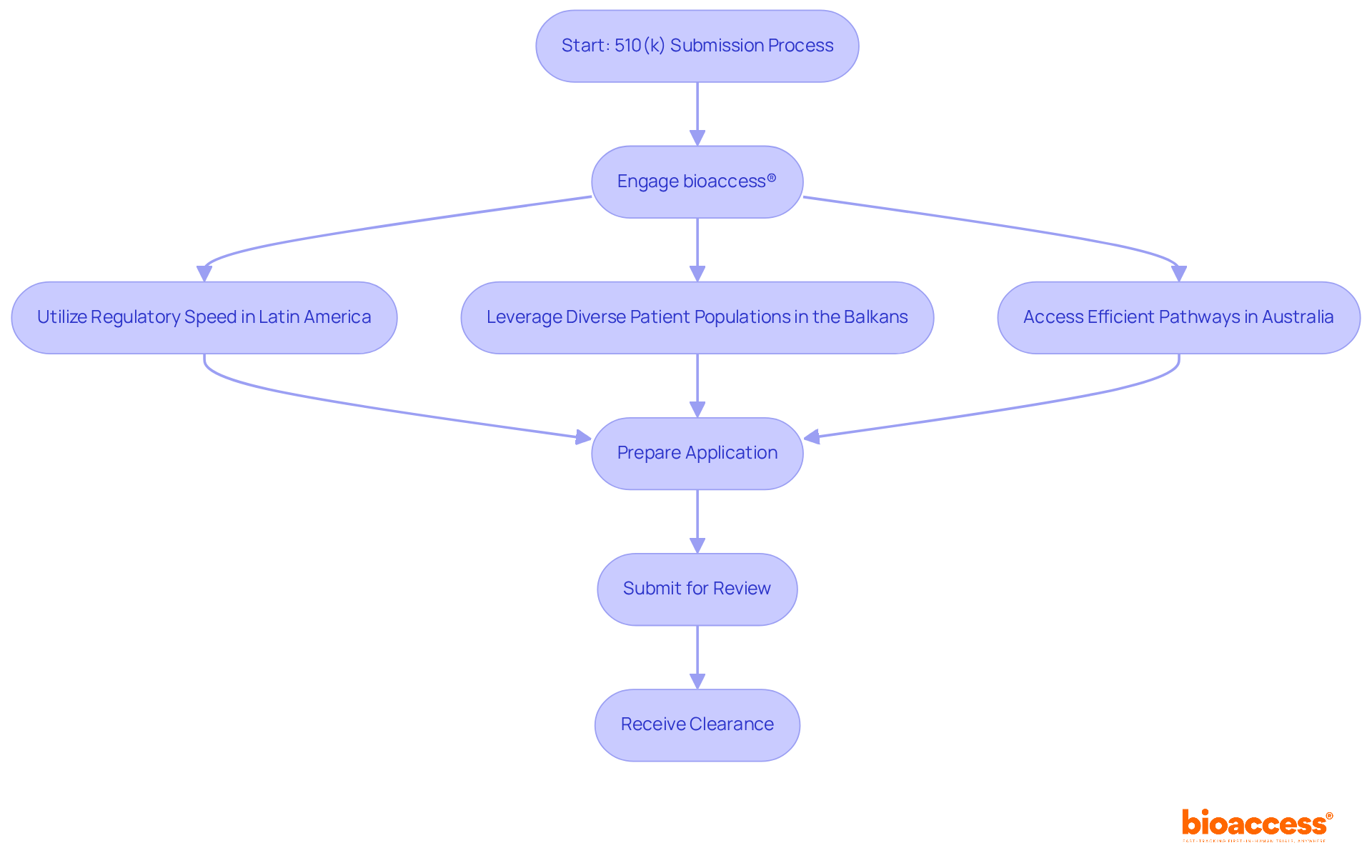
bioaccess® excels in expediting the 510 k premarket notification submissions by harnessing the regulatory speed of Latin America, the diverse patient populations in the Balkans, and Australia's efficient pathways. This strategic method facilitates ethical approvals in an impressive 4-6 weeks, significantly reducing the time to market for innovative medical solutions. By collaborating with bioaccess®, Medtech innovators can adeptly navigate the complexities of the 510 k premarket notification process, achieving faster enrollment and ensuring compliance with regulatory standards.
Successful case studies, including Avantec Vascular's first-in-human clinical trial of an innovative vascular instrument in Latin America, demonstrate that well-prepared applications enhance the likelihood of receiving a Substantially Equivalent decision. This not only provides a competitive advantage in a rapidly evolving market but also emphasizes the importance of leveraging regulatory speed. Timely submissions significantly reduce back-and-forth with reviewers, ultimately accelerating clearance and facilitating quicker product launches.
