


The article titled "10 Key Insights on Antibody Fragments for Clinical Research" underscores the pivotal role of antibody fragments in clinical research and their therapeutic applications. It emphasizes that antibody fragments, including Fab and scFv, are becoming increasingly vital in targeted therapies. This is attributed to their enhanced specificity, reduced immunogenicity, and superior tissue penetration capabilities, which collectively support their rising adoption in the treatment of complex diseases such as cancer.
Antibody fragments are revolutionizing the landscape of clinical research and therapeutic development, presenting unique advantages in precision medicine and targeted therapies. As the biopharmaceutical industry confronts the escalating demand for innovative treatment options, comprehending the structure, types, and applications of these fragments is of paramount importance.
However, their promise is accompanied by a series of challenges that researchers must navigate, leading to critical inquiries regarding the future of antibody fragment technology. What insights can be gleaned from the latest advancements in this field, and how might they reshape the future of medical treatments?
bioaccess® excels in accelerating clinical research for antibody fragments by leveraging its extensive expertise across Latin America, the Balkans, and Australia. With ethical approvals secured in a remarkable 4-6 weeks and enrollment processes that are 50% faster than traditional markets, bioaccess® provides a significant competitive advantage for Medtech, Biopharma, and Radiopharma innovators. This clinical agility is vital for companies aiming to swiftly launch therapeutic solutions, enabling them to seize emerging opportunities in the fast-evolving biopharmaceutical industry.
Furthermore, bioaccess® delivers end-to-end acceleration for global trials, having activated over 50 pre-qualified sites in under 8 weeks, ensuring FDA/EMA/MDR-ready datasets with centralized monitoring. As the demand for personalized medicine rises, bioaccess®'s capacity to navigate regulatory landscapes efficiently positions it as a key partner for organizations striving to enhance patient outcomes through innovative treatments.

Antigen-binding pieces represent specialized, smaller components of immunoglobulins, crucial for their ability to attach to specific antigens. Typically produced through enzymatic cleavage or genetic engineering of full-length antibodies, these antibody fragments include:
Each type exhibits distinct structural characteristics that significantly influence binding affinity and therapeutic applications. For instance, antibody fragments, such as Fab sections, are recognized for their high specificity and are increasingly employed in targeted therapies, particularly in oncology, where precision is paramount. The smaller size of scFv components, as an antibody fragment, allows them to infiltrate hard-to-access tumor cells, enhancing their efficacy in cancer therapies.
Furthermore, these antibody fragments are being explored for applications in immunodeficiency and other medical fields, underscoring their versatility. This structural diversity emphasizes the significance of immune system proteins in diagnostics and treatments while showcasing their growing relevance in the rapidly expanding immune system proteins market, valued at USD 7.19 billion in 2022 and projected to grow at a CAGR of 5.6% through 2032, with a revenue forecast of USD 11.36 billion by 2030.
As the demand for innovative therapies rises—especially in light of the nearly 1.9 million new cancer cases identified in the U.S. in 2022—the unique characteristics of these components position them as essential tools in the ongoing battle against illnesses, particularly cancer.
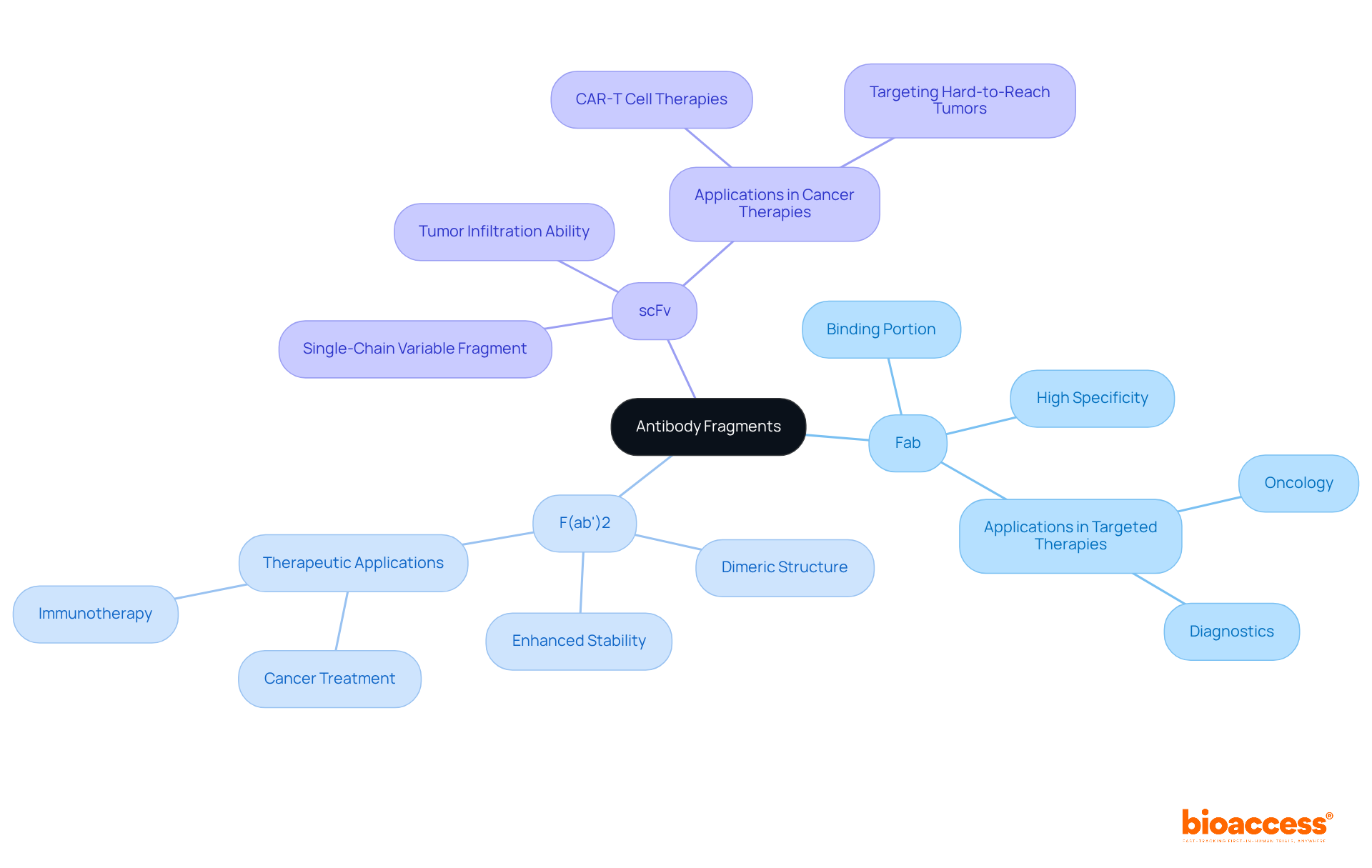
The primary types of antibody fragments are crucial in therapeutic applications, each possessing distinct characteristics that cater to various treatment approaches.
