


The primary focus of the article titled "10 Key Insights on PK Analysis for Clinical Research Success" is to emphasize critical insights that enhance the effectiveness of pharmacokinetic (PK) analysis within clinical research. It is clear that robust PK analysis plays a vital role in optimizing drug development. This analysis informs dosing strategies, predicts patient responses, and ultimately increases the success rates of clinical trials, especially when advanced statistical techniques and real-time data adjustments are integrated. By understanding these insights, clinical researchers can navigate the complexities of drug development more effectively.
Pharmacokinetic (PK) analysis plays a pivotal role in the success of clinical research, acting as a cornerstone for understanding how drugs behave within the body. This intricate field not only informs dosing strategies and treatment efficacy but also shapes the regulatory landscape that governs drug approval processes. As the demand for innovative therapies grows, the challenge lies in effectively integrating PK insights into clinical trials to ensure robust outcomes.
What are the key factors that can enhance the effectiveness of PK analysis in clinical research? How can stakeholders leverage these insights to navigate the complexities of drug development? These questions underscore the critical need for a comprehensive approach to PK analysis that not only addresses current challenges but also anticipates future developments in the Medtech landscape.
By focusing on collaboration and the strategic use of PK data, stakeholders can significantly improve their clinical trial outcomes, ultimately leading to more successful drug development initiatives.
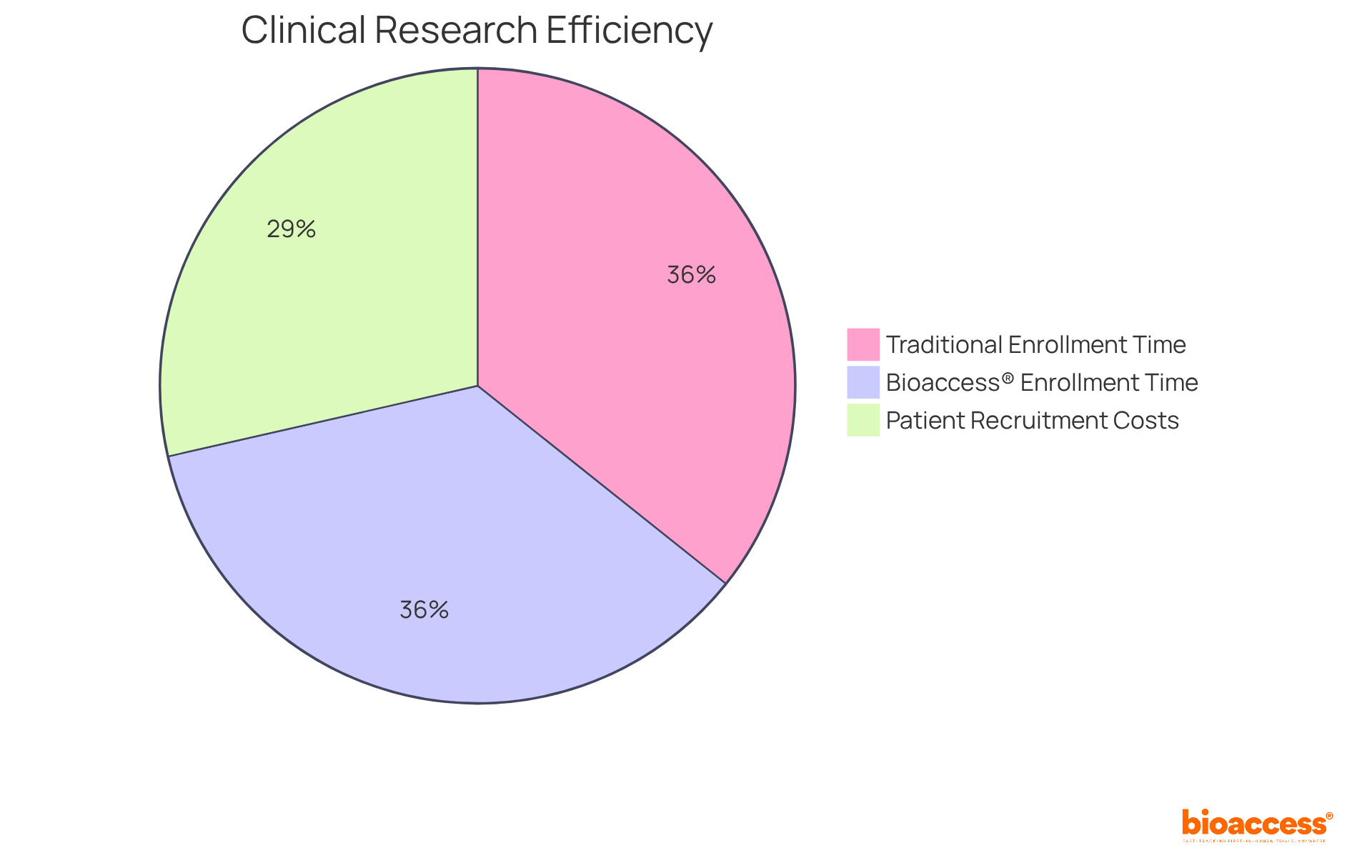
bioaccess® leverages the regulatory efficiency of Latin America, enhanced by the diverse patient demographics in the Balkans and Australia’s streamlined ethical approval procedures, to provide research services that are both effective and compliant. This agility facilitates ethical approvals within an impressive 4-6 weeks and accelerates enrollment by 50% compared to traditional markets. Such efficiency is crucial for Medtech, Biopharma, and Radiopharma innovators striving for rapid advancements in research studies, particularly those centered around pk analysis.
With over 70% of the population in Latin America living two hours or more from an academic medical center, the region presents a unique opportunity for distributed studies, thereby improving patient access and involvement. Furthermore, the average cost of patient recruitment, which constitutes approximately 40% of the overall research budget, can be significantly reduced in Latin America due to lower operational costs when compared to North America and Europe. As the demand for clinical trials continues to rise, especially in light of the anticipated increase in cancer cases in the region, bioaccess® emerges as a strategic partner for organizations seeking to navigate the complexities of clinical research efficiently.