Engaging a consultant for the 510 k premarket notification during FDA staffing shortages has proven beneficial, helping to prevent strategic missteps that could lead to delays. With a 95% success rate for the FDA's 510(k) consultants and a 97% client satisfaction rate for consulting services, the effectiveness of bioaccess®'s approach to the 510 k premarket notification is underscored. Additionally, bioaccess® offers access to pre-qualified networks and FDA/EMA/MDR-ready datasets, further enhancing its capabilities in supporting Medtech innovations.
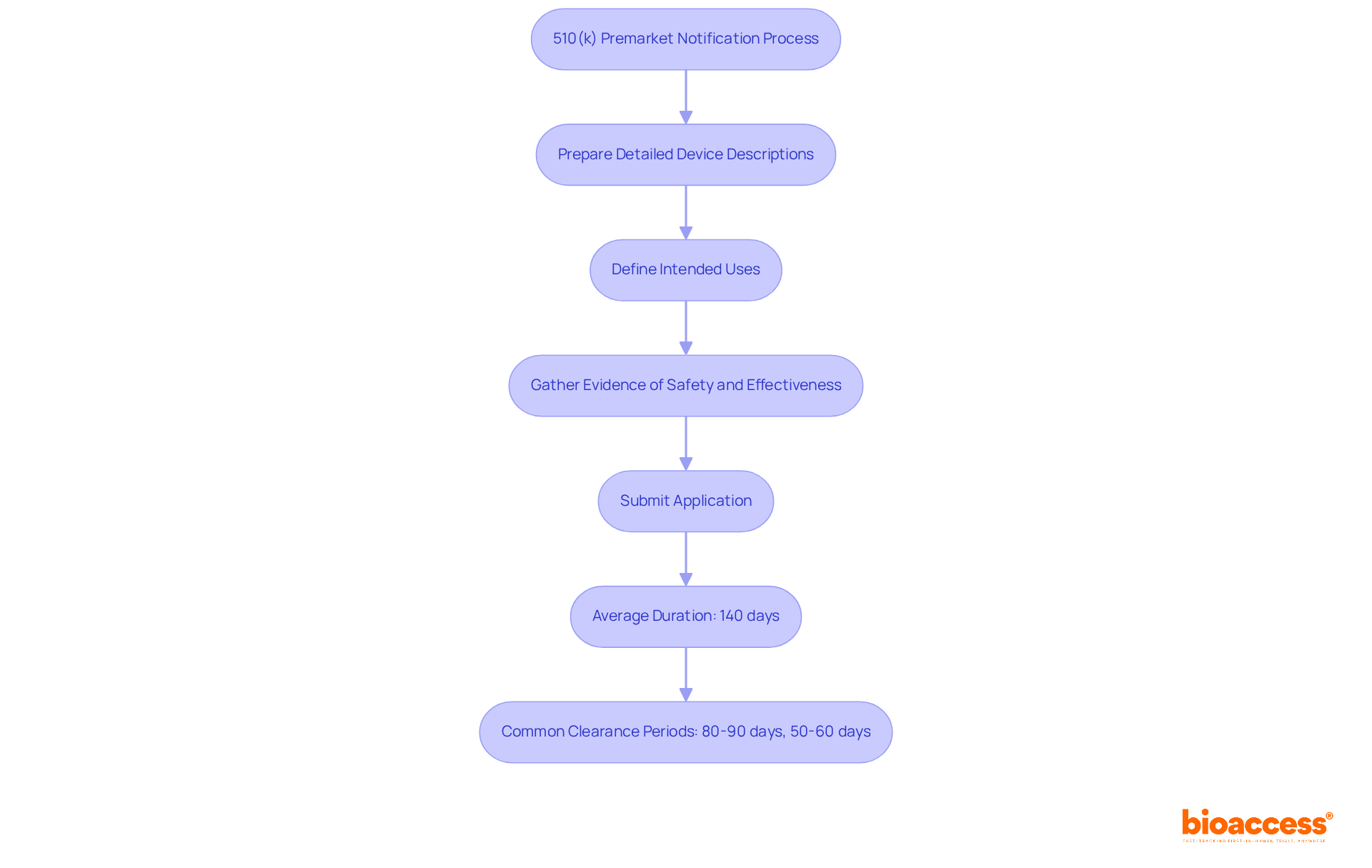
The 510 k premarket notification procedure serves as a vital regulatory pathway for manufacturers to demonstrate that their medical instrument is significantly comparable to an existing product. This process is particularly critical for Class II products, which often require a more streamlined approach for market entry. Manufacturers are required to submit comprehensive documentation, including:
Currently, the average duration for 510(k) applications is approximately 140 days, with the most common clearance periods being:
Regulatory experts underscore the importance of a thorough understanding of the 510 k premarket notification requirements for Medtech leaders aiming to expedite their innovations to market. Successful examples of 510(k) filings highlight the importance of meticulous preparation and strict adherence to regulatory standards, ultimately facilitating quicker access to the healthcare market. Recent updates in FDA guidelines further stress the necessity for manufacturers to remain informed about changes in the 510 k premarket notification process, ensuring compliance and enhancing the likelihood of successful filings.