Antibody fragments, specifically Fab fragments, comprise one heavy and one light chain, retaining the antigen-binding site, which makes them highly effective in therapeutic applications. They represented more than 85% of the market for antibody fragments in 2023, propelled by their extensive therapeutic uses in oncology and autoimmune disorders. The antibody fragment Fab segment captured a revenue share of over 85% in 2023, reinforcing its significance in the market.
The scFv fragments are a type of antibody fragment that consist of a single chain combining the variable regions of both heavy and light chains, facilitating easier production and modification. Their compact size enhances tissue penetration, making antibody fragments particularly valuable in cancer immunotherapy. The demand for antibody fragment forms, such as scFv, is swiftly rising, particularly in CAR-T cell applications, reflecting their increasing significance in targeted cancer treatments. The monoclonal antibody segment is forecasted to reach USD 7.5 billion by 2032, indicating a robust market for these therapies.
F(ab')2 portions, which are a type of antibody fragment created through the dimerization of two Fab sections, provide improved stability and binding abilities. This makes them appropriate for applications needing stronger interactions with antigens, particularly in intricate treatment environments where antibody fragments are utilized.
Each category of immune protein segment, such as antibody fragments, supports different treatment approaches, including cancer therapy and the control of autoimmune disorders. Recent advancements in immune protein technology, such as the creation of bispecific immunoglobulins and antibody fragments, are further broadening their uses in precision medicine. However, challenges such as concerns regarding stability in single-chain variable sections and high development costs remain significant. As the worldwide market for these immune protein sections is anticipated to reach around USD 12.96 billion by 2033, incorporating them into cutting-edge treatments is expected to be vital in tackling the increasing prevalence of chronic illnesses, such as cancer.

Antibody fragments offer numerous advantages in therapeutic applications.
Enhanced tissue penetration is achieved due to the smaller size of antibody fragments, which facilitates superior penetration into tissues, making them particularly effective in targeting solid tumors. This capability is crucial in oncology, where reaching tumor cells is essential for treatment efficacy.
Reduced Immunogenicity: Due to their compact structure, antibody fragments are less likely to provoke an immune response, thereby enhancing patient tolerance. This characteristic is vital for maintaining patient safety and comfort during treatment.
Flexible Engineering: Antibody fragments can be easily designed to improve their binding affinity and specificity. This flexibility allows for the development of tailored therapeutic approaches that can be adapted to individual patient needs, increasing the likelihood of successful outcomes.
Cost-Effective Production: The simpler structure of these antibody fragments often leads to reduced manufacturing expenses in comparison to complete antibodies. This cost-effectiveness makes them more accessible for clinical use, particularly in resource-limited settings.
Targeted Therapy Benefits: The distinctive mechanisms of action of antibody fragments allow them to be utilized in targeted treatments, improving their efficacy while protecting healthy cells. This targeted approach is increasingly important in precision medicine, where the goal is to maximize therapeutic benefits while minimizing side effects.

The production of antibody fragments employs several advanced techniques that are pivotal in clinical research:
Enzymatic Digestion: Enzymes such as papain and pepsin are utilized to cleave full-length antibodies into functional fragments, including Fab and F(ab')2. This method is essential for producing specific immune protein formats, such as antibody fragments, that maintain binding capabilities. Insights from biomanufacturing experts emphasize the importance of optimizing these enzymatic processes to enhance yield and functionality.