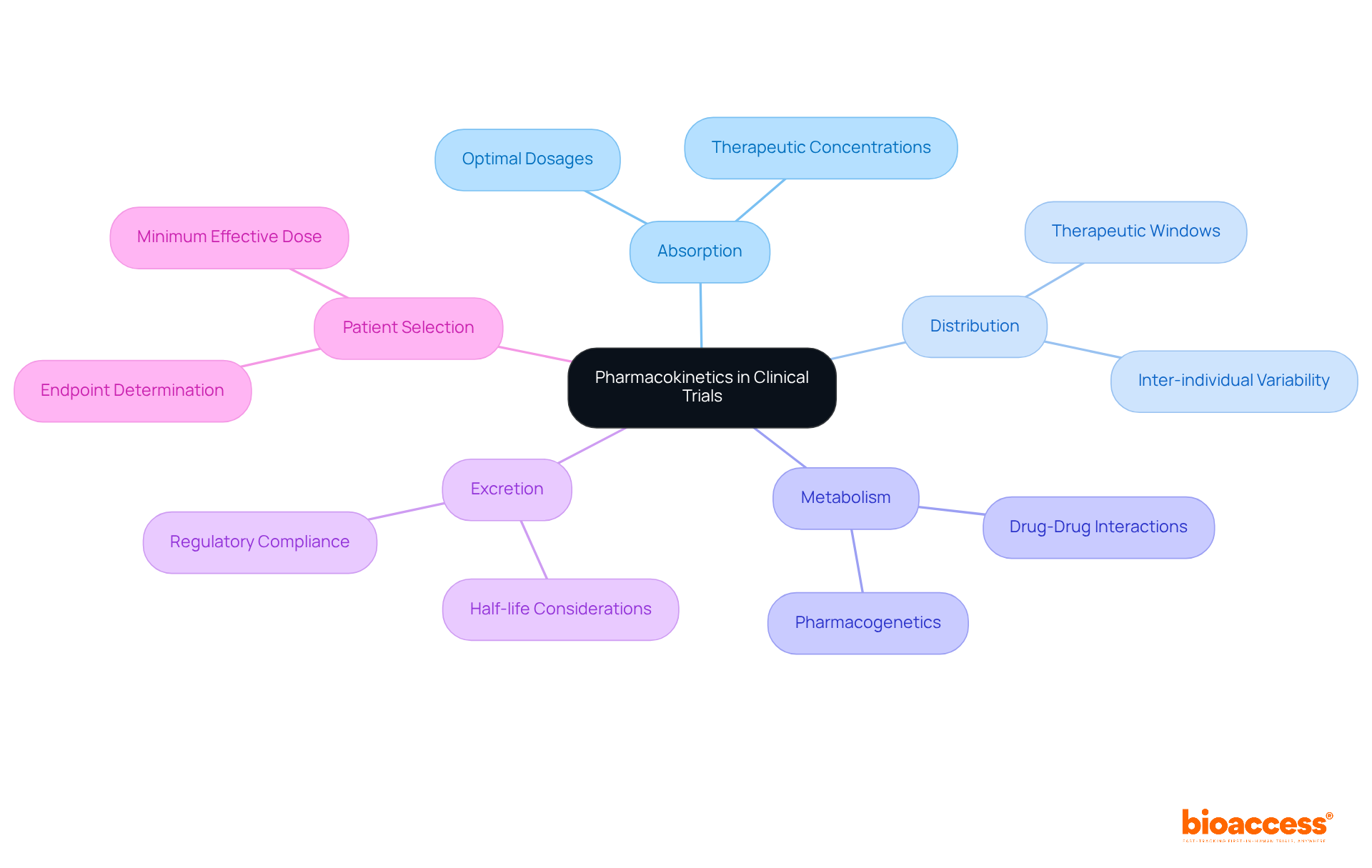
Pharmacokinetics (PK) encompasses the essential processes of substance absorption, distribution, metabolism, and excretion (ADME) within the body. Mastery of these processes is crucial for establishing optimal dosages and administration schedules, which directly influence the success of pharmaceutical development. Effective PK analysis empowers researchers to predict how various populations will respond to medications, which is a necessity for designing studies that yield dependable and relevant outcomes.
Experts assert that robust PK studies are indispensable in early-phase clinical research, particularly in critical fields such as oncology and pain management. For example, drugs demonstrating favorable exposure ratios—ideally between 50- to 100-fold—are favored for their broader therapeutic windows, thereby minimizing the risk of adverse effects. Furthermore, insights derived from PK data significantly shape the design of subsequent-phase studies, guiding patient selection and endpoint determination based on the minimum effective dose. As highlighted by Zenovel, "PK data informs the design of later-phase trials, including patient selection and endpoint determination."
Moreover, the SGS clinical pharmacology team underscores that a comprehensive understanding of the pharmacological characteristics of a compound is vital for accurately designing PK analysis. As the pharmaceutical development landscape evolves, the integration of advanced analytical techniques and adherence to regulatory guidelines will enhance the reliability and efficacy of PK analysis, ultimately resulting in improved patient outcomes. However, challenges in PK studies, such as optimizing sampling schedules and managing inter-individual variability, must also be addressed to ensure robust data collection and analysis.