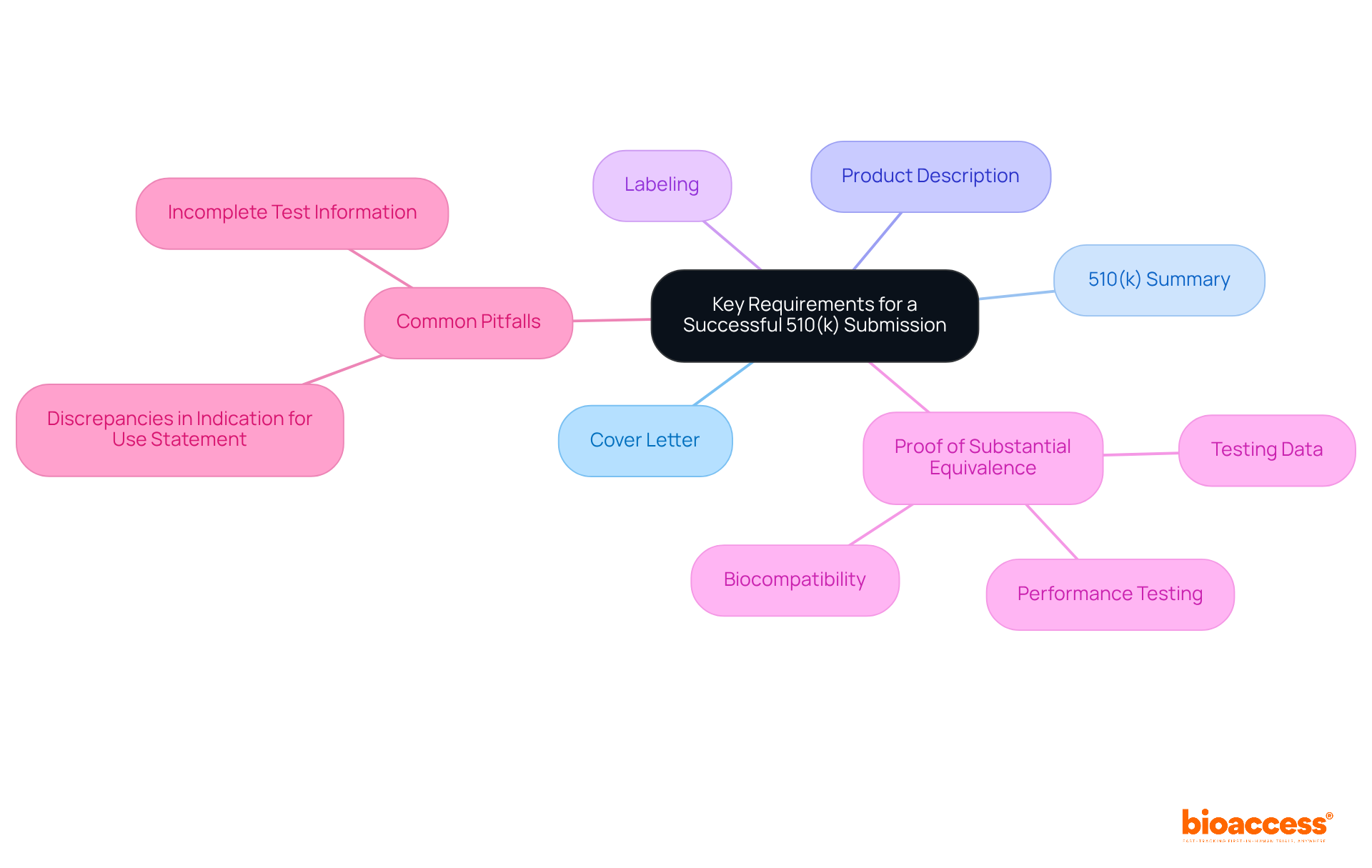
A successful 510 k premarket notification application necessitates several critical elements:
Manufacturers must also include comprehensive testing data, particularly biocompatibility and performance testing results, which are vital for establishing safety and efficacy. Notably, over 30% of submissions are rejected before review due to non-compliance with the Refusal To Accept (RTA) checklist, underscoring the necessity for meticulous documentation.