Recombinant DNA Technology: This method allows the expression of single-chain variable fragments (scFv) and other protein fragments in various host cells, including yeasts and filamentous fungi, facilitating large-scale production. The technology has successfully generated more than ten recombinant proteins authorized for medical application, demonstrating its efficiency in creating treatment agents. However, challenges persist concerning the stability of scFv molecules in comparison to complete antibodies, which can affect the medical uses of antibody fragments.
Cell-Free Systems: Recent advancements in cell-free expression systems have transformed the production of immune protein sections by enabling swift synthesis without the limitations of living cells. These systems can produce functional proteins in as little as 3-5 hours, significantly reducing the time required for traditional methods. This efficiency is especially advantageous for high-throughput applications and the swift advancement of treatment candidates. Furthermore, the possible uses of antibody components extend beyond medical treatments, also being relevant in consumer goods.

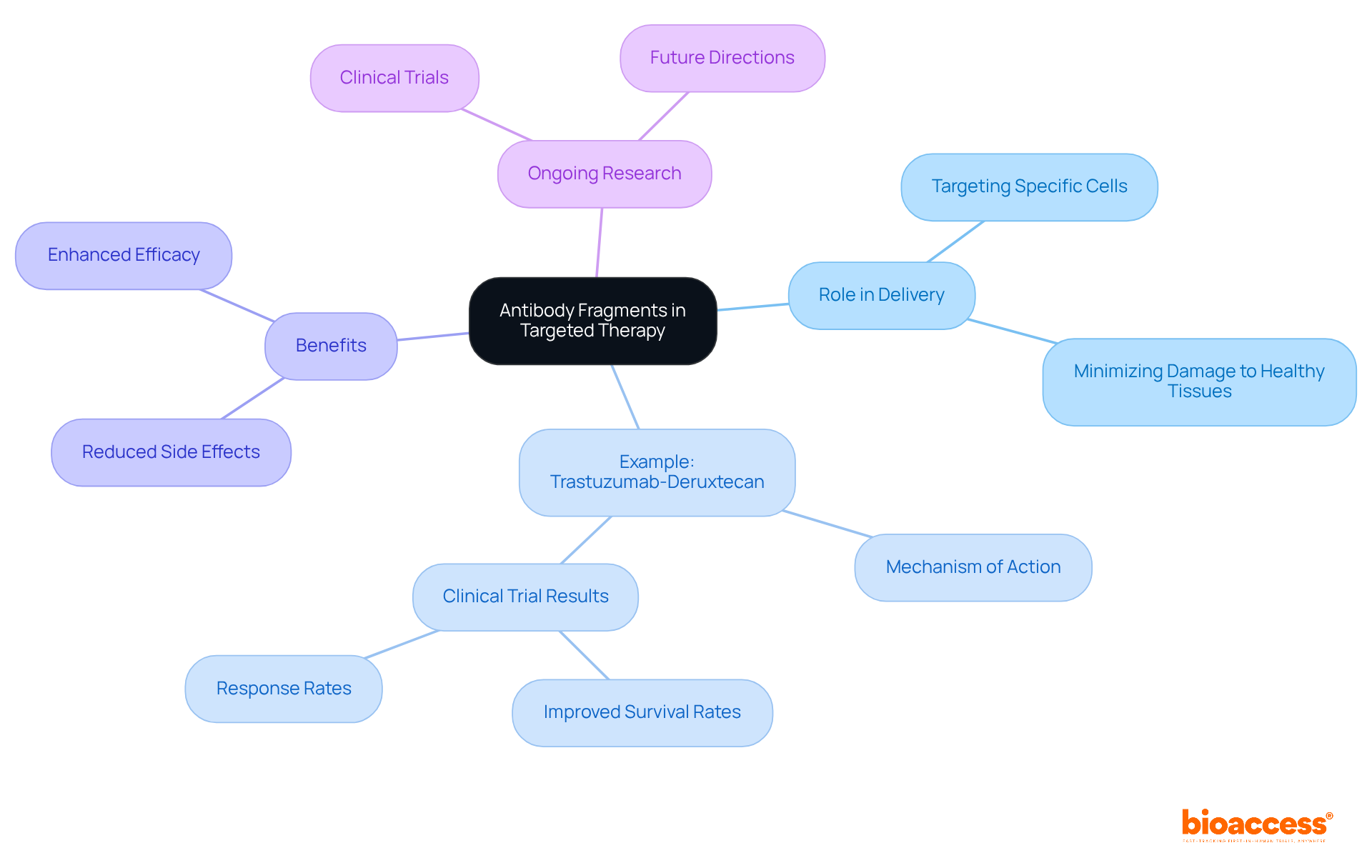
Antibody fragments play a crucial role in targeted treatment by enabling the precise delivery of therapeutic agents to specific cells or tissues. Their ability to bind specifically to antigens on cancer cells facilitates the development of targeted therapies that minimize damage to healthy tissues. This specificity is essential for reducing side effects and enhancing treatment efficacy, particularly concerning drug conjugates (ADCs) that combine the targeting capabilities of protein components with potent cytotoxic agents.