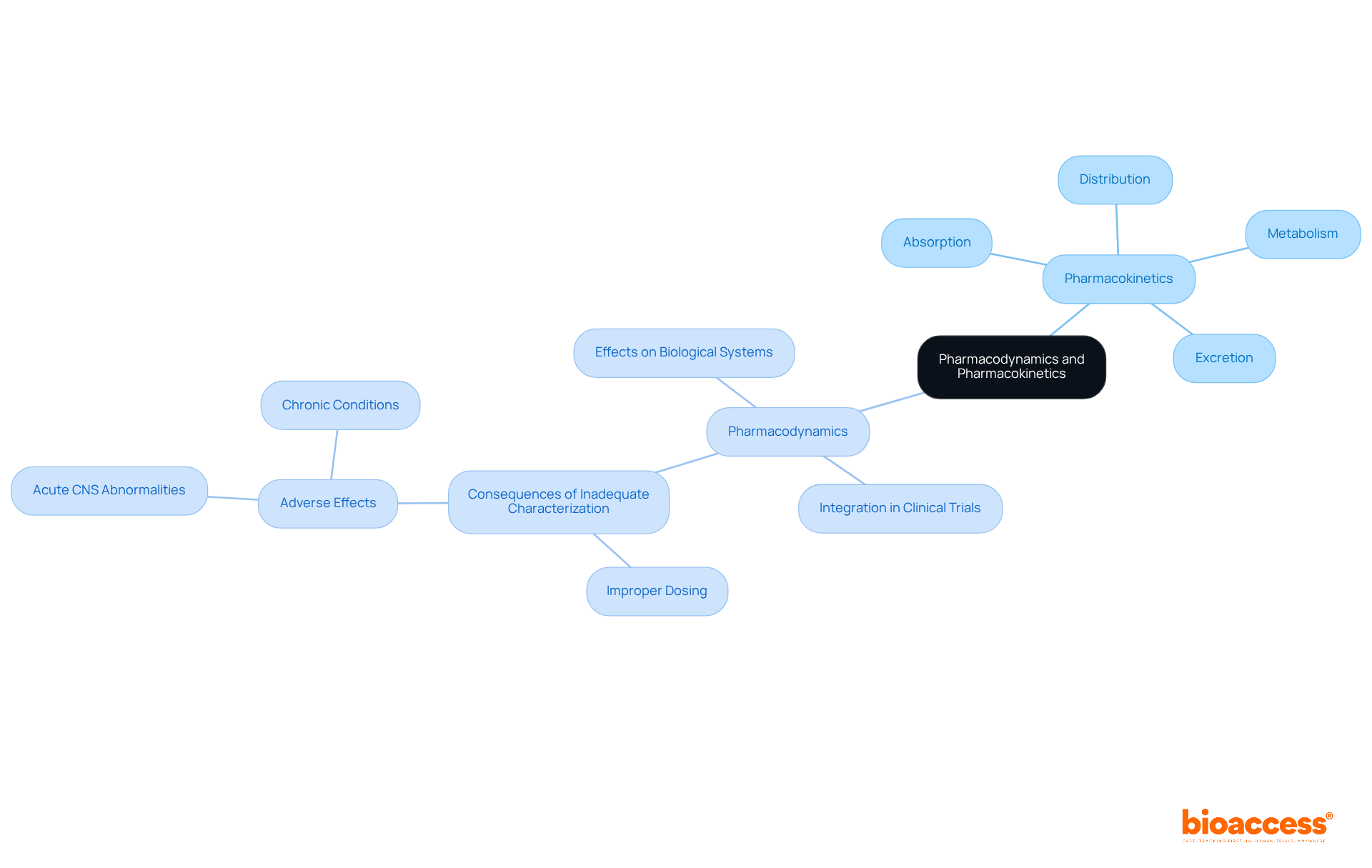
Pharmacokinetics (PK) and pharmacodynamics (PD) represent two interconnected domains that collectively provide a comprehensive understanding of drug action. PK focuses on the journey of a compound through the body—encompassing absorption, distribution, metabolism, and excretion—while PD examines the effects of the substance on biological systems. This synergy is crucial for determining not only the appropriate dosage but also the therapeutic efficacy and safety profile of treatments.
Recent research emphasizes the importance of integrating PK analysis and PD analyses within clinical trials. Understanding PD endpoints allows researchers to delineate the onset, extent, and duration of a substance's effects, which cannot be fully captured by PK parameters alone. As noted by experts in the field, 'Pharmacodynamics is a crucial component of the development puzzle when it comes to understanding exposure-response relationships, optimal dosing, and confirming safety and efficacy.'
The successful integration of PK analysis and PD in clinical trials enhances the predictive accuracy of interactions and therapeutic outcomes. Utilizing physiologically based pharmacokinetic (PBPK) models can simulate realistic scenarios that incorporate both PK and PD factors, leading to more informed dosing regimens. This approach is particularly vital in the context of polypharmacy, where multiple substances may interact, necessitating a thorough understanding of both pharmacokinetic and pharmacodynamic processes.
Furthermore, recent findings indicate that inadequate characterization of a medication's PD can result in improper dosing, leading to adverse effects that range from acute central nervous system abnormalities to chronic conditions. Thus, the relationship between PK analysis and PD transcends mere academic interest; it is a critical consideration in the design and execution of clinical trials aimed at developing safe and effective therapies.