Common pitfalls include:
Collaborating with regulatory advisors can offer perspectives on documentation needs, ensuring that all components precisely represent the product's features and adhere to FDA regulations. By emphasizing thoroughness in these areas, manufacturers can enable a smoother evaluation and improve their chances of timely approval.
The distinction between the 510 k premarket notification and Premarket Approval (PMA) pathways is primarily based on the level of scrutiny and the type of evidence required. A 510 k premarket notification enables manufacturers to demonstrate that their product is substantially equivalent to an already marketed item, making it a quicker and more affordable option. In contrast, the PMA procedure necessitates extensive clinical information to ascertain the safety and efficacy of high-risk instruments, which can take months or even years to finalize.
In 2024, approximately 3,052 products are anticipated to be approved through the 510 k premarket notification pathway, representing about 99% of all approvals. The 510 k premarket notification pathway is particularly favored for its expedited review process, with average decision times around 168.9 days, compared to the PMA's average of 181 days. Notably, products authorized through the 510(k) pathway are 5.32 times more prone to being recalled than those sanctioned through PMA, underscoring the significance of thorough evaluation when choosing a regulatory path.
Medtech firms frequently opt for the 510 k premarket notification pathway when their products resemble existing ones, which facilitates faster market entry. For instance, surgical instruments predominantly utilize the 510 k premarket notification route, with 81% of 8,985 surgical instruments approved through this pathway. Regulatory specialists emphasize that while the 510(k) pathway is advantageous for efficiency and cost-effectiveness, it is crucial to ensure that safety protocols are adequately managed to mitigate potential hazards associated with product recalls.
As the landscape of medical apparatus regulation evolves, understanding these pathways becomes essential for Medtech leaders aiming to navigate the complexities of bringing innovative products to market efficiently.

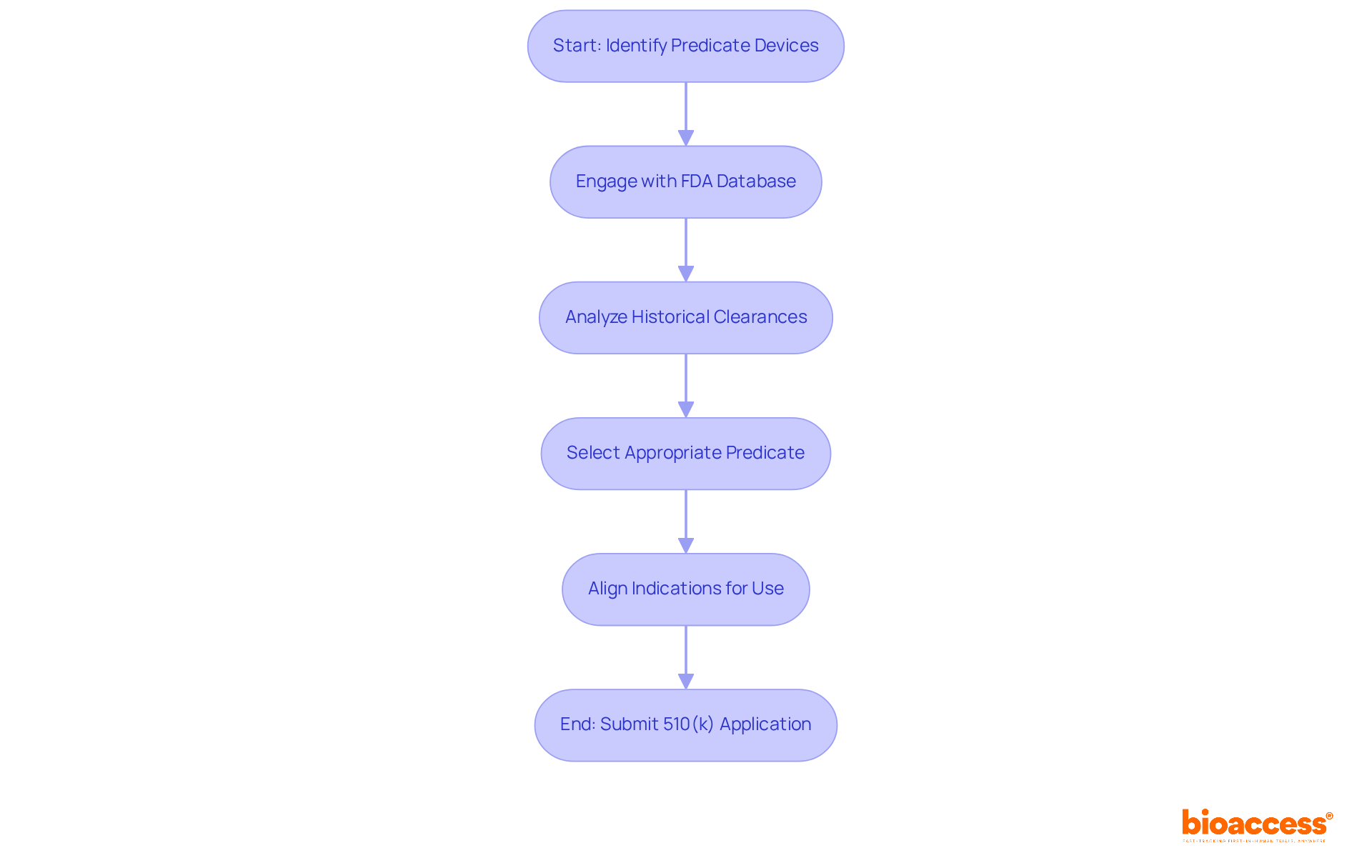
Selecting an appropriate predicate instrument is vital in the 510 k premarket notification process, as it establishes the foundation for demonstrating substantial equivalence. The predicate device must possess the same intended use and technological characteristics as the new device. To identify suitable predicates, manufacturers can leverage the FDA's comprehensive database, which encompasses records of previously approved products. This database acts as a strategic asset, allowing manufacturers to analyze historical clearances and comprehend FDA expectations.