For instance, trastuzumab-deruxtecan exemplifies how ADCs utilize antibody fragments to transport powerful cytotoxic agents directly to HER2-positive tumors, demonstrating improved overall survival rates compared to conventional treatments. Oncology experts have noted that incorporating immune protein sections into ADCs not only enhances the therapeutic index but also diminishes adverse effects, making treatments more tolerable for patients.
Data indicates that therapies leveraging immune protein sections can lead to a significant reduction in side effects, with some studies reporting a decrease in severe adverse events by as much as 30%. Furthermore, ongoing clinical trials are investigating various ADCs targeting specific antigens across different cancer types, underscoring the evolving landscape of cancer treatment.
Overall, the application of antibody fragments in ADCs represents a significant advancement in cancer therapy, offering a more precise and effective approach for targeting malignant cells.
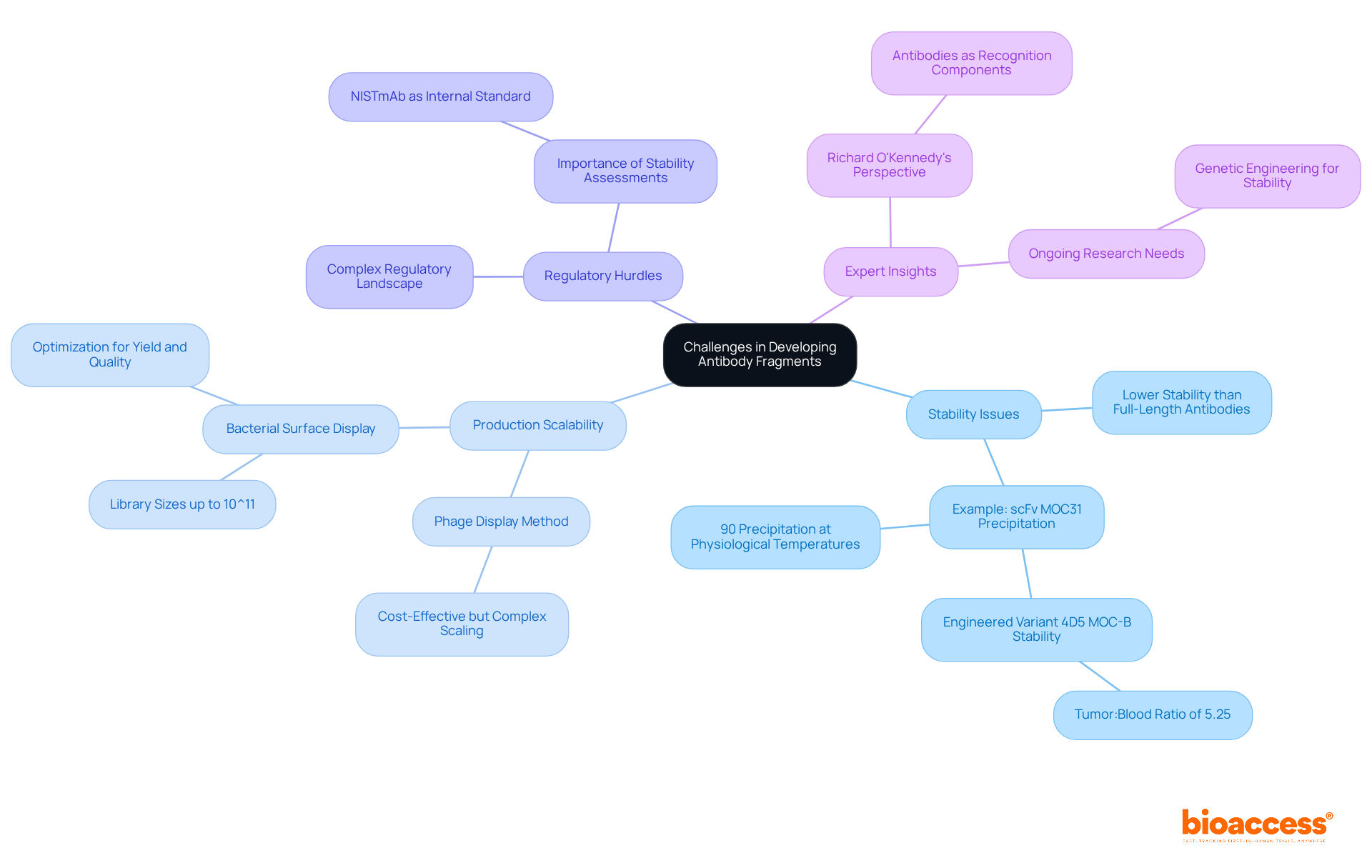
Developing antibody fragments poses several significant challenges that must be addressed, especially in the context of clinical research.
Stability Issues: Antibody fragments frequently demonstrate lower stability than full-length antibodies, which raises concerns about their shelf life and therapeutic efficacy. For instance, studies indicate that approximately 90% of scFv MOC31 precipitated significantly at physiological temperatures, impacting their performance in clinical applications. However, the engineered variant 4D5 MOC-B demonstrated improved thermal stability, achieving a tumor:blood ratio of 5.25 after 24 hours. This underscores the importance of stability in therapeutic effectiveness.