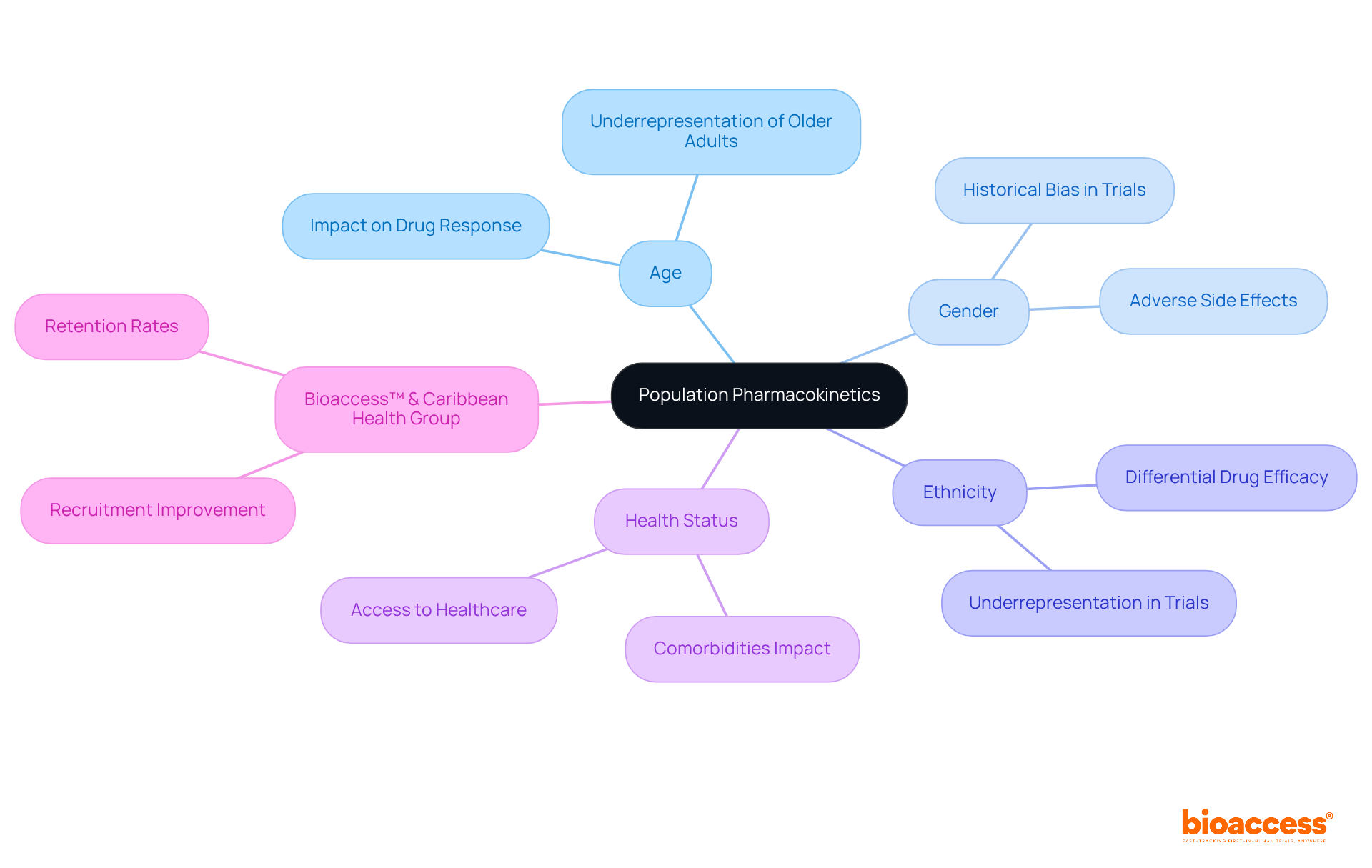
Pk analysis investigates how demographic factors—such as age, gender, ethnicity, and health status—impact medication behavior across diverse populations. This pk analysis enhances researchers' ability to forecast medication performance in varied groups, ultimately leading to more effective and safer treatments. This approach is particularly crucial in regions like Latin America, where the rich diversity of patient populations significantly influences research outcomes. Grasping these dynamics facilitates successful drug development through pk analysis tailored to the unique characteristics of various demographic segments, ensuring that treatments are both effective and equitable.
The partnership between bioaccess™ and Caribbean Health Group to enhance research services in Barranquilla exemplifies a commitment to improving recruitment and retention rates, achieving over a 50% reduction in recruitment time and 95% retention rates. This initiative has garnered strong support from Colombia's Minister of Health, underscoring its significance. To implement these insights effectively, clinical research directors must prioritize diversity in trial design and actively seek to include underrepresented groups. This effort not only contributes to economic growth but also enhances healthcare improvement in the region.
Regulatory agencies, such as INVIMA (Colombia National Food and Drug Surveillance Institute), play a pivotal role in the assessment of new medications, necessitating extensive PK analysis to evaluate safety and effectiveness. Established in 1992 under Colombia's Ministry of Health and Social Protection, INVIMA is responsible for overseeing the marketing and manufacturing of health products, ensuring compliance with health standards. This regulatory authority is integral to the approval process for medical devices and pharmaceuticals, as it meticulously monitors and controls pre- and post-market activities, which include PK analysis in pharmacokinetic studies.
The classification of INVIMA as a Level 4 health authority by the Pan American Health Organization/World Health Organization reinforces its credibility in safeguarding the safety, efficacy, and quality of medicines. PK analysis is crucial for elucidating a substance's behavior within the body, which includes its absorption, distribution, metabolism, and excretion (ADME). This information is vital for shaping dosing recommendations and identifying potential side effects, ultimately influencing the success of drugs during regulatory reviews.
Medications with well-documented PK analysis profiles are significantly more likely to navigate regulatory reviews successfully, as they provide essential evidence to substantiate claims of safety and efficacy. The approval success rate for these medications is markedly higher, underscoring the critical nature of pharmacokinetic studies in the medication development process. Furthermore, regulatory agencies highlight that comprehensive PK analysis can expedite the approval process, facilitating quicker access to innovative therapies for patients.
Additionally, adherence to rigorous ethical standards in PK analysis is imperative, ensuring that all research is conducted with responsibility and transparency. This commitment to ethical practices not only enhances the credibility of the research but also fosters trust among stakeholders in the clinical research landscape.