The FDA underscores that a judiciously selected predicate device can significantly bolster the chances of a successful submission. Engaging with the database not only aids in locating appropriate predicates but also enables manufacturers to anticipate potential reviewer inquiries, thereby streamlining the review process. Notably, over 64% of applications for 510 k premarket notification are rejected, frequently due to poor predicate selection or inadequate documentation.
To adeptly navigate the selection process, manufacturers should focus on recent clearances, as innovations in medical technology may render older predicates obsolete. Furthermore, employing advanced search techniques within the FDA database can enhance the identification of pertinent predicates, ensuring adherence to the necessary criteria for substantial equivalence. Additionally, manufacturers must ensure that the 'Indications for Use' statement aligns with the application to mitigate the risk of FDA rejections. By prioritizing comprehensive research and strategic predicate selection, manufacturers can elevate their application quality and amplify their prospects for obtaining FDA clearance.
To establish substantial equivalence in a 510 k premarket notification, manufacturers must provide compelling evidence that their device aligns with the safety and effectiveness of a predicate device. This requires comprehensive comparisons of intended use, technological characteristics, and performance data. Notably, successful entries often showcase robust performance data that supports claims of equivalence, as evidenced by the 85 percent of applications that received a Substantially Equivalent decision in the year preceding September 2022. Conversely, a significant 35 percent of entries failed to meet the acceptance for review criteria, primarily due to inadequate demonstration of substantial equivalence.
Manufacturers should meticulously address any differences between their device and the predicate, providing clear justifications for why these differences do not undermine safety or effectiveness. This strategy not only enhances the application but also builds trust with regulators and end-users. As emphasized by industry specialists, effective documentation is paramount; a thoroughly prepared 510 k premarket notification can greatly streamline the FDA evaluation process, which typically lasts 90 days but may extend to six months or longer depending on the quality of the application. Therefore, clear and comprehensive documentation is vital for a successful demonstration of equivalence, ensuring that all necessary evidence is readily available for review.