Production Scalability: Although some production methods, such as phage display, are cost-effective and facilitate the generation of large immunoglobulin libraries, scaling these processes for commercial use can be complex. The bacterial surface display technique can accommodate library sizes up to 10^11, yet translating this into a viable production model necessitates careful optimization of conditions to maintain yield and quality.
Regulatory Hurdles: Navigating the regulatory landscape for therapeutic protein therapies requires meticulous planning and adherence to stringent guidelines. This complexity can lead to delays in development timelines, as evidenced by various case studies where stability assessments were crucial for meeting regulatory standards. The incorporation of internal standards, such as NISTmAb, has been shown to enhance the accuracy of stability assessments, thereby facilitating smoother regulatory approval processes.
Expert Insights: Industry specialists emphasize that addressing stability challenges is vital for the successful commercialization of therapeutic proteins. As Richard O'Kennedy noted, "Antibodies are excellent recognition components for use in numerous biosensor-based applications," highlighting the necessity for strong stability profiles to ensure their efficacy in medical contexts. Ongoing research into improving the stability of antibody fragments through genetic engineering and formulation strategies is essential for overcoming these challenges.
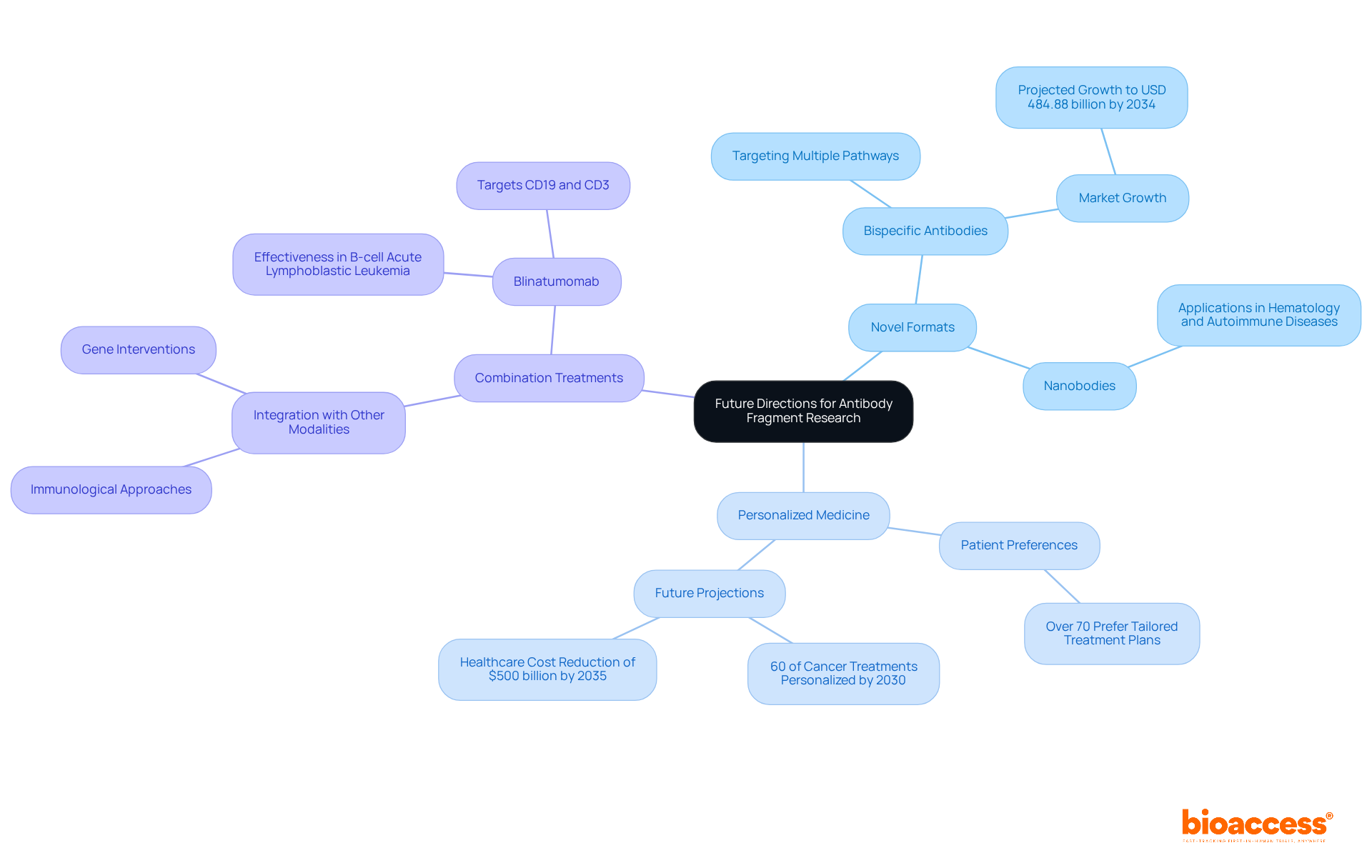
The future of antibody fragment research is promising, with several key directions anticipated:
Novel Formats: The exploration of new antibody fragment formats, including bispecific antibodies and nanobodies, is set to enhance therapeutic options significantly. These formats are gaining traction due to their ability to target multiple pathways, which is particularly beneficial in treating complex diseases like cancer.