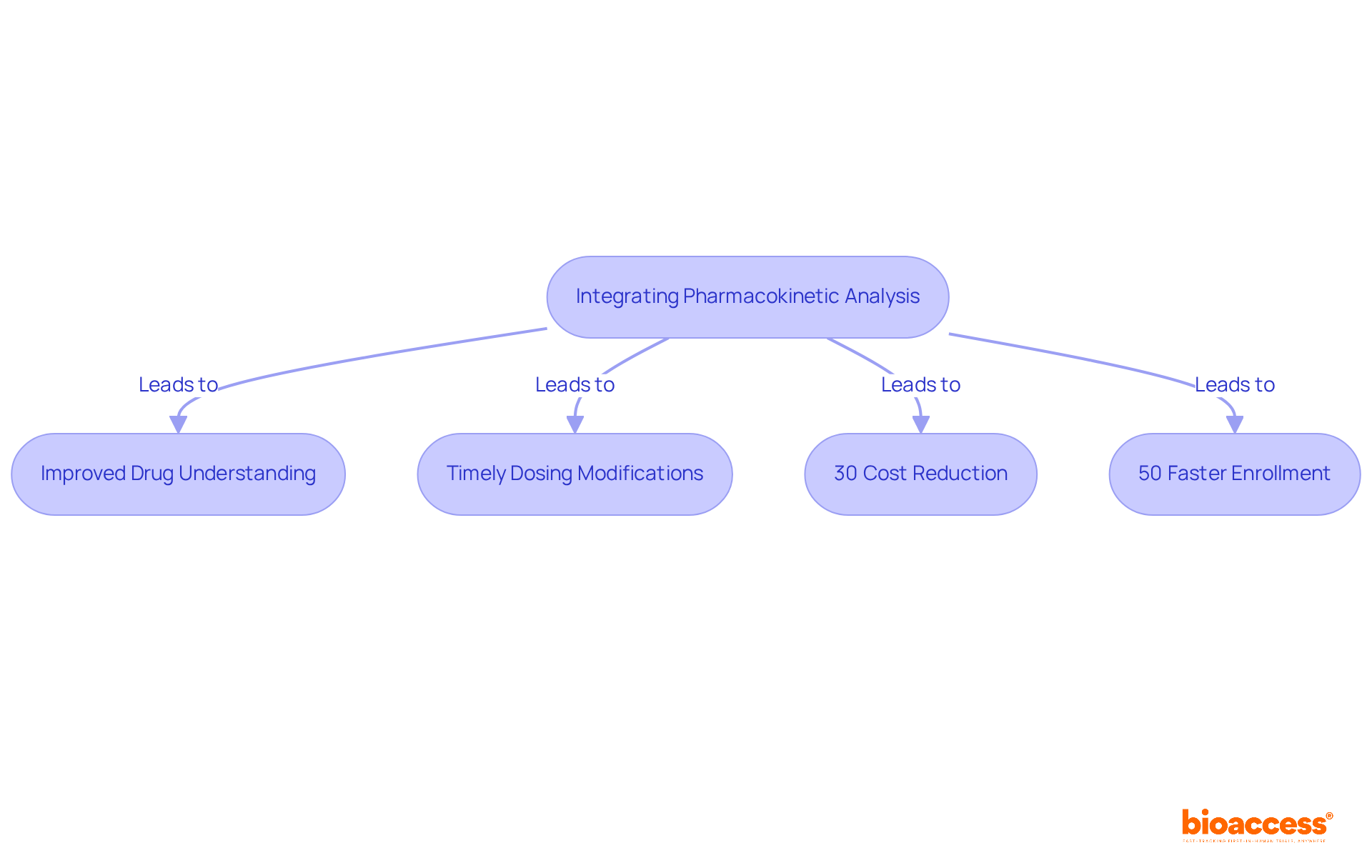
In early-stage clinical studies, incorporating pk analysis is essential for comprehending how a drug is absorbed, distributed, metabolized, and expelled by the body. This proactive method enables researchers to gather crucial information from the outset, facilitating timely modifications to dosing schedules and study designs based on real-time insights. Such flexibility significantly enhances the likelihood of success in later phases.
Recent discoveries underscore the advantages of real-time pk analysis, suggesting that studies employing this data can achieve success rates significantly higher than those relying solely on conventional approaches. For instance, studies indicate that incorporating pk analysis can lead to a 30% reduction in costs and a 50% faster enrollment process, particularly in regions like Colombia, where regulatory approvals are secured in just 4-6 weeks.
Experts in the field emphasize the transformative impact of real-time pk analysis on study outcomes. As one specialist remarked, 'The capacity to modify experimental parameters based on pharmacokinetic information not only simplifies the research process but also ensures that we are making informed decisions that can lead to improved therapeutic outcomes.' Successful early-phase clinical trials, such as those conducted by bioaccess®, demonstrate that utilizing real-time pk analysis can greatly enhance the efficiency and effectiveness of clinical research, ultimately accelerating the journey to market for innovative therapies.
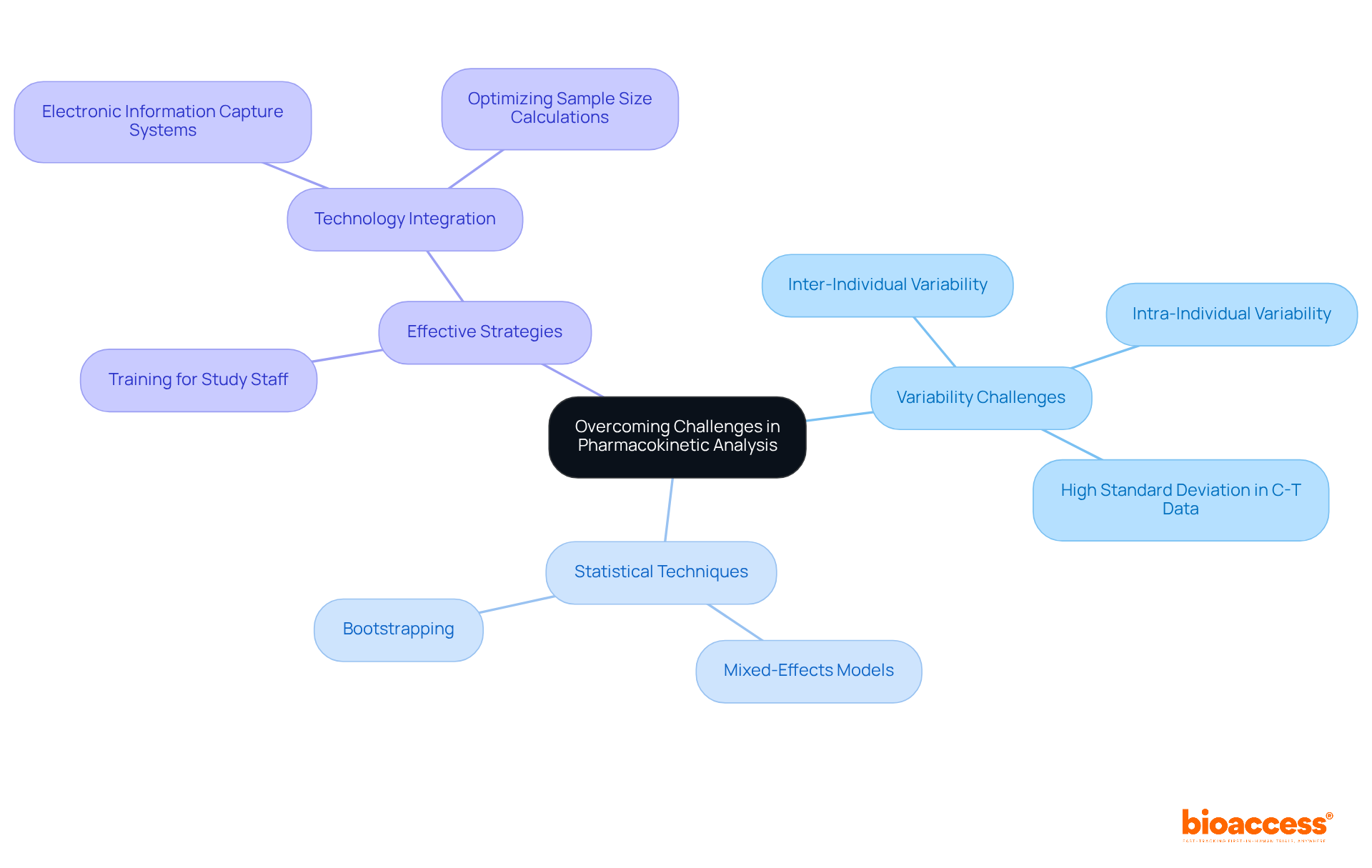
Variability in patient responses presents significant challenges in PK analysis, complicating the interpretation and modeling of pharmacokinetic parameters. To effectively manage this variability, researchers are increasingly employing advanced statistical techniques, such as mixed-effects models and bootstrapping, which enhance the robustness of analysis and improve the reliability of outcomes. These techniques facilitate a more nuanced understanding of inter- and intra-individual variability, which is essential for ensuring the integrity of information.
Moreover, effective strategies for addressing variability include:
For instance, the use of electronic information capture systems can minimize human errors and enhance the precision of gathered data. Additionally, optimizing sample size calculations based on intrasubject variability can significantly boost the power of studies, particularly in first-in-man trials where participant numbers are often constrained.
Recent research underscores the importance of minimizing standard deviation (SD) in concentration-time (C-T) data, which can lead to a clearer pharmacokinetic profile. By transforming C-T data, studies have shown more than a twofold reduction in SD, enabling better assessment of substance absorption and distribution phases. This approach not only enhances the quality of PK analysis but also assists in designing future bioequivalence trials, ensuring that the observed differences in PK parameters are statistically significant instead of being due to random variation.