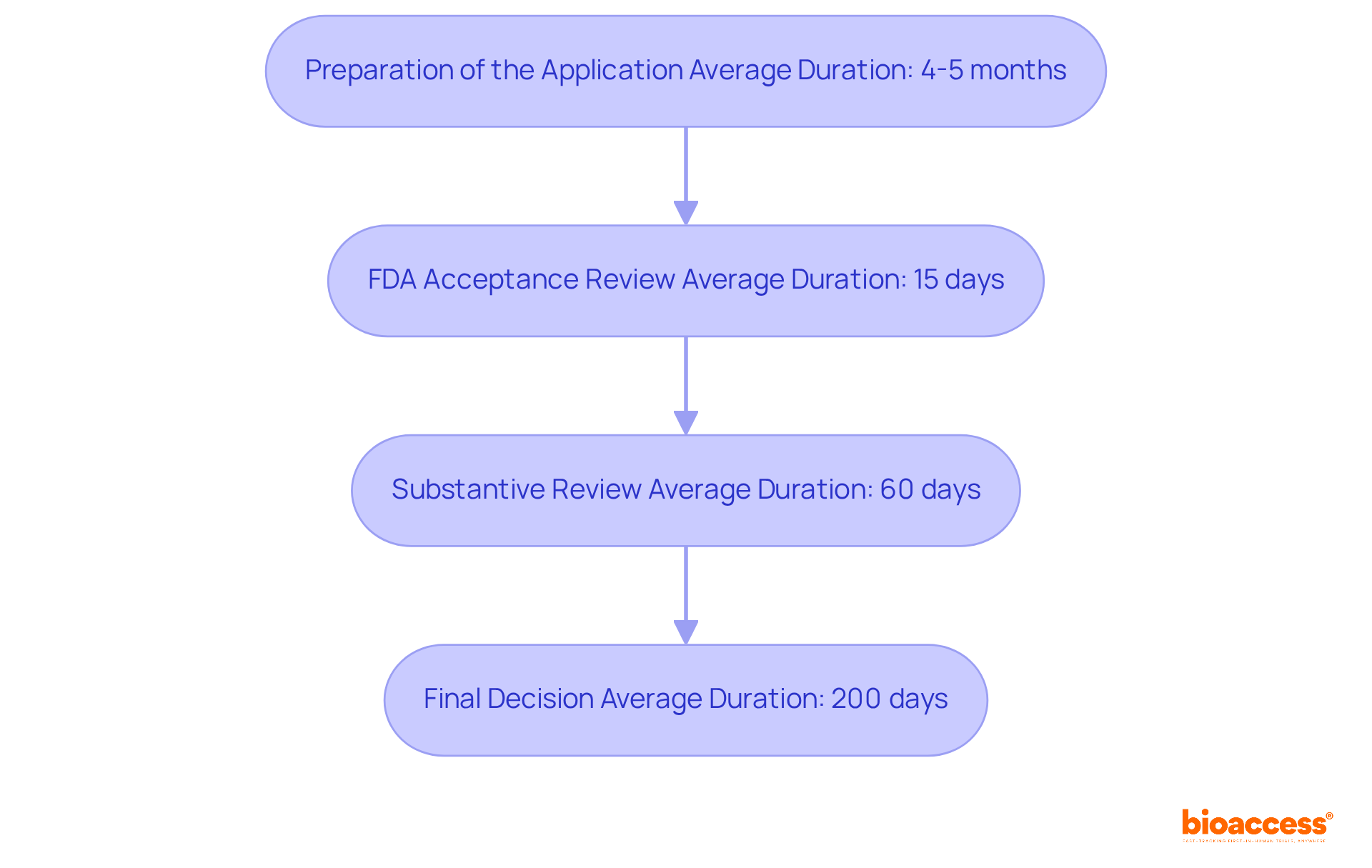
The procedure for the 510 k premarket notification application encompasses several critical phases:
While the FDA aims to finalize its review within 90 days, manufacturers should realistically prepare for an average duration of approximately 200 days. This extended timeframe accounts for the complexity of their applications and the likelihood of additional information requests.
In 2021, the average duration for the 510 k premarket notification approval was 175 days, with 67 percent of applications leading to requests for further information during the evaluation process.
To streamline this procedure, it is essential for producers to ensure that all documentation is thorough and well-organized, as nearly 32 percent of submissions failed to pass the initial acceptance review in the year leading up to September 2022. By anticipating potential delays and preparing adequately, manufacturers can significantly enhance their chances of securing timely approval.
The 510 k premarket notification process presents significant challenges that can adversely affect the timeline and success of medical device approvals. Notably, inadequate documentation has been identified as a frequent issue, impacting nearly 32% of entries, which leads to delays and increased costs. In the year leading up to September 2022, 67% of submissions resulted in requests for Additional Information (AI), highlighting a systemic problem with documentation quality.
Furthermore, the improper selection of predicate products can hinder the ability to demonstrate substantial equivalence, a critical requirement for clearance. Insufficient testing data is another common pitfall; the FDA mandates comprehensive performance data to substantiate safety and effectiveness. Additionally, formatting issues can complicate matters, as poorly organized submissions obstruct FDA reviewers from accessing essential information, potentially prolonging the review process.
To comply with FDA requirements, submissions must include page numbers and an eCopy. To navigate these challenges effectively, manufacturers should prioritize meticulous documentation, ensure appropriate predicate device selection, and adhere to clear formatting guidelines.
As Elexes Medical Consulting states, 'The 510 k premarket notification procedure may be intricate, but avoiding these common errors will improve your chances of securing a successful FDA approval.' A well-organized entry not only facilitates a smoother evaluation but also enhances the likelihood of prompt FDA approval.