Personalized Medicine: Advances in genomics and proteomics are paving the way for more tailored approaches in immune system treatments. With over 70% of patients expressing a preference for tailored treatment plans, the shift towards personalized medicine is not just a trend but a fundamental change in healthcare. By 2030, it is projected that more than 60% of cancer treatments will be personalized, significantly improving patient outcomes. Furthermore, the rise in personalized medicine could help reduce overall healthcare costs by $500 billion globally by 2035.
Combination treatments that involve the integration of antibody fragments with other treatment modalities, such as immunological approaches and gene interventions, are anticipated to enhance results for complex diseases. This synergistic method is especially pertinent in oncology, where bispecific agents such as Blinatumomab, which targets CD19 and CD3 for treating B-cell acute lymphoblastic leukemia, are already showing remarkable effectiveness in targeting multiple tumor-associated antigens. As the bispecific proteins market is expected to increase from USD 12.47 billion in 2024 to USD 484.88 billion by 2034, at an astonishing CAGR of 44.2% from 2025 to 2034, the potential for combination treatments will likely broaden, providing new hope for individuals with difficult conditions.
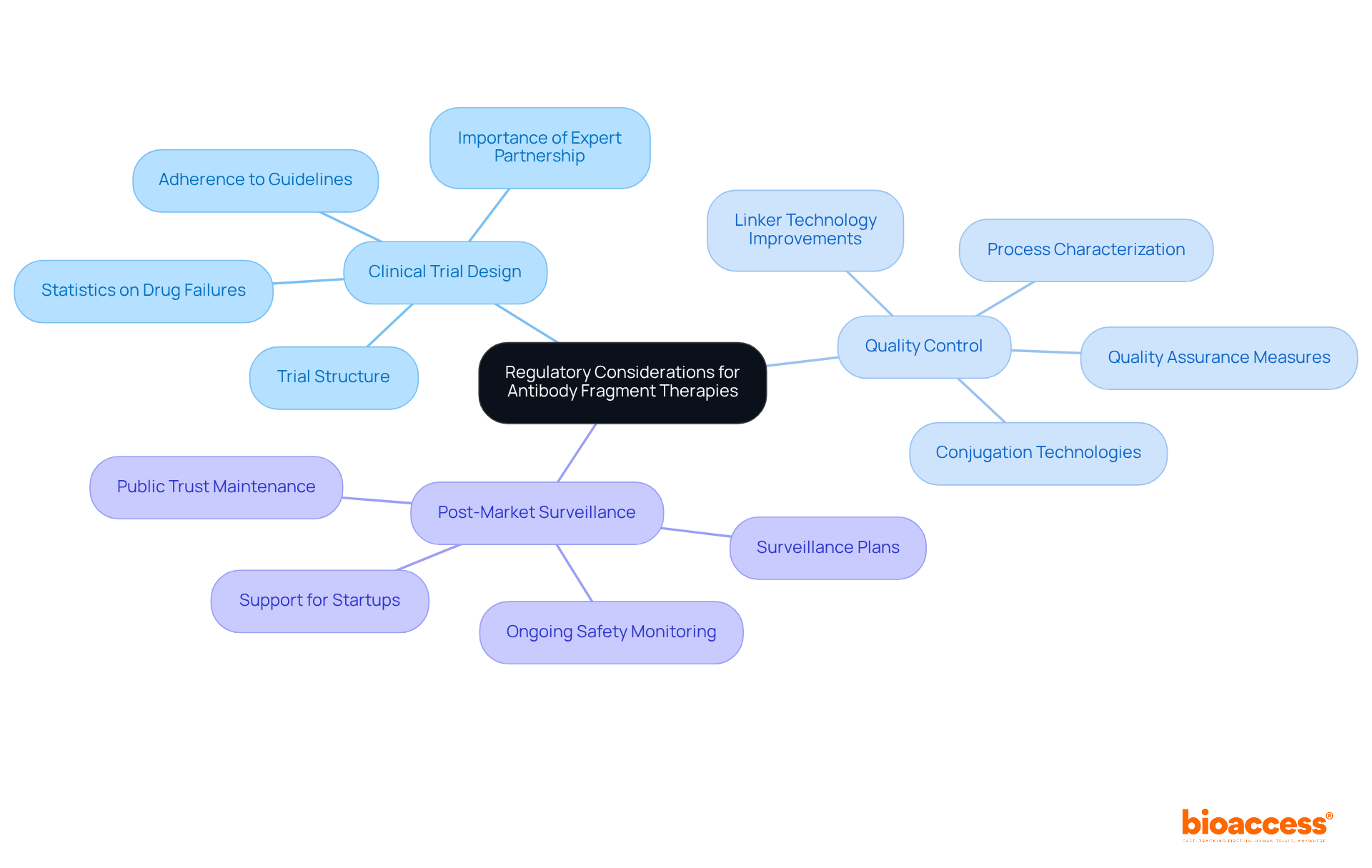
Regulatory considerations for antibody fragment therapies include several critical aspects that require attention.