As emphasized by specialists in the field, addressing variability in PK studies is critical for advancing therapeutic development and ensuring patient safety. By implementing these strategies and approaches, researchers can enhance the integrity of PK analysis, ultimately resulting in more reliable health outcomes.
Interpreting pk analysis is essential for informed healthcare decisions, demanding a comprehensive understanding of the principles governing substance absorption, distribution, metabolism, and elimination. The precise interpretation of PK analysis information directly influences dosing strategies, treatment duration, and the management of potential drug interactions. For example, the elimination half-life of midazolam can significantly vary based on the presence of inducers, showing a notable reduction from 2.16 hours to 1.47 hours. This underscores the necessity for precise dosing adjustments in clinical practice.
Clinicians who adeptly utilize pharmacokinetic information can tailor treatment plans, thereby enhancing therapeutic efficacy and minimizing adverse effects. Successful applications of pk analysis in personalized medicine have demonstrated that customized dosing regimens can lead to improved patient outcomes, particularly in critically ill populations where pharmacokinetic variability is pronounced. Population pharmacokinetic models, for instance, have proven their capability to optimize meropenem dosing, ensuring that substance concentrations remain above the minimum inhibitory concentration (MIC) required for effective treatment. A study highlighted that a significant percentage of patients required dose modifications based on estimated concentrations, illustrating the value of therapeutic medication monitoring (TDM) in managing antibiotic dosing.
Moreover, the impact of PK information on healthcare decision-making extends into pharmaceutical development, where precise interpretation is crucial for regulatory compliance and successful market entry. Recent studies emphasize that integrating robust PK analysis into drug development processes can significantly reduce the risks of underdosing or overdosing, ultimately enhancing patient safety and treatment effectiveness. In fact, companies can lose approximately $5.2 million in earnings due to unutilized information, underscoring the financial implications of inefficiently leveraging PK data in healthcare environments. Additionally, the dropout rate is often established at 20% in pharmacokinetic studies, presenting challenges in the reliability of information. As we approach 2025, the emphasis on the interpretation of precise PK analysis information is set to increase, further solidifying its importance in influencing clinical practices and fostering therapeutic innovations. As Hilary Mason aptly states, at the core of data analytics lies curiosity and learning—essential elements for understanding and applying PK data effectively.