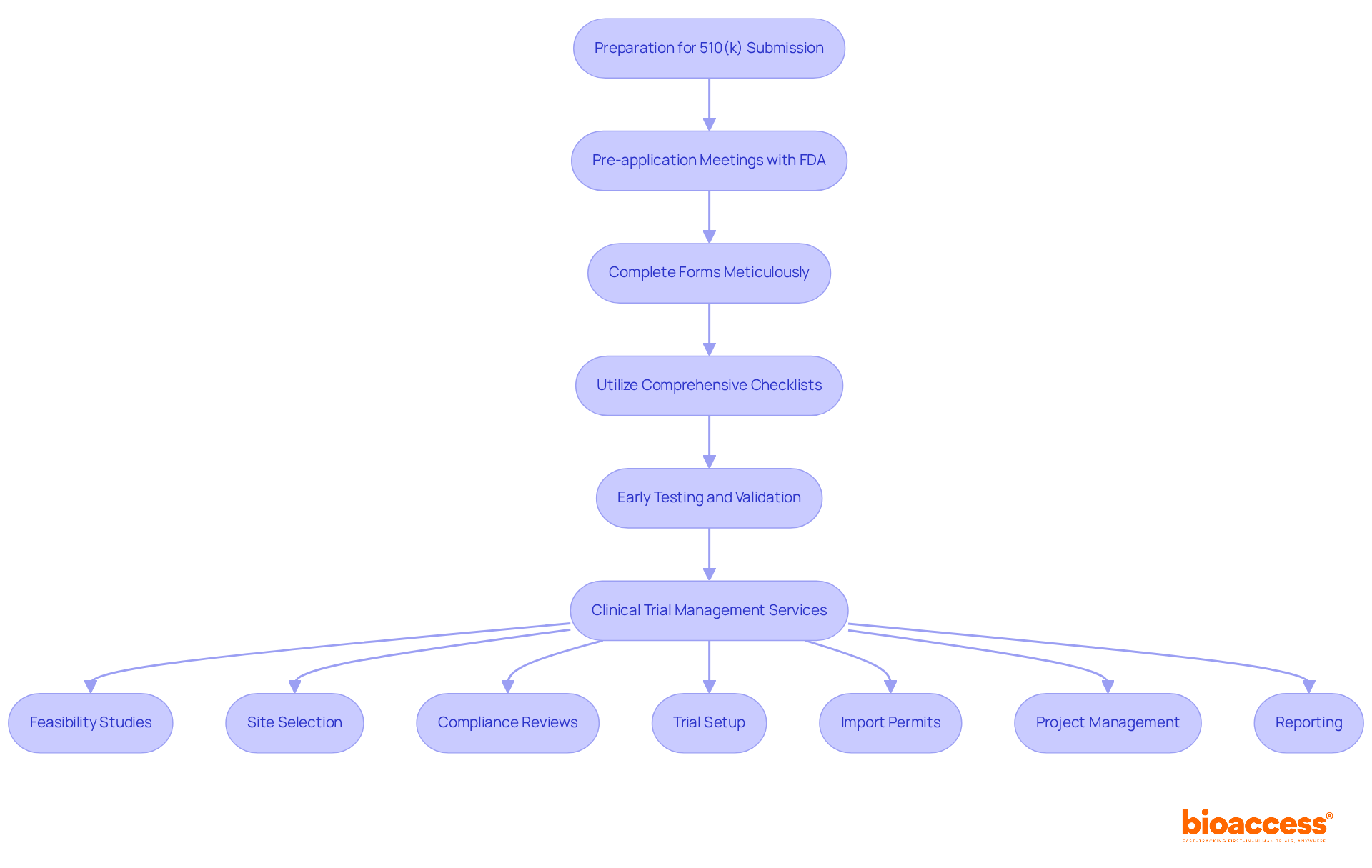
Effective preparation for a 510 k premarket notification is crucial, hinging on several best practices, with pre-application meetings with the FDA being paramount. Engaging in these discussions allows manufacturers to clarify expectations and address potential issues early in the process. Regulatory consultants emphasize that these meetings can significantly improve the chances of a successful 510 k premarket notification. One consultant remarked, 'Delays are unavoidable without a clear predicate and well-crafted document,' underscoring the necessity for thorough preparation.
In addition to pre-submission meetings, manufacturers must ensure that all forms are meticulously completed and utilize comprehensive checklists to verify that all required documentation, including review and feedback on study documents to comply with country requirements, is included. This attention to detail can prevent common pitfalls that lead to delays in the 510 k premarket notification process. Furthermore, early testing and validation are crucial for gathering robust data that supports claims of substantial equivalence for the 510 k premarket notification. As one specialist noted, 'Requirements must be unambiguous and verifiable,' emphasizing the significance of clarity in entries.
Including extensive clinical trial management services—such as:
can further enhance the application workflow. Consistent interaction among team members is also essential to maintain the workflow. By fostering a collaborative environment, teams can effectively address emerging challenges and streamline their efforts. Overall, these practices not only promote a more seamless submission method but also enhance the likelihood of achieving FDA clearance.