Clinical Trial Design: Well-structured clinical trials are vital for demonstrating the safety and efficacy of antibody fragment therapies. Adherence to regulatory guidelines is essential, as improper designs can lead to significant quality control failures. Statistics indicate that approximately 9 out of 10 drugs fail during clinical development, underscoring the importance of meticulous trial design to mitigate risks and enhance success rates. Partnering with experts like bioaccess can accelerate this process, ensuring that trials are set up efficiently and in compliance with regulatory requirements, including feasibility and the selection of research sites and principal investigators.
Quality Control: Consistent quality in production processes is paramount for regulatory approval. Strong quality assurance measures must be implemented to ensure that protein segments meet stringent safety and efficacy standards. Advanced process characterization can validate impurity removal, which is crucial for maintaining product integrity. Significantly, contemporary conjugation technologies have enhanced reproducibility, frequently leading to reduced amounts of unconjugated monoclonal proteins, thus improving overall product quality. Moreover, progress in linker technology has aided in enhanced quality control in protein segment production. bioaccess's comprehensive project management services can help maintain these quality standards throughout the trial process.
Post-Market Surveillance: Ongoing observation of treatments derived from antibody fragments after approval is essential to assess long-term safety and efficacy. Regulatory bodies require comprehensive post-market surveillance plans to identify any adverse effects that may arise once the product is in widespread use. This ongoing evaluation is essential for maintaining public trust and ensuring that treatment agents remain safe and effective over time. bioaccess supports startups in developing these plans, addressing potential challenges in the post-market phase.
In the words of regulatory affairs specialists, 'A scientifically grounded approach to clinical trial design is essential for navigating the complexities of therapies involving antibody fragments and ensuring compliance with evolving regulatory expectations.' This emphasizes the necessity for a proactive approach in clinical research to tackle potential challenges and enhance treatment results.
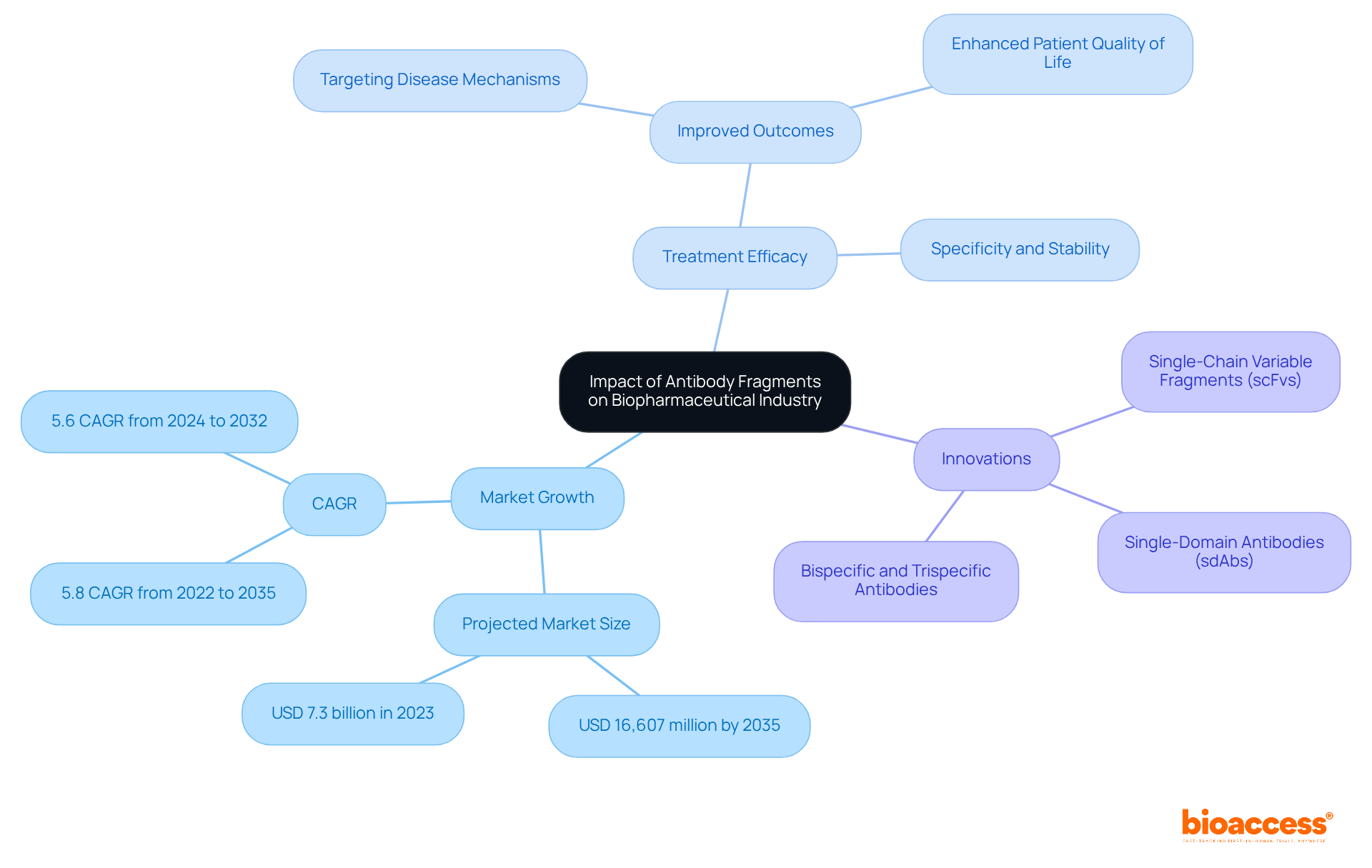
Antibody fragments are significantly reshaping the biopharmaceutical industry by fostering innovation, market growth, and improving treatment efficacy. Their unique properties catalyze the development of novel therapeutics that effectively address unmet medical needs, particularly in oncology and autoimmune diseases. The introduction of next-generation immune system proteins, such as single-chain variable units (scFvs) and single-domain immunoglobulins (sdAbs), exemplifies this trend, providing improved stability and specificity due to their low molecular weight and heterologous production.