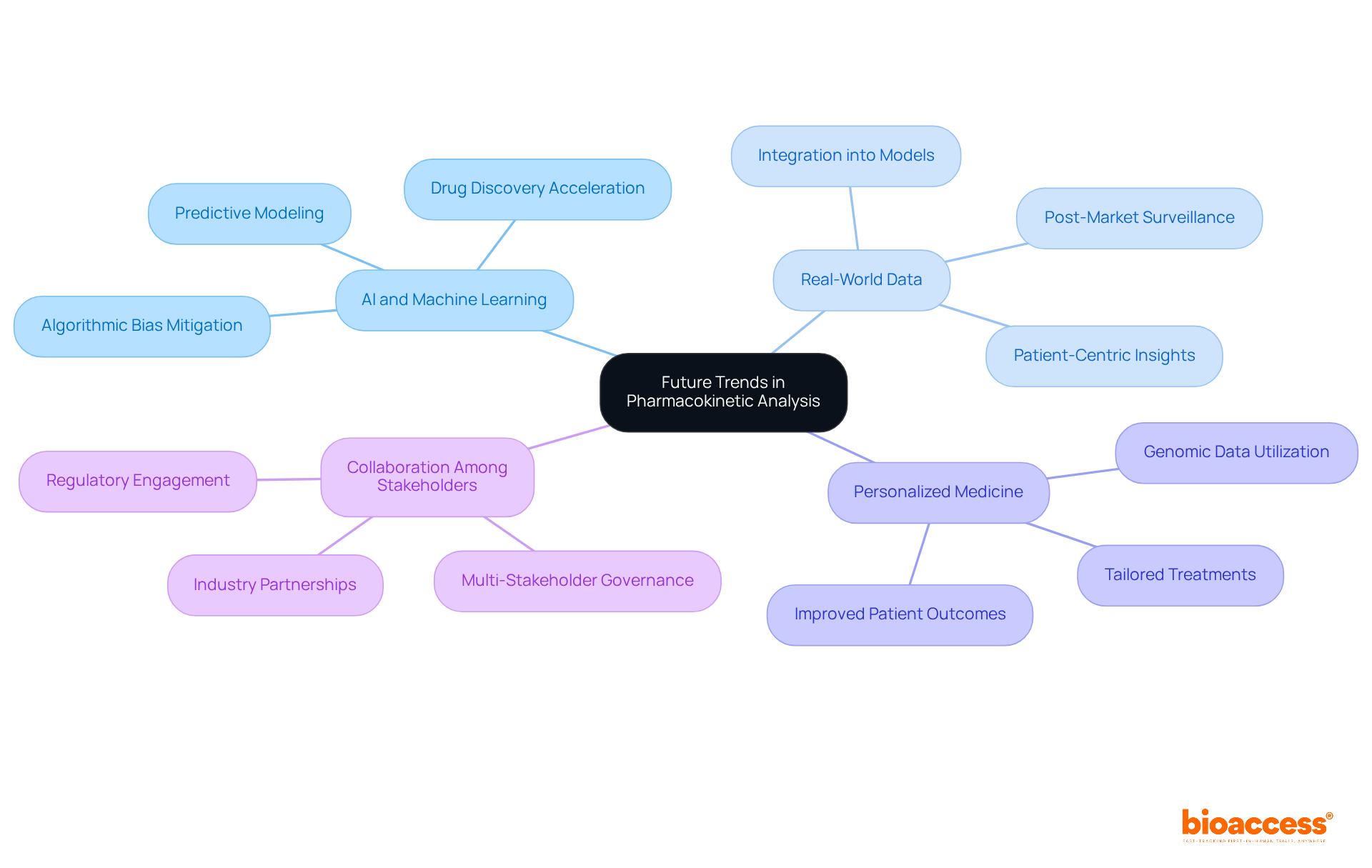
Future trends in pharmacokinetic analysis are poised to transform the landscape of clinical research, particularly through the increasing integration of artificial intelligence and machine learning to predict drug behavior. This focus resonates with the innovative approach championed by Dr. Sergio Alvarado, Clinical Trial Manager at bioaccess, whose commitment to cutting-edge technology exemplifies a broader industry trend. Moreover, the incorporation of real-world data into pharmacokinetic models, combined with an emphasis on personalized medicine, is expected to significantly enhance the accuracy of pk analysis.
These advancements are likely to catalyze more effective drug development and improve patient outcomes, underscoring bioaccess's dedication to regulatory excellence and innovation within the Latin American medical framework. As the industry evolves, collaboration among stakeholders will be crucial to navigating the complexities of clinical trials and ensuring that these innovative methodologies are effectively implemented.
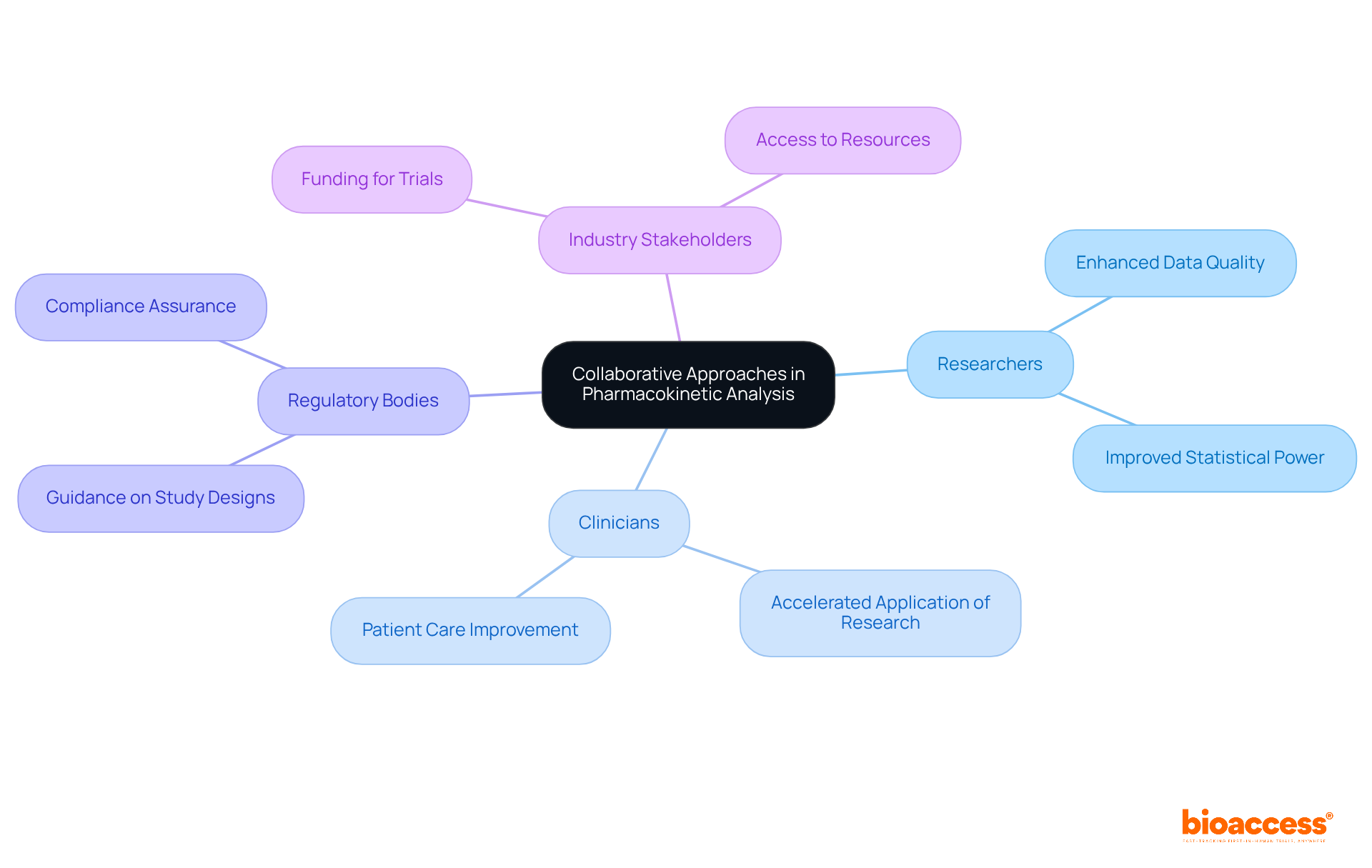
Collaboration among researchers, clinicians, regulatory bodies, and industry stakeholders is vital for successful PK analysis. By sharing information, insights, and resources, these groups enhance the quality of PK analysis, ensuring findings are relevant across diverse populations. A significant instance is the partnership between bioaccess™ and Caribbean Health Group, which seeks to establish Barranquilla as a premier location for medical studies in Latin America, backed by Colombia's Minister of Health. Such cooperative methods not only enhance the strength of data but also aid in the application of research discoveries to medical practice.