Post-market surveillance serves as a vital element of the 510(k) premarket notification process, concentrating on the ongoing monitoring of a product's safety and effectiveness once it reaches the market. Manufacturers are required to report any adverse events and may need to conduct post-market studies, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), to mitigate potential safety concerns.
A well-structured post-market surveillance plan not only ensures compliance with FDA regulations but also significantly enhances patient safety by enabling the early identification of issues. For instance, data reveals that 41.8% of 510(k) Medical Equipment Reports (MDRs) were linked to items that experienced recalls, emphasizing the necessity for diligent oversight related to the 510(k) premarket notification.
Industry leaders assert that regulatory compliance is not merely an obstacle but a pledge to patient safety and product quality. By proactively tackling regulatory challenges and implementing comprehensive surveillance strategies, manufacturers can protect their products and cultivate trust among stakeholders.
Effective post-market studies, such as those conducted by bioaccess® that monitor adverse event reporting rates, yield invaluable insights into product performance and can lead to timely interventions, ultimately enhancing patient outcomes. To bolster your post-market surveillance efforts, consider establishing a systematic approach for collecting and analyzing data on device performance, which can aid in identifying trends and informing necessary adjustments.
Katherine Ruiz's expertise in regulatory affairs further fortifies these initiatives, ensuring that all facets of compliance are thoroughly addressed.

The 510(k) premarket notification process serves as a vital pathway for Medtech leaders aiming to efficiently introduce innovative medical devices to the market. By comprehensively understanding the intricacies of this regulatory framework and employing best practices, manufacturers can significantly bolster their chances of success. The insights presented throughout this article underscore the necessity of meticulous preparation, strategic predicate selection, and effective documentation in navigating the complexities associated with 510(k) submissions.
Key arguments articulated emphasize the importance of a thorough understanding of the requirements, alongside the advantages of engaging with regulatory experts and consultants. Successful case studies illustrate that timely and well-prepared applications not only expedite the approval process but also provide a competitive advantage in an ever-evolving marketplace. Furthermore, the significance of post-market surveillance is paramount, as it ensures ongoing compliance and enhances patient safety.
In summary, Medtech leaders are urged to adopt a proactive stance towards the 510(k) premarket notification process. By leveraging the insights shared, they can streamline their submissions and mitigate common challenges. Prioritizing regulatory compliance and embracing best practices will empower manufacturers to foster innovation while ensuring the safety and effectiveness of their medical devices. The journey towards successful 510(k) submissions is paved with diligence and strategic planning, ultimately paving the way for advancements that benefit both the industry and patients alike.
What is bioaccess® and how does it assist with 510(k) submissions?
bioaccess® specializes in expediting the 510(k) premarket notification submissions by utilizing regulatory speed in Latin America, diverse patient populations in the Balkans, and efficient pathways in Australia. This approach allows for ethical approvals in about 4-6 weeks, helping Medtech innovators navigate the complexities of the 510(k) process for faster market entry.
What are the benefits of using bioaccess® for 510(k) submissions?
By collaborating with bioaccess®, Medtech innovators can achieve faster enrollment, ensure compliance with regulatory standards, and enhance their chances of receiving a Substantially Equivalent decision. This results in a competitive advantage and quicker product launches due to reduced back-and-forth with reviewers.
What is the average duration for 510(k) applications?
The average duration for 510(k) applications is approximately 140 days, with common clearance periods ranging from 50-60 days to 80-90 days.
What are the key requirements for a successful 510(k) submission?
A successful 510(k) submission requires a cover letter, a 510(k) premarket notification summary, a comprehensive product description, labeling, and proof of substantial equivalence to a predicate instrument. Additionally, comprehensive testing data, particularly biocompatibility and performance testing results, must be included.
What common pitfalls should manufacturers avoid in their 510(k) submissions?
Common pitfalls include incomplete test information and discrepancies in the Indication for Use Statement, which can lead to delays or rejections. Ensuring thorough documentation and compliance with the Refusal To Accept (RTA) checklist is crucial.
How does engaging a consultant benefit the 510(k) submission process?
Engaging a consultant during FDA staffing shortages can prevent strategic missteps that could lead to delays. Consultants have a high success rate for 510(k) submissions and can provide valuable insights into documentation needs to ensure compliance with FDA regulations.
Why is it important to stay updated on FDA guidelines for the 510(k) process?
Staying informed about recent updates in FDA guidelines is essential for manufacturers to ensure compliance and enhance the likelihood of successful filings. Understanding these changes can facilitate quicker access to the healthcare market.