The market for therapeutic proteins is projected to reach USD 16,607 million by 2035, expanding at a compound annual growth rate (CAGR) of 5.8%. This growth is driven by the increasing need for focused treatments and advancements in production technologies that enhance the development process. By enabling more accurate targeting of disease mechanisms, protein segments are improving treatment outcomes and enhancing patient quality of life. Their capacity to attach uniquely to antigens enables more efficient treatments, especially in cancer care, where the market for immune protein sections is anticipated to experience the quickest expansion due to the rising occurrence of cancer globally. Approximately 1.9 million new cancer diagnoses were reported in the U.S. in 2022. This shift is reshaping the therapeutic landscape, establishing antibody fragment as a cornerstone of modern biopharmaceutical innovation.
Antibody fragments are revolutionizing clinical research and therapeutic applications, showcasing their critical role in the advancement of modern medicine. Their unique structural characteristics and advantages, including enhanced tissue penetration and reduced immunogenicity, position them as essential components in targeted therapies, particularly in oncology. As the biopharmaceutical industry evolves, the importance of antibody fragments in developing innovative treatments cannot be overstated.
This article highlights key insights into the following aspects of antibody fragments:
With projected market growth and increasing demand for personalized medicine, these fragments are not only improving treatment efficacy but are also driving advancements in therapeutic strategies. Collaboration with organizations like bioaccess® emphasizes the importance of efficient clinical research processes that facilitate the rapid development of these vital therapeutic agents.
Looking ahead, the future of antibody fragment research appears promising, with emerging trends pointing towards novel formats and combination therapies that could enhance treatment outcomes for complex diseases. As the industry embraces these advancements, stakeholders are encouraged to prioritize the integration of antibody fragments into therapeutic development, ensuring that innovative solutions continue to meet the pressing healthcare needs of patients worldwide.
What is bioaccess® and what role does it play in clinical research?
bioaccess® is an organization that accelerates clinical research for antibody fragments by utilizing its expertise across Latin America, the Balkans, and Australia. It offers significant advantages such as securing ethical approvals in 4-6 weeks and faster enrollment processes, which are 50% quicker than traditional markets.
How does bioaccess® enhance the efficiency of clinical trials?
bioaccess® enhances clinical trial efficiency by providing end-to-end acceleration for global trials, activating over 50 pre-qualified sites in under 8 weeks, and ensuring that datasets are FDA/EMA/MDR-ready with centralized monitoring.
What are antibody fragments?
Antibody fragments are specialized, smaller components of immunoglobulins that can bind to specific antigens. They are typically produced through enzymatic cleavage or genetic engineering of full-length antibodies and include types such as Fab, F(ab')2, and single-chain variable sections (scFv).
What are the different types of antibody fragments and their characteristics?
The primary types of antibody fragments include: - Fab: Comprising one heavy and one light chain, effective in therapeutic applications, especially in oncology. - scFv: A single chain that combines the variable regions of heavy and light chains, allowing for easier production and better tissue penetration. - F(ab')2: Created by dimerizing two Fab sections, providing improved stability and binding abilities.
What is the market significance of antibody fragments?
Antibody fragments, particularly Fab fragments, represented over 85% of the market in 2023 due to their extensive therapeutic uses. The overall market for immune system proteins is valued at USD 7.19 billion in 2022, projected to grow at a CAGR of 5.6%, reaching USD 11.36 billion by 2030.
What therapeutic applications do antibody fragments have?
Antibody fragments are increasingly used in targeted therapies, particularly in oncology, for conditions such as cancer and autoimmune disorders. Their unique characteristics make them valuable tools in the development of innovative therapies.
What challenges do antibody fragments face in development?
Challenges include concerns regarding the stability of single-chain variable sections and high development costs, which can impact the broader adoption and application of these therapeutic tools.
What is the future outlook for the antibody fragment market?
The global market for antibody fragments is anticipated to reach around USD 12.96 billion by 2033, indicating a growing demand for these components in advanced treatments for chronic illnesses, particularly cancer.