For example, research has demonstrated that successful collaborations can result in enhanced statistical power, aiming for 80% or greater in medical studies. Moreover, the incorporation of regulatory insights frequently leads to more efficient study designs, as demonstrated by the FDA's guidance on enrichment strategies for research. Furthermore, bioaccess™ provides extensive services including feasibility studies, compliance reviews, setup, and project management, which are essential for enhancing outcomes. The collaboration between GlobalCare Clinical Studies and bioaccess™ has improved ambulatory services for research in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates.
As one researcher noted, "Collaboration between industry and academics is common in the development of vaccines, drugs, and devices, as it can be mutually beneficial." This synergy not only enhances the credibility of the research but also accelerates the path from discovery to application, ultimately benefiting patient care. Moreover, it is noteworthy that 21% of published trials were solely funded by industry over the past 20 years, highlighting the significant role of industry partnerships in clinical research. However, while these collaborations offer substantial benefits, they may also lead to concerns regarding academic freedom, as discussed in the case study on the impact of industry funding on academic independence.
The exploration of pharmacokinetic (PK) analysis reveals its pivotal role in enhancing the success of clinical research. By understanding the absorption, distribution, metabolism, and excretion of drugs, researchers can tailor studies to yield reliable and relevant outcomes. The integration of PK with pharmacodynamics (PD) further enriches this understanding, ensuring that dosing strategies are optimized for diverse populations, ultimately leading to safer and more effective therapies.
Key insights from the article underscore the importance of robust PK studies in early-phase clinical trials, the necessity of addressing patient variability, and the value of collaborative approaches in research. The strategic partnership between organizations like bioaccess® and Caribbean Health Group demonstrates how leveraging diverse patient demographics can significantly improve patient recruitment and retention, while also expediting regulatory approvals. Moreover, the incorporation of advanced technologies and real-world data is set to revolutionize PK analysis, enhancing the predictive accuracy of drug behavior and therapeutic outcomes.
In light of these insights, it is crucial for stakeholders in the clinical research field to prioritize the integration of pharmacokinetic analysis in their studies. As the landscape of drug development continues to evolve, embracing collaborative efforts and innovative methodologies will be essential for overcoming challenges and ensuring that new therapies reach patients in a timely and efficient manner. The future of pharmacokinetic analysis holds great promise, and a commitment to these principles will ultimately lead to improved patient care and successful clinical outcomes.
What is bioaccess® and how does it contribute to clinical research?
bioaccess® is a research service provider that leverages the regulatory efficiency of Latin America, diverse patient demographics in the Balkans, and streamlined ethical approval procedures in Australia. It facilitates ethical approvals within 4-6 weeks and accelerates patient enrollment by 50%, making it an effective partner for Medtech, Biopharma, and Radiopharma innovators.
Why is Latin America a significant region for clinical trials?
Over 70% of the population in Latin America lives two hours or more from an academic medical center, presenting opportunities for distributed studies that enhance patient access and involvement. Additionally, the lower operational costs in Latin America can significantly reduce the average cost of patient recruitment, which is about 40% of the overall research budget.
What is pharmacokinetics (PK) and why is it important in clinical trials?
Pharmacokinetics (PK) involves the processes of absorption, distribution, metabolism, and excretion (ADME) of substances within the body. Mastery of PK is crucial for establishing optimal dosages and administration schedules, which directly impacts the success of pharmaceutical development and the design of reliable studies.
How do PK studies influence early-phase clinical research?
Robust PK studies are essential in early-phase clinical research, especially in fields like oncology and pain management. They help predict how different populations will respond to medications and inform the design of later-phase studies, including patient selection and endpoint determination based on the minimum effective dose.
What role does pharmacodynamics (PD) play in clinical trials?
Pharmacodynamics (PD) examines the effects of a substance on biological systems, complementing PK. Understanding PD endpoints helps researchers determine the onset, extent, and duration of a substance's effects, which is crucial for establishing therapeutic efficacy and safety profiles.
How can integrating PK and PD analyses improve clinical trial outcomes?
Integrating PK and PD analyses enhances the predictive accuracy of interactions and therapeutic outcomes. Utilizing physiologically based pharmacokinetic (PBPK) models can simulate realistic scenarios that incorporate both PK and PD factors, leading to more informed dosing regimens, especially in cases of polypharmacy.
What challenges exist in conducting PK studies?
Challenges in PK studies include optimizing sampling schedules and managing inter-individual variability to ensure robust data collection and analysis. Addressing these challenges is vital for enhancing the reliability and efficacy of PK analysis.